Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 240-243 doi:10.4236/ampc.2012.24B061 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Experimental Studies on the Influence of HCO3- on Absorption and Desorption of CO2 from Ammonia Solution Shaojian Jiang, Wei Zhong, Rui Peng, Yong Jiang, Jun Zha ng School of Energy Science and Engineering, Central South University, Changsha, China Received 2012 ABSTRACT With aqueous ammonia in the process of CO2 absorption and desorption to join sodium bicarbonate, the influence of HCO3- on CO 2 absorption and desorption from ammonia solution was investigated through the experimental analysis of the desorption quantity of CO2, desorption rate, CO2 loading and the absorption rate. The experimental results showed that, in experimental conditions, The desorption rate decreased gr adually with increasing ammonia concentrations. The desorption rate increased 12%, 17%, 19% and 28.8% when 1 mol/L of ammonia solution is added in 0.1 mol/L, 0.3 mol/L, 0.5 mol/L and 1 mol/L of sodium bicarbonate. The higher concentration of ammonium bicarbonate solution which was added sodium bicarbonate,the more observably the effect of CO2 desorption was promoted. The absorption rate had dropped when absorption process added 0.3 mol/L sodium bicarbonate, the CO2 loadin g was a little change. Keywords: Ammonia Desorption; the Desorption Rate; CO2 Absorption;CO2 Loading 1. Introduction As the rapid development of modern industry, a large number of the use of fossil fuels leads to increased CO2 emissions, and CO2 is climate change and the main cause of global warming. Therefore, carbon capture and storage (CCS) projects have been reported[1]. The chemical absorption capture of CO2 has been studied and widely used as a reliable and cost-efficient method for CO2 because of its characteristics such as higher absorption rate, higher CO2 recovery rate, no absorbent degra- tion and lower desorption energy requirement, etc[2]. The tra- ditional chemical absorption methods include alcohol amine solution, alkali solution, aqueous alkanolamine solution, etc. Among the m, MEA, as a r epresen tative of the alco hol amine i s the most traditional absorbent[3], it is used in industry early in the 19 th century. However, because its higher desorption en- gy, amine degradation by O2, SO2 in flue gas which induces a high absorbent makeup rate and high equipment corrosion rate[ 4], o n the actual plant there is still a limit used in decarbu- rization. Compared with MEA, aqueous ammonia which has higher CO2 load ing capacity (g C O2 absorbed per g absorbent), no corrosion problem, no absorbent degradation problem, lower desorption energy[5,6], and the ability to capture all three major acid gases(S O 2, NOX, CO2), is becoming a hot research 7]. It has been concluded that the maximum CO2 removal effi- ciency by NH3 absorbent can achieve 95% and the major pro- duced from the Aqua Ammonia Process including ammonium bicarbonate and ammonium carbonate etc[8]. Due to the process is reversible, therefore, a way to heat its bicarbonate salts to desorb the solution is used to make sorbent recycling. The process to CO2 desorption which is not desorbed com- pletely has resulted in absorption capacity is reduced. Liu Fang[9] researched the p rocess of CO2 desorption from ammo- nium bicarbonate, Zeng Qing[10] studied the characteristics of CO2 desorption from Carbonated ammonia solution, which showed that desorption rate was influenced by temperature, CO2 loading. Houping Huang[11] investigated a method to regenerate ammonia so as to allow for the ammonia scrubbing technique to be practical in the capture of CO2 that a weakly basic io n-exchange resin co ntaining amine functional groups is used. How to improve the desorption rate, and in the process of absorption recycling, keep the CO2 loading capacity the same are a key research d irection. Research h as shown that[12], with the increasing of the con- ntration of bicarbonate ions, CO2 is easier to release from the solution. This paper studied the influence of HCO3- (put NaH- CO3 into solution as a additives) on CO2 absorption and de- sorption from ammonia solution which keep the concentration of NH4+ the same and change the solution of HCO3- concentra- on. 2. Experiment 2.1. Theory The desorption reaction of CO2 into ammonia solution can be described as the following equations (1), (2), (3). it is shown the lowest enthalpy of dissociation for CO2 release from (1). Ammonium carbonate can be further dissociated to release more CO2, but at higher cost per mole CO2 desorbed. Therefore, in the process of the most favorable desorption is to transfer ammonium carbonate solution from the absorber and use it as CO2 absorbent, as shown in (7). Moreover, it can reduce the NH3 escape. 2NH4HCO3(aq) ⇔ (NH4)2CO3(aq)+CO2(g)+H2O(l) (1) NH4HCO3(aq) ⇔ NH3(aq)+CO2(g)+H2O(l) (2) (NH4)2CO3(aq) ⇔ 2NH3(aq)+CO2(g)+H2O(l) (3) For abso rpti on react ion ,it is typical ch emical reacti on p rocess as following equations (4), (5) ,(6), (7). 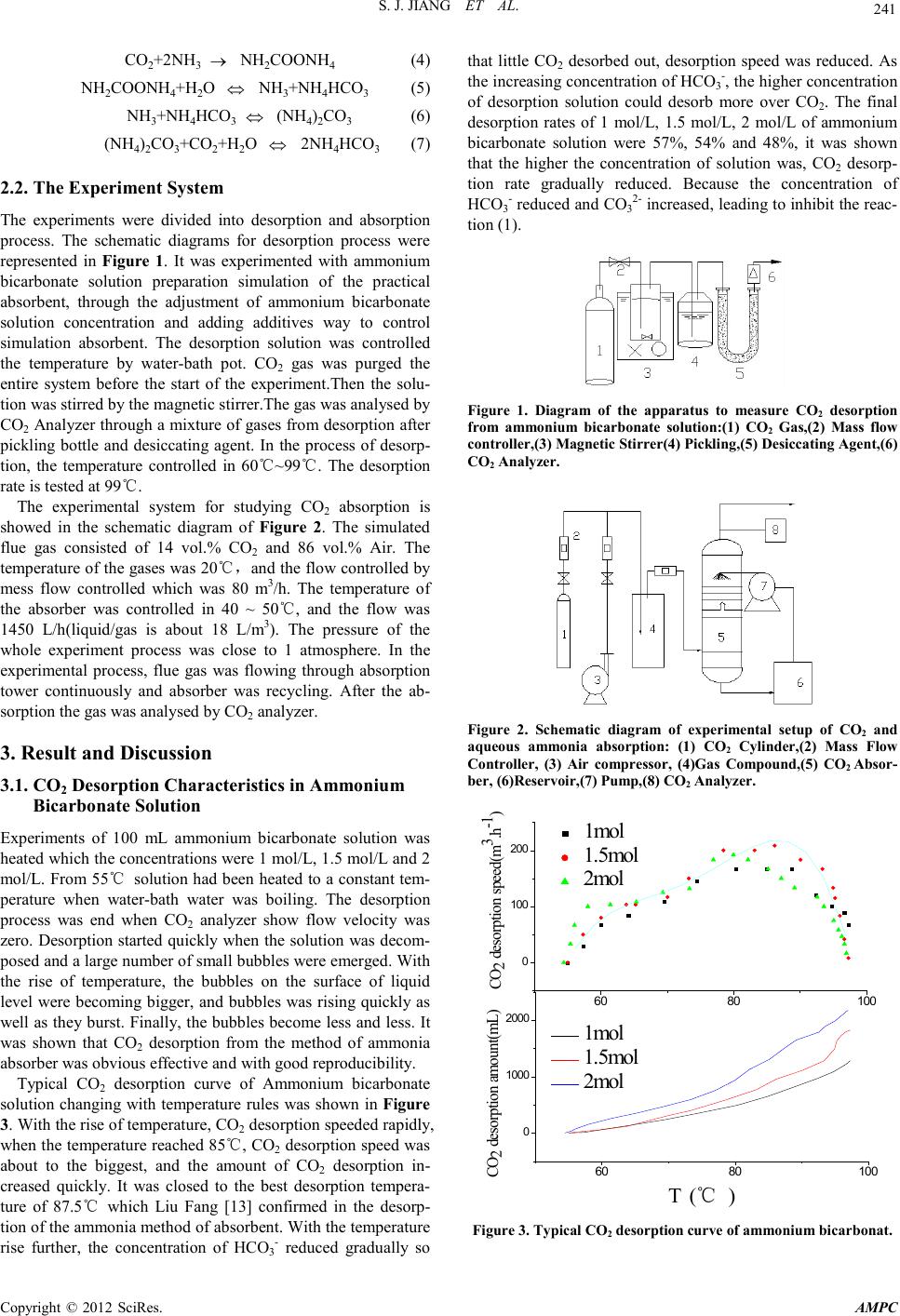 S. J. JIAN G ET AL. Copyright © 2012 SciRes. AMPC 241 CO2+2NH3 → NH2COONH4 (4) NH2COONH4+H2O ⇔ NH3+NH4HCO3 (5) NH3+NH4HCO3 ⇔ (NH4)2CO3 (6) (NH4)2CO3+CO2+H2O ⇔ 2NH4HCO3 (7) 2.2. The Experiment Sy stem The experiments were divided into desorption and absorption process. The schematic diagrams for desorption process were represented in Figure 1. It was experimented with ammonium bicarbonate solution preparation simulation of the practical absorbent, through the adjustment of ammonium bicarbonate solution concentration and adding additives way to control simulation absorbent. The desorption solution was controlled the temperature by water-bath pot. CO2 gas was purged the entire system before the start of the experiment.Then the solu- tion was stirred by the magnetic s tirrer. Th e gas was analysed by CO2 Analyzer through a mixture of gas es from desorp tion after pickling bottle and desiccating agent. In the process of desorp- tion, the temperature controlled in 60℃~99℃. The desorption rate is tested at 99℃. The experimental system for studying CO2 absorption is showed in the schematic diagram of Figure 2. The simulated flue gas consisted of 14 vol.% CO2 and 86 vol.% Air. The temperatu re of the gases was 20℃,and the flow controlled by mess flow controlled which was 80 m3/h. The temperature of the absorber was controlled in 40 ~ 50℃, and the flow was 1450 L/h(liquid/gas is about 18 L/m3). The pressure of the whole experiment process was close to 1 atmosphere. In the experimental process, flue gas was flowing through absorption tower continuously and absorber was recycling. After the ab- sorption th e gas was analysed by CO2 anal yzer. 3. Result and Discussion 3.1. CO2 Desorptio n C haracteri st i c s in Ammonium Bicarbo nate Solution Experiments of 100 mL ammonium bicarbonate solution was heated which th e concentr ations were 1 mol/L, 1.5 mol/L and 2 mol / L. F ro m 55 ℃ solution had been heated to a constant tem- perature when water-bath water was boiling. The desorption process was end when CO2 analyzer show flow velocity was zero. Desorption started quickly when the solution was decom- posed and a large number of small bubb les were emerged . With the rise of temperature, the bubbles on the surface of liquid level were becoming bigger, and bubbles was rising quickly as well as they burst. Finally, the bubbles become less and less. It was shown that CO2 desorption from the method of ammonia absorber was obvious effective and with good reproducibility. Typical CO2 desorption curve of Ammonium bicarbonate solution changing with temperature rules was shown in Figure 3. With the r ise o f temperat ur e, CO2 desorption speeded rapidly, when th e temperature r eached 8 5℃, CO2 desorption speed was about to the biggest, and the amount of CO2 desorption in- creased quickly. It was closed to the best desorption tempera- ture of 87.5℃ which Liu Fang [13] confirmed in the desorp- tion of the ammonia method of absorbent. With the temperature rise further, the concentration of HCO3- reduced gradually so that little CO2 desorbed out, desorption speed was reduced. As the in creasing con centrat ion of HCO3-, the higher concentration of desorption solution could desorb more over CO2. The final desorption rates of 1 mol/L, 1.5 mol/L, 2 mol/L of ammonium bicarbonate solution were 57%, 54% and 48%, it was shown that the higher the concentration of solution was, CO2 desorp- tion rate gradually reduced. Because the concentration of HCO3- reduced and CO32- increased, leading to inhibit the reac- tion (1). Figure 1. Diagram of the apparatus to measure CO2 desorption from ammonium bicarbonate solution:(1) CO2 Gas,(2) Mass flow controller,(3) Magnetic Stirrer(4) Pickling,(5) De sicca ting Agent, (6 ) CO2 Analyzer. Figure 2. Schematic diagram of experimental setup of CO2 and aqueous ammonia absorption: (1) CO2 Cylinder,(2) Mass Flow Controller, (3) Air compressor, (4)Gas Compound,(5) CO2 Absor- ber, (6)Reservoir,(7) Pump,(8) CO 2 Analyzer. 6080 100 0 100 200 1mol 1.5m ol 2m ol 6080 100 0 1000 2000 1m ol 1.5m ol 2m ol CO 2 desorption speed(m3.h-1) CO 2 desorption amount (mL) T (℃ ) Figure 3. Typica l CO2 desorption curve of ammonium bicarbonat. 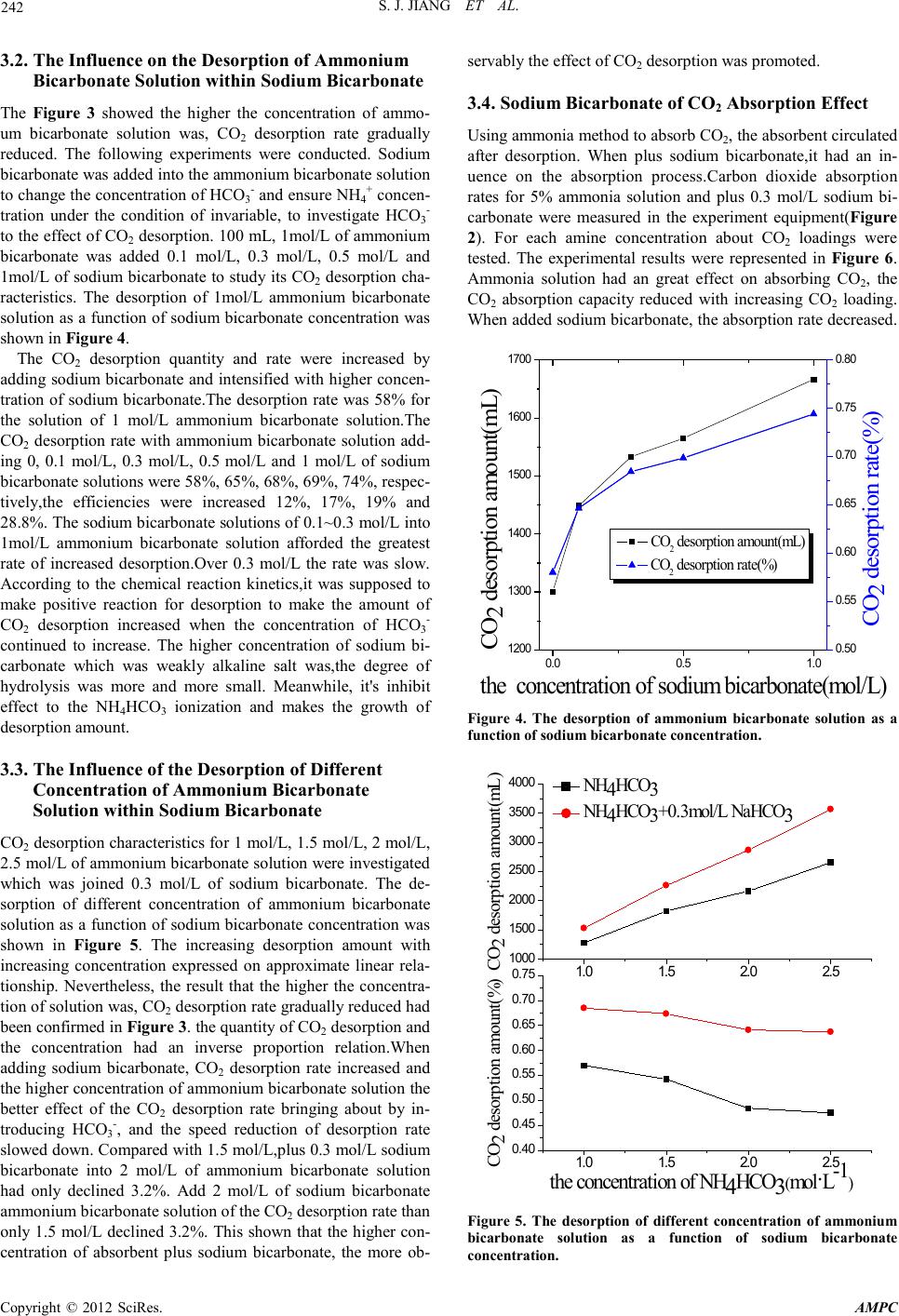 S. J. JIAN G ET AL. Copyright © 2012 SciRes. AMPC 242 3.2. The Influence on the Desorpt ion of Ammonium Bicar bonate Solution w ithin Sodiu m Bic arbonate The Figure 3 showed the higher the concentration of ammo- um bicarbonate solution was, CO2 desorption rate gradually reduced. The following experiments were conducted. Sodium bicarbonate was added into the ammonium bicarbonate solution to ch ange the co ncentrat ion of HCO3- and ensure NH4+ concen- tration under the condition of invariable, to investigate HCO3- to the effect o f CO2 desorption. 100 mL, 1mol/L of ammonium bicarbonate was added 0.1 mol/L, 0.3 mol/L, 0.5 mol/L and 1mol/L of sodium bicarbonate to study its CO2 desorption cha- racteristics. The desorption of 1mol/L ammonium bicarbonate solution as a function of sodium bicarbonate concentrati on was shown in Figure 4. The CO2 desorption quantity and rate were increased by adding sodium bicarbonate and intensified with higher concen- tration of sodium bicarbonate.The desorption rate was 58% for the solution of 1 mol/L ammonium bicarbonate solution.The CO2 desorption rate with ammonium bicarbonate solution add- ing 0, 0.1 mol/L, 0.3 mol/L, 0.5 mol/L and 1 mol/L of sodium bicarbonate solutions were 58%, 65%, 68%, 69%, 74%, respec- tively,the efficiencies were increased 12%, 17%, 19% and 28.8%. The sodium bicarbonate solutions of 0.1~0.3 mol/L into 1mol/L ammonium bicarbonate solution afforded the greatest rate of increased desorption.Over 0.3 mol/L the rate was slow. According to the chemical reaction kinetics,it was supposed to make positive reaction for desorption to make the amount of CO2 desorption increased when the concentration of HCO3- continued to increase. The higher concentration of sodium bi- carbonate which was weakly alkaline salt was,the degree of hydrolysis was more and more small. Meanwhile, it's inhibit effect to the NH4HCO3 ionization and makes the growth of desorption amount. 3.3. The Influence of the Desorption of Different Concentration of Ammonium Bicarbonate Solution within So di um Bicarbona te CO2 desorption characteristics for 1 mol/L, 1.5 mol/L, 2 mol/L, 2.5 mol/L of ammonium bicarbonate solution were investigated which was joined 0.3 mol/L of sodium bicarbonate. The de- sorption of different concentration of ammonium bicarbonate solution as a function of sodium bicarbonate concentration was shown in Figure 5. The increasing desorption amount with increasing concentration expressed on approximate linear rela- tionship. Nevertheless, the result that the higher the concentra- tion of solution was, CO2 desorption rate gradually reduced had been con firmed i n Figure 3. the quantity of CO2 desorption and the concentration had an inverse proportion relation.When adding sodium bicarbonate, CO2 desorption rate increased and the higher concentration of ammonium bicarbonate solution the better effect of the CO2 desorption rate bringing about by in- troducing HCO3-, and the speed reduction of desorption rate slowed down. Compared with 1.5 mol/L,plus 0.3 mol/L sodium bicarbonate into 2 mol/L of ammonium bicarbonate solution had only declined 3.2%. Add 2 mol/L of sodium bicarbonate ammonium bicarbonate solution of the CO2 desorption rate than only 1.5 mol/L declined 3.2%. This shown that the higher con- centration of absorbent plus sodium bicarbonate, the more ob- servably the effect o f CO2 desorption was promoted. 3.4. Sodium Bicarbonate of CO2 Absorption Effect Using ammonia method to absorb CO2, the absorbent circulated after desorption. When plus sodium bicarbonate,it had an in- uence on the absorption process.Carbon dioxide absorption rates for 5% ammonia solution and plus 0.3 mol/L sodium bi- carbonate were measured in the experiment equipment(Figure 2). For each amine concentration about CO2 loadings were tested. The experimental results were represented in Figure 6. Ammonia solution had an great effect on absorbing CO2, the CO2 absorption capacity reduced with increasing CO2 loading. When added sodium bicarbonate, the absorption rate decreased. 0.00.5 1.0 1200 1300 1400 1500 1600 1700 CO 2 desorption amount(m L ) CO 2 desorption rate(% ) the concentration of sodium bicarbonate(m ol/L ) CO 2 desorption amount(m L ) 0.50 0.55 0.60 0.65 0.70 0.75 0.80 CO 2 desorption rate(% ) Figure 4. The desorption of ammonium bicarbonate solution as a function of sodium bicarbonate concentration. 1.0 1.5 2.0 2.5 1000 1500 2000 2500 3000 3500 4000 the concentration of NH 4HCO3 ( mol.L-1 ) CO2 desorption amount(m L) 1.0 1.5 2.0 2.5 0.40 0.45 0.50 0.55 0.60 0.65 0.70 0.75 NH 4 HCO 3 NH 4 HCO 3 +0.3mol/L NaHCO 3 CO2 desorption amount(% ) Figure 5. The desorption of different concentration of ammonium bicarbo nate solution as a function of sodium bicarbonate concentration. 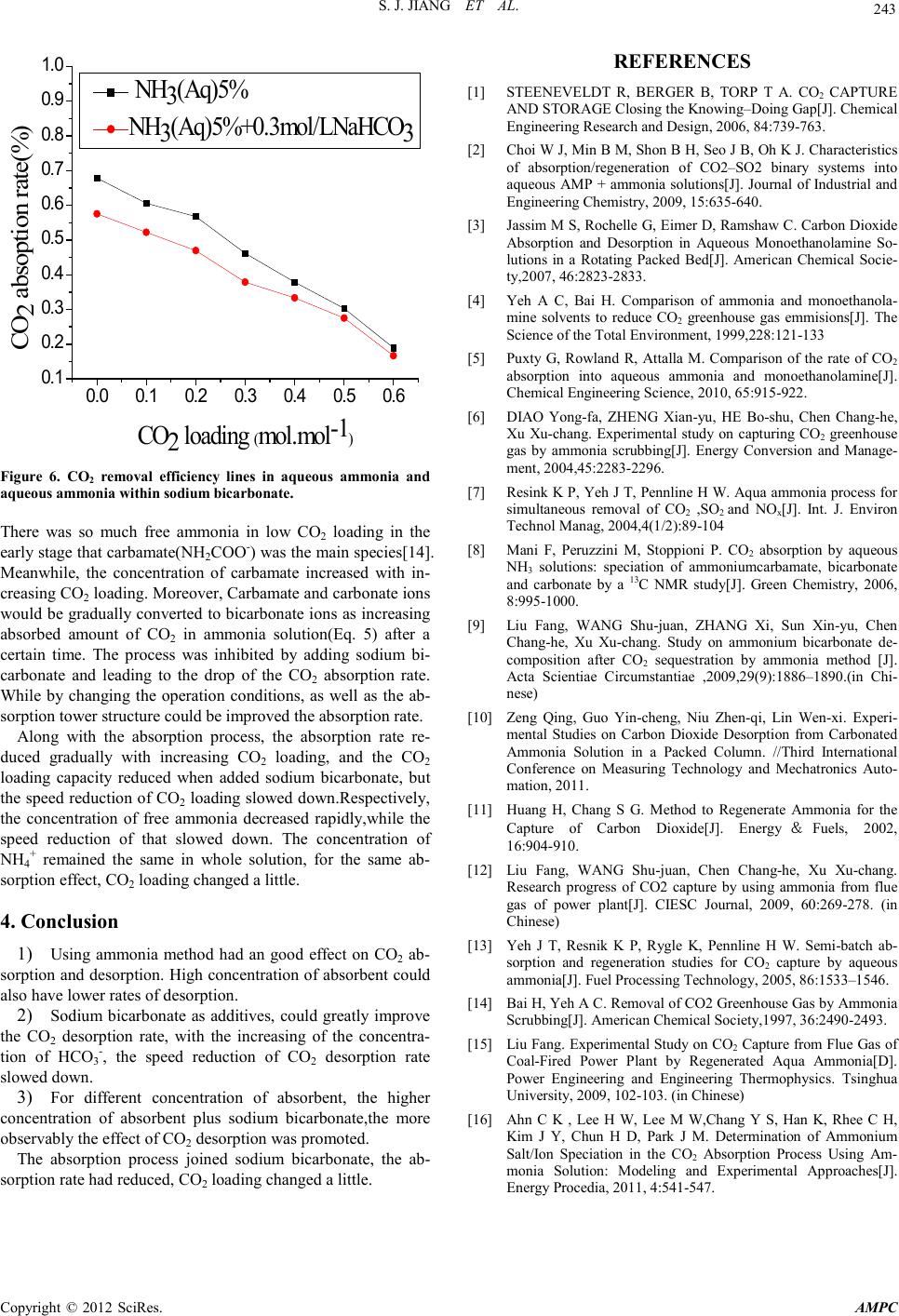 S. J. JIAN G ET AL. Copyright © 2012 SciRes. AMPC 243 0.0 0.1 0.2 0.3 0.40.5 0.6 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 CO2 absoption rate(% ) CO2 loading ( mol.mol-1 ) NH3(Aq)5% NH3(Aq)5%+0.3mol/LNaHCO3 Figure 6. CO2 removal efficiency lines in aqueous ammonia and aqueous am mon ia within sodium bicarbonate. There was so much free ammonia in low CO2 loading in the early stage th at carbamate( NH2COO-) was th e main species[1 4]. Meanwhile, the concentration of carbamate increased with in- creasing CO2 loadi ng. Moreo ver, Carb amate an d carbonate ions would be gradually converted to bicarbonate io ns as increasin g absorbed amount of CO2 in ammonia solution(Eq. 5) after a certain time. The process was inhibited by adding sodium bi- carbonate and leading to the drop of the CO2 absorption rate. While by changing the operation conditions, as well as the ab- sorption tower structure could be improved the absorption rate. Along with the absorption process, the absorption rate re- duced gradually with increasing CO2 loading, and the CO2 loading capacity reduced when added sodium bicarbonate, but the speed reduction of CO2 loading slowed down.Respectively, the concentration of free ammonia decreased rapidly,while the speed reduction of that slowed down. The concentration of NH4+ remained the same in whole solution, for the same ab- sorption effect, CO2 loading changed a little. 4. Conclusion 1) Using ammonia method had an good effect on CO2 ab- sorption and desorption. High concentration of absorbent could also have lower rates of desorption. 2) Sodium bicarbonate as additives, could greatly improve the CO2 desorption rate, with the increasing of the concentra- tion of HCO3-, the speed reduction of CO2 desorption rate slowed down. 3) For different concentration of absorbent, the higher concentration of absorbent plus sodium bicarbonate,the more obser vably the effect of CO2 desorption was promoted. The absorption process joined sodium bicarbonate, the ab- sorptio n rate had reduced, C O 2 load ing changed a little. REFERENCES [1] STEENEVELDT R, BERGER B, TORP T A. CO2 CAPTURE AND ST O RA GE C l o s i ng t he Kno wing –Doi n g Gap [ J] . Ch emi ca l Engin eering Research and Des ign, 2006, 84:739-763. [2] C hoi W J, Min B M, Shon B H, Seo J B, Oh K J . Characteristics of absorption/regeneration of CO2–SO2 binary systems into aqueous AMP + ammonia solutions[J]. Journal of Industrial and Engineering Chemistry, 2009, 15:635-640. [3] Jassim M S, Roch elle G, Ei mer D, Rams haw C. Ca rbon Dioxide Absorption and Desorption in Aqueous Monoethanolamine So- lutions in a Rotating Packed Bed[J]. American Chemical Socie- ty,2007, 46:2823-2833. [4] Yeh A C, Bai H. Comparison of ammonia and monoethanola- mine solvents to reduce CO2 greenhouse gas emmisions[J]. The Science of the Total Environment, 1999,228:121-133 [5] Puxty G, Rowland R, Attalla M. Comparison of the rate of CO2 absorption into aqueous ammonia and monoethanolamine[J]. Chemical Engin eeri ng Science, 201 0, 65:915-922. [6] DIAO Yong-fa, ZHENG Xian-yu, HE Bo-shu, Chen Chang-he, Xu Xu-chang. Experimental study on capturing CO2 greenhouse gas by ammonia scrubbing[J]. Energy Conversion and Manage- ment, 2004,45:2283-2296. [7] Resink K P, Yeh J T, Pennline H W. Aqua ammonia process for simultaneous removal of CO2 ,SO2 and NOx[J]. Int. J. Environ Te c hno l Manag, 2004, 4(1/2) :89-104 [8] Mani F, Peruzzini M, Stoppioni P. CO2 absorption by aqueous NH3 solutions: speciation of ammoniumcarbamate, bicarbonate and carbonate by a 13C NMR study[J]. Green Chemistry, 2006, 8:995-1000. [9] Liu Fang, WANG Shu-juan, ZHANG Xi, Sun Xin-yu, Chen Chang-he, Xu Xu-chang. Study on ammonium bicarbonate de- composition after CO2 sequestration by ammonia method [J]. Acta Scientiae Circumstantiae ,2009,29(9):1886–1890.(in Chi- nese) [10] Zeng Qing, Guo Yin-cheng, Niu Zhen-qi, Lin Wen-xi. Experi- mental Studies on Carbon Dioxide Desorption from Carbonated Ammonia Solution in a Packed Column. //Third International Conference on Measuring Technology and Mechatronics Auto- mation, 2011. [11] Huang H, Chang S G. Method to Regenerate Ammonia for the Capture of Carbon Dioxide[J]. Energy&Fuels, 2002, 16:904-910. [12] Liu Fang, WANG Shu-juan, Chen Chang-he, Xu Xu-chang. Research progress of CO2 capture by using ammonia from flue gas of power plant[J]. CIESC Journal, 2009, 60:269-278. (in Chinese) [13] Yeh J T, Resnik K P, Rygle K, Pennline H W. Semi-batch ab- sorption and regeneration studies for CO2 capture by aqueous ammonia[J]. Fuel Processing Technology, 2005, 86:1533–1546. [14] Ba i H, Yeh A C. Removal of C O2 Greenh ou se Gas b y Ammoni a Scrubbing[J]. American Chemical Society,1997, 36:2490-2493. [15] Liu Fan g. Experim ental Study on CO2 Capture from Flue Gas of Coal-Fired Power Plant by Regenerated Aqua Ammonia[D]. Power Engineering and Engineering Thermophysics. Tsinghua University, 20 09, 102-103. (in Chinese) [16] Ahn C K , Lee H W, Lee M W,Chang Y S, Han K, Rhee C H, Kim J Y, Chun H D, Park J M. Determination of Ammonium Salt/Ion Speciation in the CO2 Absorption Process Using Am- monia Solution: Modeling and Experimental Approaches[J]. Energy Procedia, 2011, 4:541-547. |

