Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 237-239 doi:10.4236/ampc.2012.24B060 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Comparison of Gas Permeability and Selectivity Between Alumi na Membrane and Vycor Glass at High Temperatur es* F. N. Tüzün, E. Ko çdemir, G.Uğuz Department of Chemical Engineering, Hitit University, Çorum, TURKEY Email: nihaltuzun@yahoo.com Received 2012 ABSTRACT In this study, gas permeability and selectivity of Vycor glass and alumina membrane were compared by using H2, CO2, CO, CH4 and N2 gases at the temperatures of 323-823 K before and afterapplying the silica coating process. H2 permeability decreased with in- creasing the temperature before silica coating both in Vycor glass and alumina membrane. However, H2 permeability increased with increasing the temperature, that was an indication of activated transport after silica coating both in Vycor glass and alumina membrane. Lo wer p er meabili t y valu es and h igher selecti viti es wer e ob tai ned in V ycor glass membr an e th an al umina membran e ha v- ing higher permeability and lower selectivities. Keywords: Membrane; Hydrogen; Permeability; Selecti vi ty 1. Introduction Membrane separation of gaseous mixtures has come to attract much a t tenti on fr om t he v iew point of energy-conservative recovery of gases, especially at hi gh temperature, wher e most o f ordin ary organi c membr anes can n ot be applied because of thei r thermal instabi lity. In cont rast, inorganic me mbranes, which seem quite stable at high temperature and against most chemicals, will preferably be applied to gas separation processes as in [1]. The preparation and application of ceramic membranes has received much at tention in the past few years as in [2]. Ceramic membranes are technically important in separation and filtra- tion as well as in catalytic reactions, because of their high thermal and chemical stability, long life time and good defoul- ing properties in comparison with polymeric membranes as in [3,4]. Hydrogen permeable an d selectiv e silica membran es have at- tracted much interest in the membrane gas separation field due to the importance of hydrogen as an industrial feedstock for the production of fuels and many chemicals as in [5, 6]. Silica membranes are attractive since they are chemically and ther- mally stable while offering high permeability and selectivity for hydrogen as in [7]. Silica fab ricated b y the so l-gel process is known to have high surface are a an d microporosity as in [8-10]. The so l-gel pr ocess, in this case, refers to the controlled hydrolysis and polymeriza- tion of tetraethylorthosilicate(TEOS) in a water-alcohol solu- tion as in [11,12]. The solution goes through an irreversible sol-gel transition. The resulting gel can be dried to a rigid sili- ca-like material . 2. Experimental The alumina membrane changing the particle size distribution from 3 µm to 70 nm with the diameter of 21 mm and the thick- ness of 1 mm was purchased from Germany. The Vycor glass having t he mean par ticl e size o f 4 0 Angst ro m with th e d iameter of 21 mm and the thickness of 1 mm was purchased from USA. Gas permeability tests of Vycor glass and alumina membrane were performed by using H2, CO2, CO, N2 and CH4 gases at constant volume-variable pressure measurement system be- tween 323 and 823 K and H2 selectivites o ver other gas es were determined. Then, alumina membrane was dipped into LUDOX colloidal silica solution at 2-3 s, subsequently, it was calcined in the oven up to the temperature of 1173 K with heating rate of 1 K/min. After two hours passed at the temperature of 1173 K, calcination was continued with cooling rate of 1 K/min. How- ever, sol-gel process was applied to Vycor glass by refluxing a solution of TEOS, H2O, C2H5OH and HNO3 in the ratio of 1: 1: 26: 11.76 respectively at 353 K for two hours. A sample of solution was diluted with C2H5OH in the ratio of 1:18 and membrane was dipped for a few seconds. It was then dried at 393 K for three hours with heating rate of 1 K/min and calcined at 673 K for three hours with the same heating rate. The dip- ping was repeated a second time with the dilution being 1:180. After silica coating processes of Vycor glass and alumina membrane were completed, they were exposed to gas permea- tion experiments with using H2, CO2, CO, N2 and CH4 gases among 323 and 823 K and H2 selectivit es o ver ot her gases wer e detected. 3. Results and Discussion Gas permeability test of samples including Vycor glass and alumina membrane before and after silica coating was fulfilled by using constant volume-variable pressure measurement sys- tem for the temperatures around 323 and 823 K and H2 selecti- vites over other gases were determined. Permeability coeffi- cient of each gas was calculated from (1) and selectivity was also d etected from (2 ) . *The scientific and technological research council of turkey gave the financial support for the preparation of this work. 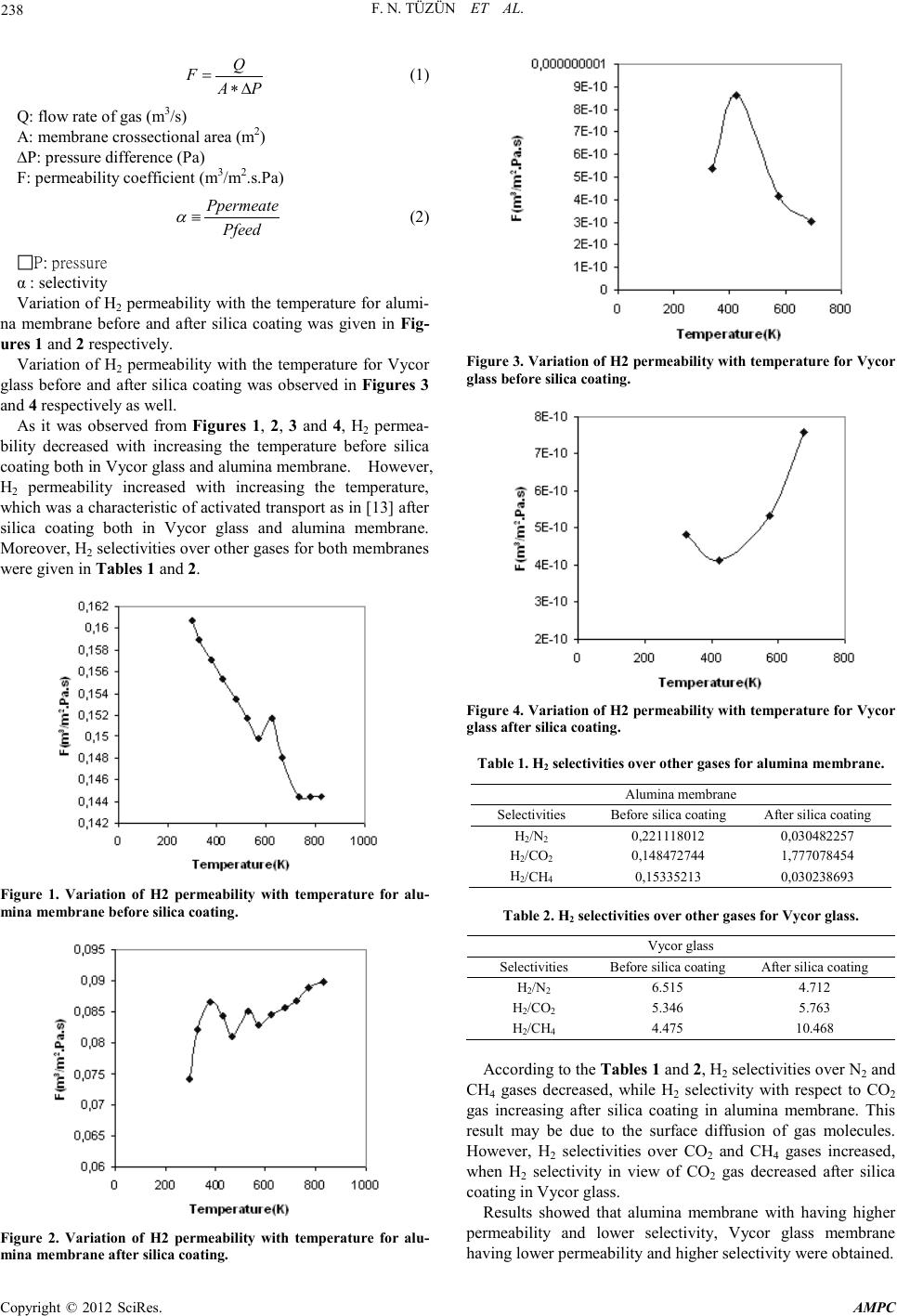 F. N. TÜZÜN ET AL. Copyright © 2012 SciRes. AMPC 238 Q FAP = ∗∆ (1) Q: flow rate of gas (m3/s) A: membrane crossect ional area (m2) ∆P: pres sure differen ce ( Pa) F: permeability coefficient ( m3/m2.s.P a ) Ppermeate Pfeed α ≡ (2) P: pressure α : selectivity Variation of H2 permeability with the temperature for alumi- na membrane before and after silica coating was given in Fig- ures 1 and 2 respectivel y. Variation of H2 permeability with the temperature for Vycor glass before and after silica coating was observed in Figures 3 and 4 respectively as well. As it was observed from Figures 1, 2, 3 and 4, H2 permea- bility decreased with increasing the temperature before silica coating both in Vycor glass and alumina membrane. However, H2 permeability increased with increasing the temperature, which was a ch aracter istic o f activated transp ort as i n [13] after silica coating both in Vycor glass and alumina membrane. Moreo ver, H 2 selecti viti es over o t h er gases for b o th membranes were given in T a bles 1 and 2. Figure 1. Variation of H2 permeability with temperature for alu- mina membrane before silica coating. Figure 2. Variation of H2 permeability with temperature for alu- min a mem b ran e aft er sil ica coatin g. Figure 3. Variation of H2 permeability with temperature for Vycor glass before silica coating. Figure 4. Variation of H2 permeability with temperature for Vycor glass after silica coating . Tabl e 1. H2 selectivities over other gases for alumina membrane. Alumina membrane Selectivities Before silic a coating After silica c oating H2/N2 0,221118012 0 ,030482257 H2/CO2 0,148472744 1 ,777078454 H2/CH4 0,15335213 0 ,030238693 Table 2. H2 selectivities over other gases for Vycor glass. Vycor glass Selectivities Before silic a coating After silica coating H2/N2 6.515 4.712 H2/CO2 5.346 5.763 H2/CH4 4.475 10.468 According to the Tables 1 and 2, H2 selectivities over N2 and CH4 gases decreased, while H2 selectivity with respect to CO2 gas increasing after silica coating in alumina membrane. This result may be due to the surface diffusion of gas molecules. However, H2 selectivities over CO2 and CH4 gases increased, when H2 selectivity in view of CO2 gas decreased after silica coating in Vycor glass. Results showed that alumina membrane with having higher permeability and lower selectivity, Vycor glass membrane having l ower per meabi lity and higher selectivity were obtain ed.  F. N. TÜZÜN ET AL. Copyright © 2012 SciRes. AMPC 239 4. Acknowledgements The authors would like to thank to the scientific and technolo- gical research council of turkey for the financial support given in 2209 Scientist Supporting Programme related to the prepara- tion of this work. REFERENCES [1] S.Kitao, H.Kameda, M.Asaeda, Membrane, 15(4), 222-227 (1990). [2] J.-H.Lee, S.-C. Choi, D.-S.Bae, K.-S. Han, Journal of Materials Science Letters, 18(1999) 1367-1369. [3] Y.S.L in, A.J.Bur g g r aaf , J.Amer .Ce r am.S o c., 7 4( 1 9 91) 2 9. [4] K.K. Chan, A.M. Brownstein, Amer.Ceram.Soc. Bull.70(1991) 703. [5] R.Ramachandran, R.K.Menon, Int.J.Hydrogen Energy 23(1998) 593. [6] T.N.V e z iroglu, Chem.Ind.53(19 99 ) 3 83. [7] A.J.Burggraaf, L.Cot, Fundamentals of Inorganic Membrane Science and Techn ology, Elsevier, Am sterdam, 1996. [8] B.E.Yoldas, J.Non-Cryst.So lids 63, 14 5(1984). [9] C.J.Brinker, K.D.Keefer, D.W.Schaefer, C.S.Ashley, J.Non-Cryst.Solids 48, 47(1982). [10] J.Zarzycki, M.Prassas, J.Phalippou, J.Mater.Sci. 17, 3371(1982). [11] R.Aelion, A.Loebel, F. Eirich, Amer.Ceram.Soc. 72, 5705(1950). [12] H.Dislich, P. Hinz, J.Non-Cryst.Solids 48, 11(1982). [13] S.Giessler, L.Jordan, J.C.D.Da Costa, Separation and Purifica- tion Technolog y, 32(2003 ) 255. |

