Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 53-56 doi:10.4236/eng.2012.410B014 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Infra-red Thermal Imaging of the Inner Canthus: Correlates with the Temperature of the Injured Human B rain Char maine C hi lds1, Mya Myint Zu 1, Aung Phyo Wai1, Yeo Tseng Tsai1, Shiqian Wu2, Wang Li3 1ALCNS and Department of Surgery, National University of Singapore (NUS), Nati onal Uni versity Hospital (NUH), Sin gapore 2Signal Processing Depar tment, Inst itute for Infocomm Research, Fusionopolis Way, #21 -01 C onnexis, Singapore 3National Metrology Centre (NMC), A*Star, Singapore Email: nurcc@nus.edu.sg Received 2012 ABSTRACT Introduction: Infra-red (IR) thermometr y is a safe and valid meth od to determine in tern al and surface temperat ure in hu man subject s. Under conditions of brain damage (head injury or stroke) knowledge of changes in the temperature of intracranial tissue is justified because of the vulnerability of neurons to accelerated damage at temperatures at the upper end of the febrile range. Aim: To deter- mine the temperatu r e at the inner cant hus (I C ) of the eye as a potential surrogate for brain temperature. Methods: Invasive monitoring of deep brai n stru ctures, lat eral ventri cle and deep white matter. IR temperat ur e read in gs obtain ed at right and left IC. Results: Strong correlat io ns were eviden t b etween R and L IC and brai n. Close, as well as poor , agreemen t b etween si tes was sho wn in so me patients and at some times. For right hemispheric lesions four had a better correlation between TbrV and TRIC when compared to TLIC. When the correlation between TbrV and TLIC was better compared to TbrV and TRIC, four had a predominant right hemispheric lesion. Conclusions: Improved techniques for IR thermal imaging accuracy at the bedside has the potential to improve temperature measure ment agreement . The p red omin ant lesion side may have a b eari ng on maximum ips ilat eral IC temperatu re F ur th er studies are ongoing in this pilot study population. Keywords: Brain Temperature; Infra-red Thermometry; Inner Canthus; Th er mal Imaging; Eye 1. Introduction Temperature changes play a major role in the survival of all animal speci es [1]. In mammals, body temperature is main- tained within a relatively ‘tight’ range with normal values of the order of 36.7oC. Nevertheless, d ifferen ces in t he te mperature o f the viscera are reported, typically the variation is small only (<0.5oC) [2]. Whilst a rise in deep body (core) temperature due to fever is co mmon a fter trauma and critical illn ess it is usually regarded as an adap tive resp onse with p oten tial survival b enefit [3] . By contrast, if damage to brain occurs due to ischaemia (stroke) or trauma (mild to moderate traumatic brain injury, TBI) the p revailin g view is that even small increases i n temper- ature will accelerate secon dary damage of vul nerabl e ischaemic neurons; the consequence being an increased risk of death and worsened neurological outcome [4]. Knowing when a rise in brain temperature has occurred is clinically important. It is generally assumed that body tempera- ture is a reliable ‘surrogate’ for brain temperature but proxy measures for brain temperature are often unreliable [5]. Direct monitoring is safe and recommended during neurocritical care [6] . However, it is customary to revert to body measurement sites (rectum, tympanum, oral, tympanic) once the patient leaves the intensive care unit (ICU). Studies from our group have shown the potential of infra-red (IR) thermometry as a proxy for brain temperature measurement. In the normal to febrile temperature range, IR temporal artery measurement is closer to brain temperature than is IR tympanic membrane temperature with average differences of 0.3oC only [7]. IR sur- face temperature measurements at sites other then ear or fore- head t hus have pot ential as a surrogate for brain temperature. The aim of the current study was to determine if IR thermal imaging of the inner canthus of the eye provides a clinically useful approach to brain temperature estimation in patients with severe intracereb ral trau ma. 2. Materials and Methods The stud y was appr oved by th e local resear ch et hi cs committee. Written informed consent was obtained from the patient’s spouse or relative before measu rements were taken. Patients: Adults aged >21 years with severe traumatic brain injury (TBI) admitted for neurocritical care were eligible for inclusion in the study. All patients were intubated and sedated and mechan icall y ventilat ed and were admitted to the I CU after neurosurgery or as direct admissions to ICU for neuromonitor- ing. The patients were treated in accordance with local Neuro- critical care guidelines and studied for a maximum of 5 days from arrival to ICU. 2.1. Monitoring Invasive brain monitoring: Temperature monitoring was ob- tained at two brain sites; within brain tissue (Tbrt) and within the lateral ventricle (Tbr V). Thermal images of facial skin tem- perature were also o btained. 2.2. Materials Tbrt was measured using a multiparameter (temperature, pres- 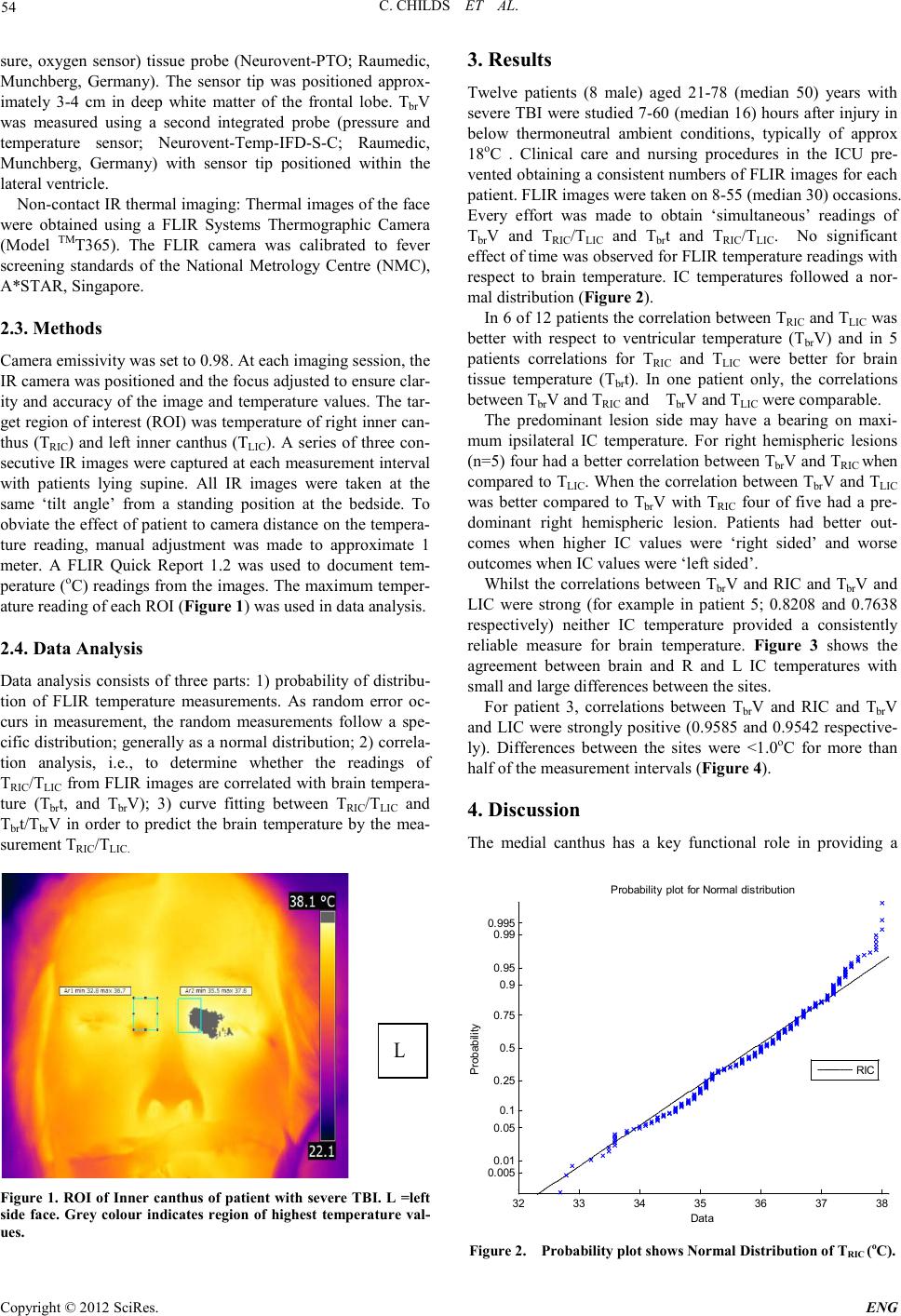 C. CHILDS ET AL. Copyright © 2012 SciRes. ENG 54 sure, oxygen sensor) tissue probe (Neurovent-PTO; Raumedic, Munchberg, Germany). The sensor tip was positioned approx- imately 3-4 cm in deep white matter of the frontal lobe. TbrV was measured using a second integrated probe (pressure and temperature sensor; Neurovent-Temp-IFD-S -C; Raumedic, Munchberg, Germany) with sensor tip positioned within the lateral ven tricle. Non-cont act IR thermal imagin g: Ther mal images of the face were obtained using a FLIR Systems Thermographic Camera (Model TMT365). The FLIR camera was calibrated to fever screening standards of the National Metrology Centre (NMC), A*STAR, Singapore. 2.3. Methods Camera emiss ivity was set to 0.98. At each imaging session, the IR camera was po sit io ned and the focus adjusted to ensure clar- ity and accuracy of the image an d temperature values. The tar- get region of interest ( ROI) was temperatu re of right inn er can- thus (TRIC) and left inner canthus (TLIC). A series of three con- secutive IR images were captu red at each measure ment in terval with patients lying supine. All IR images were taken at the same ‘tilt angle’ from a standing position at the bedside. To obviat e the effect o f patient to camera distan ce on the t empera- ture reading, manual adjustment was made to approximate 1 meter. A FLIR Quick Report 1.2 was used to document tem- peratu re (oC) readings fro m the images . The maximu m temper- ature reading of each ROI (Figure 1) was used in data analysis. 2.4. Data Analysis Data analysis consists of three parts: 1) probability of distribu- tion of FLIR temperature measurements. As random error oc- curs in measurement, the random measurements follow a spe- cific distribution; generally as a normal distribution; 2) correla- tion analysis, i.e., to determine whether the readings of TRIC/TLIC fro m FLIR images are correlated with br ain tempera- ture (Tbrt, and TbrV); 3) curve fitting between TRIC/TLIC and Tbrt/Tbr V in order to predict the brain temperature by the mea- surement TRI C/TLIC. L Figur e 1. R OI of Inner c anthus of patient with severe TBI. L =left side face. Grey colour indicates region of highest temperature val- ues. 3. Results Twelve patients (8 male) aged 21-78 (median 50) years with severe TBI were studi ed 7-60 (median 16) hours after injury in below thermoneutral ambient conditions, typically of approx 18oC . Clinical care and nursing procedures in the ICU pre- vented o btaini ng a consis tent numbers o f FLIR i mages for each patient. FLIR images were taken on 8-55 (median 30) occasions. Every effort was made to obtain ‘simultaneous’ readings of TbrV and TRIC/TLIC and Tbrt and TRIC/TLIC. No significant effect of ti me was ob served for FLIR te mperatu re r eadi ngs with respect to brain temperature. IC temperatures followed a nor- mal distribution (Figure 2). In 6 of 12 patients the correlation between TRIC and TLIC was better with respect to ventricular temperature (TbrV) and in 5 patients correlations for TRIC and TLIC were better for brain tissue temperature (Tbrt). In one patient only, the correlations between Tbr V and TRIC and TbrV and TLIC were comparable. The predominant lesion side may have a bearing on maxi- mum ipsilateral IC temperature. For right hemispheric lesions (n=5) four had a better co rrelation bet ween TbrV and TRIC when compared to TLIC. Wh en the correlation between Tbr V and TLIC was better compared to Tbr V with TRIC four of five had a pre- dominant right hemispheric lesion. Patients had better out- comes when higher IC values were ‘right sided’ and worse out comes when IC valu es were ‘l eft sided’. Whilst the correlations between TbrV and RIC and TbrV and LIC were strong (for example in patient 5; 0.8208 and 0.7638 respectively) neither IC temperature provided a consistently reliable measure for brain temperature. Figure 3 shows the agreement between brain and R and L IC temperatures with small and large di fferences between the sites . For patient 3, correlations between TbrV and RIC and TbrV and LIC were strongly positive (0.9585 and 0.9542 respective- ly). Differences between the sites were <1.0oC for more than half of the meas urement interval s (Figure 4). 4. Discussion The medial canthus has a key functional role in providing a 32 33 34 35 36 37 38 0. 005 0. 01 0. 05 0. 1 0. 25 0. 5 0. 75 0. 9 0. 95 0. 99 0. 995 Data P robabil i t y P robabil i ty plot for Normal di stri buti on RIC Figure 2. Probability plot shows Normal Distribution of TRIC (oC). 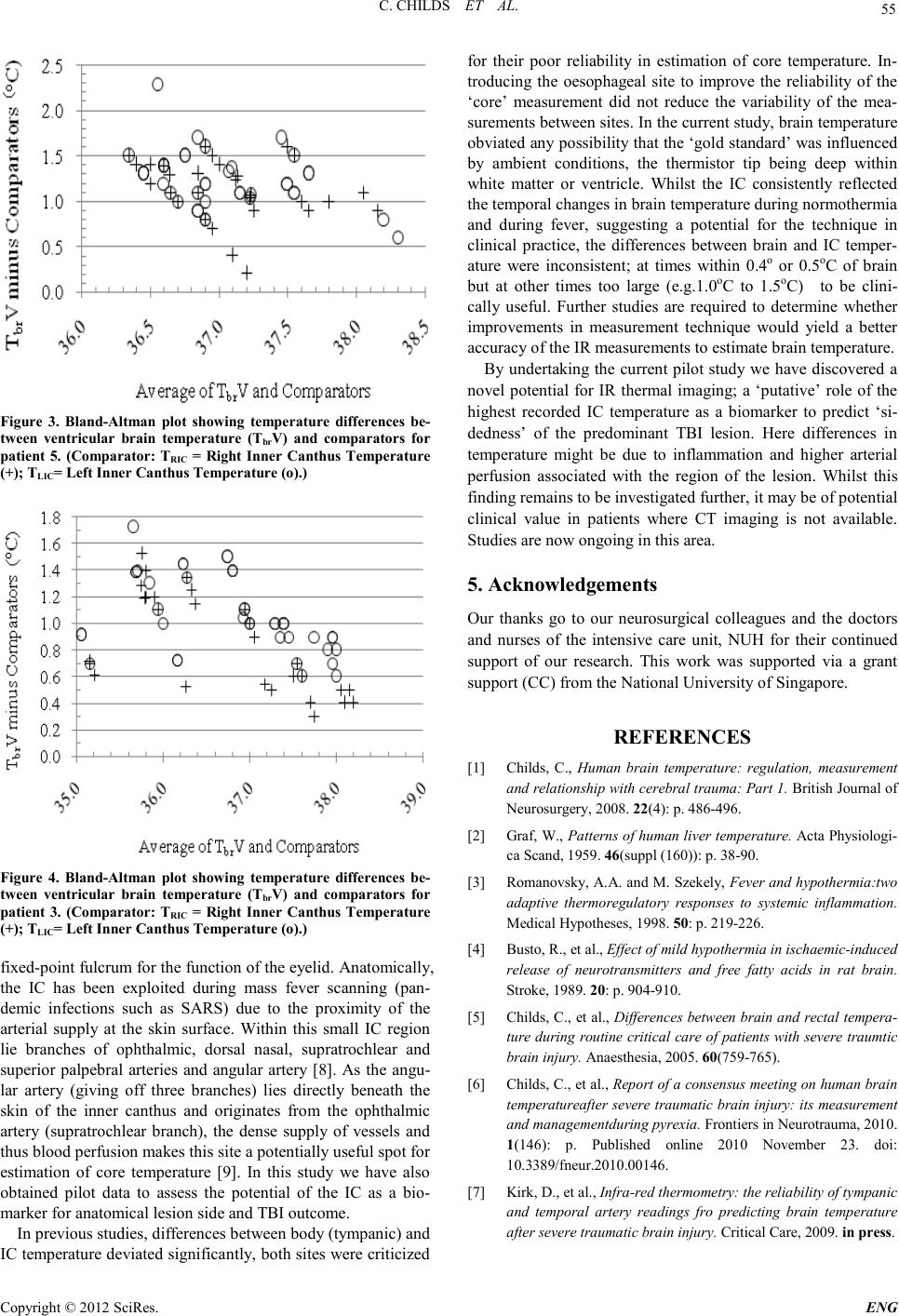 C. CHILDS ET AL. Copyright © 2012 SciRes. E NG 55 Figure 3. Bland-Altman plot showing temperature differences be- tween ventricular brain temperature (TbrV) and comparators for patient 5. (Comparator: TRIC = Right Inner Canthus Temperature (+); TLIC= Left Inner Canthus Temperature (o).) Figure 4. Bland-Altman plot showing temperature differences be- tween ventricular brain temperature (TbrV) and comparators for patient 3. (Co mparator: TRIC = Right Inner Canthus Temperature (+); TLIC= Left Inner Canthus Temperature (o).) fix ed -point fulcrum for the function of the eyelid. Anatomically, the IC has been exploited during mass fever scanning (pan- demic infections such as SARS) due to the proximity of the arterial supply at the skin surface. Within this small IC region lie branches of ophthalmic, dorsal nasal, supratrochlear and superior palpebral arteries and angular artery [8]. As the angu- lar artery (giving off three branches) lies directly beneath the skin of the inner canthus and originates from the ophthalmic artery (supratrochlear branch), the dense supply of vessels and thus blood perfusion makes this site a potentially useful spot for estimation of core temperature [9]. In this study we have also obtained pilot data to assess the potential of the IC as a bio- marker for anatomic al lesio n side and TBI outcome. In previous studies, differences between body (tympanic) and IC temperature deviated significantly, both sites were criticized for their poor reliability in estimation of core temperature. In- troducing the oesophageal site to improve the reliability of the ‘core’ measurement did not reduce the variability of the mea- surement s bet ween sit es. In th e cu rren t stud y, brai n t emperatu re obviated any possibility that the ‘gold standard’ was influenced by ambient conditions, the thermistor tip being deep within white matter or ventricle. Whilst the IC consistently reflected the temporal changes in brain temperature during normothermia and during fever, suggesting a potential for the technique in clinical practice, the differences between brain and IC temper- ature were inconsistent; at times within 0.4o or 0.5oC of brain but at other times too large (e.g.1.0oC to 1.5oC) to be clini- cally useful. Further studies are required to determine whether improvements in measurement technique would yield a better accurac y of the IR measurements to esti mate b r ain temperature. By undertaking the current pilot study we have discovered a novel potential for IR thermal imaging; a ‘putative’ role of the highest recorded IC temperature as a biomarker to predict ‘si- dedness’ of the predominant TBI lesion. Here differences in temperature might be due to inflammation and higher arterial perfusion associated with the region of the lesion. Whilst this finding remains to be investigated further, it may be of potential clinical value in patients where CT imaging is not available. Studi es are now ongoing in this area. 5. Acknowledgements Our thanks go to our neurosurgical colleagues and the doctors and nurses of the intensive care unit, NUH for their continued support of our research. This work was supported via a grant support (CC) from the National University of Singapore. REFERENCES [1] Childs, C., Human brain temperature: regulation, measurement and relationship with cerebral trauma: Part 1. British Journal of Neurosurgery, 2008. 22(4): p. 486-496. [2] Graf, W., Patterns of human liver temperature. Acta Ph ys iologi- ca Scand, 19 5 9. 46(suppl (160)): p. 38-90. [3] Romanovsky, A.A. and M. Szekely, Fever and hypothermia:two adaptive thermoregulatory responses to systemic inflammat io n. Medical Hypotheses, 1998. 50: p. 2 19-226. [4] Busto, R., et al., Effect of mild hypo thermia in ischaemic-induced release of neurotransmitters and free fatty acids in rat brain. Stroke, 1989. 20: p. 904-910. [5] C hilds, C ., et al., Differences between brain and rectal tempera- ture during routine critical care of patients with severe traumtic brain injury. Anaesth esia, 200 5 . 60(75 9 -765). [6] Childs, C., et al., Report of a consensus meeting on human brain temperatureafter severe traumatic brain injury: its measurement and managementduring pyrexia. Fronti ers in N eu rotrauma, 2 0 10. 1(146): p. Published online 2010 November 23. doi: 10.3389/fneur.2010.00146. [7] Kirk, D., et al., Infra-red thermometry: the reliability of tympanic and temporal artery readings fro predicting brain temperature after se vere traumat ic brain in j ur y. Critical Care, 2009. in press.  C. CHILDS ET AL. Copyright © 2012 SciRes. ENG 56 [8] Teunissen, L.P.J. and H.A.M. Daanen, Infrared thermal imaging of the inner canthus of the eye as an estimator of body core tem- perature. J. Medical Engineering & technology, 2011. 35(3-4): p. 134-138. [9] Erdogmus, S. and F. Govsa, Arterial features of inner canthus region: confirming the dafety for the flap region. The journal of craniofacial surgery, 2006. 17( 5 ) : p. 8 64-868 |

