Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 68-71 doi:10.4236/eng.2012.410B017 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Ultrasound Mediated Delivery of Liposomal Doxorubicin in Mice with Glioma Feng-Yi Yang1, Shih-C heng H or ng2 1Department of Biomedical, Imaging and Radi o lo g ical Sciences, S chool of Biomed i cal Science and Engi neering, Nati onal Yang-Ming University, Taipei, Taiwan 2Department of Compu ter Science & In f o r mation , Engineering, Chaoyang University of Tech nology, Taichung, Taiw an Email: fyyang@ym.edu.tw, schong@cyut.edu.tw Received 2012 ABSTRACT Malignant brain tumors remain difficult to treat with chemotherapy because the blood–brain barrier (BBB) limits the amounts of pot ent agents th at can reach the tumor , such that the d rugs are un able to reach ther apeut ic dosage. Alth ough variou s targeted carriers that encapsulate chemotherapeutic agents have been shown to improve drug delivery to brain tumors, the BBB is still a major ob- stacle in the use of chemotherapy for the treatment of these tumors. Human glioblastoma-bearing mice were injected intravenously with doxorubicin (Dox) encapsulated in atherosclerotic plaque-specific peptide-1 (AP-1)-conjugated liposomes or unconjugated li- posome. These treatments took place with or without BBB disruption induced by transcranial pulsed high-intensity focused ultra- sound (pulsed HIFU). This study showed that the treatment with Dox encapsulated in AP-1-conjugated liposomes followed by pulsed HIFU enhanced the accumulation of the cytotoxic drug in cells and inhibited the growth of brain tumors in vivo. Combining pulsed HIFU with cytotoxic agents might improve their efficacy in patients with brain tumors while simultaneously reducing the drug side effects. Further investigation is required to provide a comprehensive physical characterization of the sonication process and to deter- mine its bioeffects. Keywords: Focused Ult ra s ound; IL-4; Blood-b rain Barrier; Brain Tumor; Drug Delivery 1. Introduction The limited amount of chemotherapeutic agent present in the circulation is able to be transported into brain tumors without the assistance of a blood-tumor barrier (BTB) delivery system due to the presen ce of the BTB. So me works have repo rted that using infusion of hyperosmotic solution of mannitol, which disrupts the BTB, the drug uptake in brain tumor could be higher than in tumor without BTB disruption [1-3]. Lipo- some-based drug delivery systems have been designed to in- crease tumor drug levels while limiting systemic drug dosage [4] . It is thought that the employment of liposomes conjugated to an tibodies o r targeti ng ligand s can enh ance the accu mulatio n and retention of drugs at the tumor region compared with the free drug [5] . The glioblastoma multiforme (GBM) is one of the most common forms of glioma. It is hard to treat gliomas completely by surgical res ection and therefore rad iotherapy and ch emothe- rapy are used to remove residual microscopic tumor material [6] . Previous reports have demonstrated that high-dose chemo- therapy may have a potential survival benefit compared to his- torical controls treated with standard-dose therapy [7-8]. It has been shown that human brain tumor cell lines express high levels of pl asma membrane i nterleukin-4 receptors (IL-4R) [9]. Thus, selective drug delivery may be achievable by binding chemotherapeutic agents to IL-4R [10]. A ligan d from atheros- clerotic plaque-specific peptide-1 (AP-1) was selected from phage display libraries that can locate atherosclerotic plaque tissue and bind to the IL-4 recept or, since i t has the s ame bin d- ing motif to the IL-4 protein. AP-1-labeled nanoparticles have been used for the targeted drug delivery to tumor in the pre- vious works [11-12]. The purpose of this study was to demonstrate that the tech- nology combines pulsed HIFU and liposomal nanoparticles as a synergistic delivery system for treating malignant brain tumors. 2. Methodology 2.1. Gl i oma Xenograft Model Male 6 to 8-week-old NOD-scid mice were anesthetized by an intraperitoneal administration of pentobarbital at a dose of 40 mg/kg of body weight. All animal experiments were performed according to the appropriate guidelines and approved by our Animal Care and Use Committee. The 2 × 105 Human brain malignant glioma (GBM8401) cells in 2 μL culture medium were injected into the brains of the mice. Magnetic Resonance imaging (MRI) was used to determine that a tumor was estab- lished. 2.2. Ultrasound Equipment Pulsed HIFU exposures were generated by a 1.0-MHz, sin- gle-element focused transducer (A392S, Panametrics, Waltham, MA, USA) with a diameter of 38 mm and a radius of curvature of 63.5 mm. T he fo cal zo ne o f the ther apeu ti c transdu cer was in the shape of an elongated ellipsoid, with a radial diameter (–6 dB) of 3 mm and an axi al length (–6 dB) of 26 mm. The trans- ducer with removab le co ne was fixed on a st ereo taxic ap par atu s 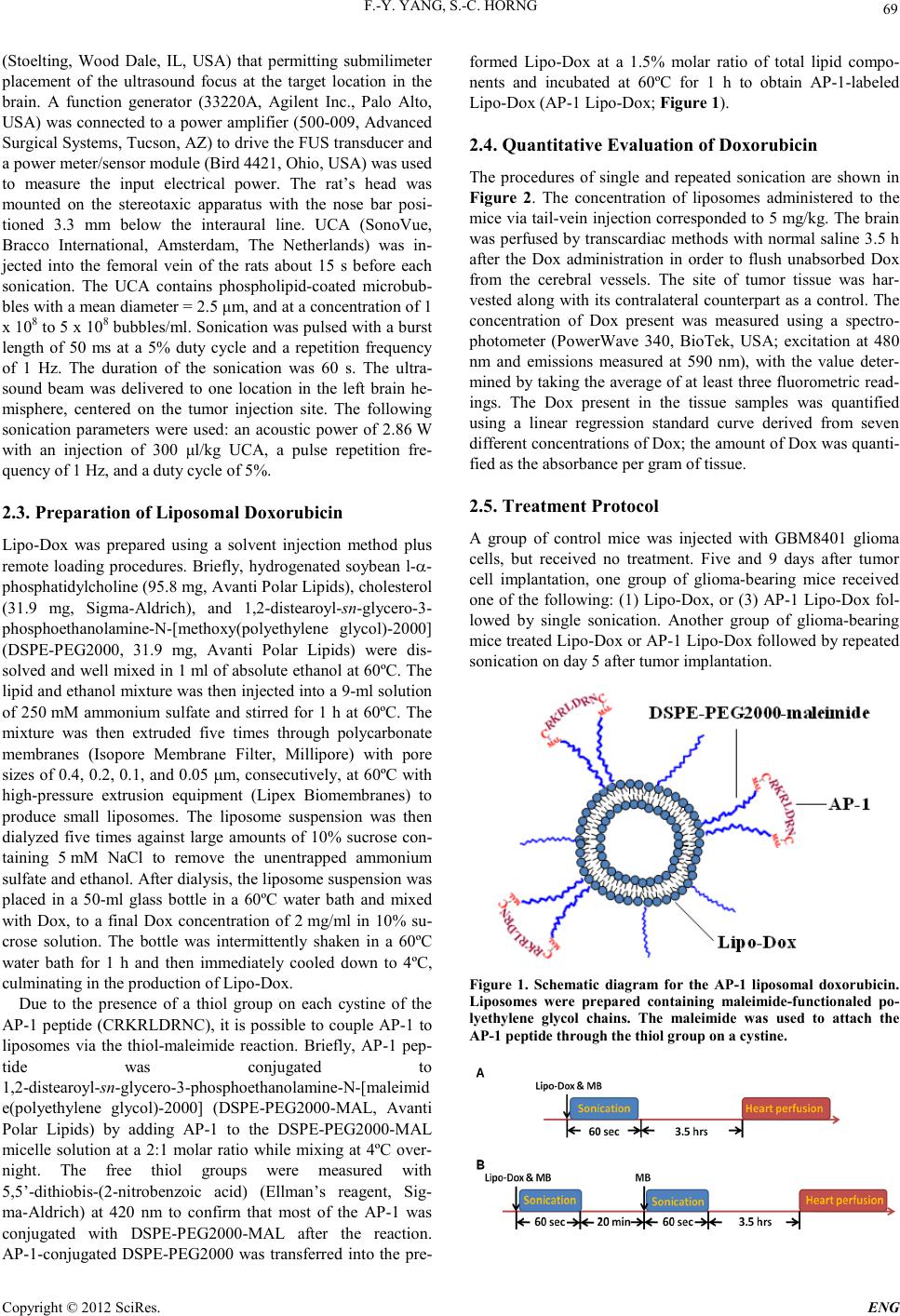 F.-Y. YANG, S.-C. HORNG Copyright © 2012 SciRes. E NG 69 (Stoelting, Wood Dale, IL, USA) that permitting submilimeter placement of the ultrasound focus at the target location in the brain. A function generator (33220A, Agilent Inc., Palo Alto, USA) was connected to a power amplifier (500-009, Advanced Surgical Syste ms, Tucso n , AZ) to drive the FUS transducer and a power meter/sensor module (Bird 4421, Ohio, USA) was used to measure the input electrical power. The rat’s head was mounted on the stereotaxic apparatus with the nose bar posi- tioned 3.3 mm below the interaural line. UCA (SonoVue, Bracco International, Amsterdam, The Netherlands) was in- jected into the femoral vein of the rats about 15 s before each sonication. The UCA contains phospholipid-coated microbub- bles with a mean diameter = 2.5 μm, and at a concentrat ion o f 1 x 108 to 5 x 108 bubbles/ml. Sonication was pulsed with a burst length of 50 ms at a 5% duty cycle and a repetition frequency of 1 Hz. The duration of the sonication was 60 s. The ultra- sound beam was delivered to one location in the left brain he- misphere, centered on the tumor injection site. The following sonication parameters were used: an acoustic power of 2.86 W with an injection of 300 μl/kg UCA, a pulse repetition fre- quency of 1 Hz, and a duty cycle of 5%. 2.3. Preparation of Liposomal Doxorubicin Lip o-Dox was prepared using a solvent injection method plus remote loading procedures. Briefly, hydrogenated soybean l-α- phosphatidylcholine (95.8 mg, Avanti Polar Lipids), cholesterol (31.9 mg, Sigma-Aldrich), and 1,2 -distearoyl-sn-glycero-3- phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000, 31.9 mg, Avanti Polar Lipids) were dis- solved and well mixed in 1 ml of absolute ethanol at 60ºC. The lipid and ethanol mixture was then injected into a 9 -ml solution of 250 mM ammonium sulfate and stirred for 1 h at 60ºC. The mixture was then extruded five times through polycarbonate membranes (Isopore Membrane Filter, Millipore) with pore sizes of 0.4, 0.2, 0.1, and 0.05 µm, consecutively, at 60ºC with high-pressure extrusion equipment (Lipex Biomembranes) to produce small liposomes. The liposome suspension was then dialyzed five times against large amounts of 10% sucrose con- taining 5 mM NaCl to remove the unentrapped ammonium sulfate and ethanol. After dialysis, the liposome suspension was placed in a 50-ml glass bottle in a 60ºC water bath and mixed with Dox, to a final Dox concentration of 2 mg/ml in 10% su- crose solution. The bottle was intermittently shaken in a 60ºC water bath for 1 h and then immediately cooled down to 4ºC, culminating in the production of Lipo-Dox. Due to the presence of a thiol group on each cystine of the AP-1 peptide (CRKRLDRNC), it is possible to couple AP-1 to liposomes via the thiol-maleimide reaction. Briefly, AP-1 pep- tide was conjugated to 1,2-distearoyl-sn-g lycer o-3-phosphoethanolamine-N-[maleimid e(polyethylene glycol)-2000] (DSPE-PEG2000-MAL, Avanti Polar Lipids) by adding AP-1 to the DSPE-PEG2000-MAL micelle solution at a 2:1 molar ratio while mixing at 4ºC over- night. The free thiol groups were measured with 5,5’-dithiobis-(2-nitrobenzoic acid) (Ellman’s reagent, Sig- ma-Aldrich) at 420 nm to confirm that most of the AP-1 was conjugated with DSPE-PEG2000-MAL after the reaction. AP-1-conjugated DSPE-PEG2000 was transferred into the pre- formed Lipo-Dox at a 1.5% molar ratio of total lipid compo- nents and incubated at 60ºC for 1 h to obtain AP-1-labeled Lip o-Dox (AP-1 Lipo-Dox; F igure 1). 2.4. Quantitative Evaluation of Doxorubicin The procedures of single and repeated sonication are shown in Figure 2. The concentration of liposomes administered to the mice via tail-vein injection corresponded to 5 mg/kg. The brain was perfused by transcardiac methods with normal saline 3.5 h after the Dox administration in order to flush unabsorbed Dox from the cerebral vessels. The site of tumor tissue was har- vested alo ng with its contralateral count erpart as a control. The concentration of Dox present was measured using a spectro- photometer (PowerWave 340, BioTek, USA; excitation at 480 nm and emissions measured at 590 nm), with the value deter- mined by ta king the average o f at least th ree fluoro metric read- ings. The Dox present in the tissue samples was quantified using a linear regression standard curve derived from seven different concentrations of Dox; the amount of Dox was quanti- fied as the absorbance per gram of tissue. 2.5. Treatment Protocol A group of control mice was injected with GBM8401 glioma cells, but received no treatment. Five and 9 days after tumor cell implantation, one group of glioma-bearing mice received one of the following: (1) Lipo-Dox, or (3) AP-1 Lipo-Dox fol- lowed by single sonication. Another group of glioma-bearing mice treated Lipo-Dox or AP-1 Lipo-Dox followed by repeated sonication on day 5 after tumor implantation. Figure 1. Schematic diagram for the AP-1 liposomal doxorubicin. Liposomes were prepared containing maleimide-functionaled po- lyethylene glycol chains. The maleimide was used to attach the AP-1 peptide through the thiol group on a cystine.  F.-Y. YANG, S.-C. HORNG Copyright © 2012 SciRes. ENG 70 Figure 2. Diagrams of the experimental time line for (A) single sonication and (B) repeated sonication. 2.6. MR Imaging Magneti c resonance i maging (MR I) was performed us ing a 3-T MRI system (TRIO 3-T MRI, Siemens MAGNETOM, Ger- many) after focused ultrasound sonication. The mice were anesthetized with isoflurane mixed with oxygen during the imaging procedure. A loop coil (Loop Flex Coil, approximately 4 cm in diameter) was used for RF reception. The imaging plane was located across the center of the tumor injection site. Tumor progression was monitored by means of T2-weighted images obtained from 4 to 16 days after tumor implantation. The parameters for T2-weighted imaging were as follows: re- petition time/echo time = 3500/75 ms, matrix = 125×256, field of view = 25×43 mm, and secti on thi ckness = 1.0 mm 3. Results Figure 3 shows the mean concentration of Dox per unit mass for the contralateral normal brain, the brain tumors and the brain tumors with single or repeated sonication after unconju- gated Lipo-Dox or AP-1 Lipo-Dox administration. The concentration of Dox was not only significantly greater in the unsonicated tumor BBB than in the contralateral normal brain region, but it was also significantly greater at the tumor site after single or repeated sonication than in the unsonicated tumor for the two forms of Lipo-Dox, especially for the re- peated sonication. In addition, the concentration of Dox was significantly greater at the tumor site with unconjugated Lip o-Dox followed by single or repeated sonication than in the unsonicated tumor treated with AP-1 Lipo -Dox without sonica- tion. Figure 3. Measurements of Lipo-Dox and AP-1 Lipo-Dox in con- tralateral normal brain, the brain tumor and the brai n tumor with single or repeated sonication. Compared with the contralateral normal brain, there was a significant differe nce for the control and sonicated tumors between the two forms of the drug. The concen- trations of Dox were significantly higher in sonicated brai n tumors than in unsonicated brain tumors. */***, #/### and ††/††† denote significant differences compared with the contralateral normal brain, the unsonicated brain tumor and single sonicated tumor, respectively. (*,#,‡P<0.05, ††P<0.01, ***,###, †††P<0.001; n=3 mice per group). Compared to the control tumor, there was a significant in- crease in the derived tumor-to-contralateral brain ratios for the single or repeated sonicated tumor treated with either drug (Figure 4). Interestingly, the derived tumor-to-contralateral brain ratio was significantly greater after single or repeated sonication for the untargeted Lipo-Dox group than for the tar- geted Lipo-Dox group without sonication. Figure 5 shows the effects of the various treatment protocols on tumor growth were monitored by MR imaging on day 12 after implantation. These results suggest that AP-1 Lipo-Dox enhan ced by pulsed HI FU at the tumor site i s more effective at inhibiting tumor growth than AP-1 Lipo-Dox alone. 4. Discussion Malignant glioma remains one of the most deadly types of tu- mor in humans, and GBM is one of the most common forms of glioma. Pulsed HIFU induced high-dose chemotherapy may have a poten tial survi val benefit compared to his torical cont rols treated with standard-dose therapy. This study showed that the treatment with Dox encapsulated in AP-1-conjugated liposomes followed by single or repeated pulsed HIFU enhanced the ac- cumulation of the cytotoxic drug in cells and inhibited the growth of brain tumors in rats. Combining pulsed HIFU with c yt otoxic agents might improve their efficacy in brain tumors with minimal systemic toxicity. This technology, employed with recent advances in targeted microbubbles, may promote Figure 4. The derived tumor-to-contralateral brain ratios with unsonication, single sonication and repeated sonic ation after drug administration. (*,#P<0.05, ##,††P<0.01, ***,†††P<0.001; n=3 mice per group). Figure 5. Representative sample of T2-weighted magnetic reson- ance imaging of a human GMB 8401 xenograft on day 12 after i mplantati on. Compared to the control tumor, there was a clear  F.-Y. YANG, S.-C. HORNG Copyright © 2012 SciRes. E NG 71 decrease in the size of the tumor in mice treated with AP-1 Lipo- Dox fo llowed by single pulsed HIFU or repeated pulsed HIFU. new approaches to be developed that make targeted brain tumor therapy possible. The results of this pilot study therefore sug- gest it would provide targeted access for chemotherapy and allow the use of recombinant pharmaceuticals for the brain diseases. 5. Acknowledgements This study was supported by grants from the National Science Council of Taiwan (no. NSC 100-2321-B-010-010 and NSC 99-2321-B-010-017), Cheng Hsin General Hospital Foundation (no. 100F117CY25), Veterans General Hospitals University System of Taiwan Joint Research Program (#VGHUST100-G1- 3-3 and V100E6-007), Yen Tjing Ling Medical Foundation (grant CI-100-17), Department of Health of Taiwan (DOH101- TD-PB-111-TM012). REFERENCES [1] R. Barth, et a l., "Boron neutron cap ture thera py of brain tumors: enhanced survival following intracarotid injection of either so- dium borocaptate or boronophenylalanine with or without blood-brain barrier disruption," Cancer Research, vol. 57, p. 1129, 1997. [2] R. F. Barth, et al., "Neutron capture therapy of intracerebral melanoma: enhanced survival and cure after blood-brain barrier opening to improve delivery of boronophenylalanine," Int J Ra- diat Oncol Biol Phys, vol. 52, pp. 858-68, Mar 1 20 02. [3] C. H. Hsieh, et al., "Evaluation of pharmacokinetics of 4-borono-2-(18)F-fluoro-L-phenylalanine for boron neutron captu re th erap y in a glioma -bearing rat model with hyperosmolar blood-brain barrier disruption," J Nucl Med, vol. 46, pp. 1858-65, Nov 2005. [4] T. M. Allen and P. R. Cullis, "Drug delivery systems: entering the mainstrea m ," Science, vol. 303, pp. 1818-22, Mar 19 200 4. [5] C. R. Dass, et al., "Enhanced anticancer therapy mediated by specialized liposomes," J Pharm Pharmacol, vol. 49, pp. 972-5, Oct 1997. [6] D. F. Deen, et al., "Brain Tumor Working Group Report on the 9th International Conference on Brain Tumor Research and Therapy. Organ System Program, National Cancer Institute," J Neurooncol, vol. 16, pp. 243-72, Jun 1993. [7] R. J. Motzer, et al., "Phase II trial of high-dose carboplatin and etoposide with autologous bone marrow transplantation in first-line therapy for patients with poor-risk germ cell tu mors," J Natl C ancer Inst, vol. 85, pp. 1828-35, Nov 17 1993. [8] R. J. Motzer, et al., "High-dose carboplatin, etoposide, and cyc- lophosphamide with autologous bone marrow transplantation in first-line therapy for patients with poor-risk germ cell tumors," J Clin Oncol, vol. 15, pp. 2546-52, J ul 1997. [9] R. K. Puri, et al., "Human neurological cancer cells express interleukin-4 (IL-4) receptors which are targets for the toxic ef- fects of IL4-Pseudomonas exotoxin chimeric protein," Int J Cancer, vol. 58, pp. 574-81, Aug 15 1994. [10] H. Y. Hong, et al., "Phage display selection of peptides that home to atherosclerotic plaques: IL-4 receptor as a candidate target in atheroscler osis," J Cell Mol Med, vol. 12, pp. 2003 -14, Oct 2008. [11] X. L. Wu, et al., "Tumor-targeting peptide conjugated pH-responsive micelles as a potential drug carrier for cancer therapy," Bioconjug Chem, vol. 21, pp. 208-13, Feb 17 2010. [12] J. H. Kim, et al., "Facilitated intracellular delivery of pep- tide-guided nanoparticles in tumor tissues," J Control Release, Sep 16 20 11. |

