Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Chemistry, 2012, 2, 216-220 doi:10.4236/ampc.2012.24B055 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC The Study of Method for Complex Processing Turgay Sub-S tandard Aluminum-Containing Raw Materials G. Sarsenbay , L. A. Myltykbaeva, R. A. Abdulwalyev, S. B. Satylganova Cen ter of Eart h Sciences, Metallurgy and O re Beneficiation, JSC, Science an d Educati on Ministry of the Republic of Kazakhstan Email: ао.cnzmo@rambler.ru Received 2012 ABSTRACT obj ects of the r esearch are Ka zakhs tan’s Tur gay clay, studied of method for alumina and potassium metasilicate obtaining from Tur- gay sub-standard aluminum raw materials. Concluded that optimal conditions for the process of Turgay clay: reaction temperature 100°C, original solution K2O concentr ati o n to 300 g/ dm3, reaction time 120 min, liquid-solid ratio of 3:1; optimized the conditions of digestion alumina concentrate: original Na2O solution concentration of 400 g/dm3, temperature 280°C, molar ratio CaO : SiO2 = 1. Recovery is 99.6% of alumina digestion under this condition; crystallized solid phase components as Na2O·Al2O3·6H2O sodium hydroaluminate crystals. Extracted of alumina from solution of sodium hydroaluminate. Keywords: Potassium Hydroxide Solution; Leaching; Clay; Alumina Concentrate; Sodium Aluminate Solution; Digestion; Desilication; Alumina 1. Introduction With the reduction of bauxite resources, the application of low- grade aluminum-containing raw materials i n aluminum produc- tion will be the key question. Kazakhstan is rich of low grade bauxite mine (burnt ash, clay), which were the storage of Tur- gay clay long enough to provide raw materials for alumina production [1]. For low grade aluminum ore, chemical benefic- iation processing – alkali leaching to removal some silicon- containing to extraction silicate mineral, and alumina produc- tion by using concentrate can be comprehensive utilization of raw materials, solve problems of production effectively. Study on alkaline leaching of high-silicon aluminum ore, treated by sodium hydroxide solution and extraction silicate widely visible [2,3], not seen on the potassium hydroxide reports. Potassium silicate as main ingredient for high-quality potassium fertilizer of chloride-free is widely used in agricultural production. For the purpose of this study to complex processing Turgay clay, potassium hydroxide solution used for the first time of low grade aluminum-containing raw materials dressing process, by planning central composite second order rotatable experiment and hydrochemical test, to find the best reaction condition of extraction from Turgay clay industrial alumina and chlorine- free po tash fertil izer processing methods. 2. Experimental 2.1. Optimization Process of Clay ore Dressing Potassium silicate solution and alumina concentrate can be obtained by baking clay leaching by potassium hydroxide solu- tion, using central composite second order rotatable test [4] to find the optimal leaching conditions. Chemical composition of Turgay’s baking clay samples for experiments is: SiO2 37%; Al2O3 42.6%; Fe2O3 13.8 %; CaO 1.3%; Na2O 0.8%; other 1.5%; A/S=1.15. The experiments an d results: using of the central composite of second order rotata- ble to develop test, factors influencing the leaching effect of three is made for the variabl e: X1 as the concen tration for K2O in original solution, g/dm3; X2 for leaching time, min; X3 for liquid-solid ratio, percentage of SiO2 into solution (y) selected optimization parameter. Experiment conditions and results of Second order rotatable as shown in Table 1. Table 1 Conditions and results of experiments. Ν Conditions, g/dm 3, min Solu tion components, g/dm3, % X1 X2 X3 K2O Al2O3 SiO2 SiO2 1 100 60 2:1 61.1 1.38 98.0 48.21 2 300 60 2:1 211.5 2.46 243.5 79.00 3 100 180 2:1 56.4 1.63 99.5 48.94 4 300 180 2:1 253.8 2.89 2 28.5 78.40 5 100 60 4:1 61.1 1.13 70.5 69.32 6 300 60 4:1 282.0 2.64 105.0 79.44 7 100 180 4:1 61.1 1.63 72.5 71.29 8 300 180 4:1 282.0 3.13 97.5 82.84 9 31.8 120 3:1 30.25 2.6 4 26.5 20.93 10 336.4 120 3:1 235.0 1.13 162.5 80.86 11 200 19 3:1 188.0 1.13 95.0 65.02 12 200 221 3:1 188.0 2.64 112.0 76.66 13 200 120 1,31:1 188.0 2.64 208.0 62.26 14 200 120 4,68:1 188.0 0.12 74.0 79.06 15 200 120 3:1 164.5 1.63 114.3 78.23 16 200 120 3:1 188.0 1.13 114.5 78.37 17 200 120 3:1 164.5 1.13 114.0 78.03 18 200 120 3:1 164.5 1.13 115.0 78.71 19 200 120 3:1 164.5 1.63 113.5 77.69 20 200 120 3:1 88.0 1.63 115.3 78.88 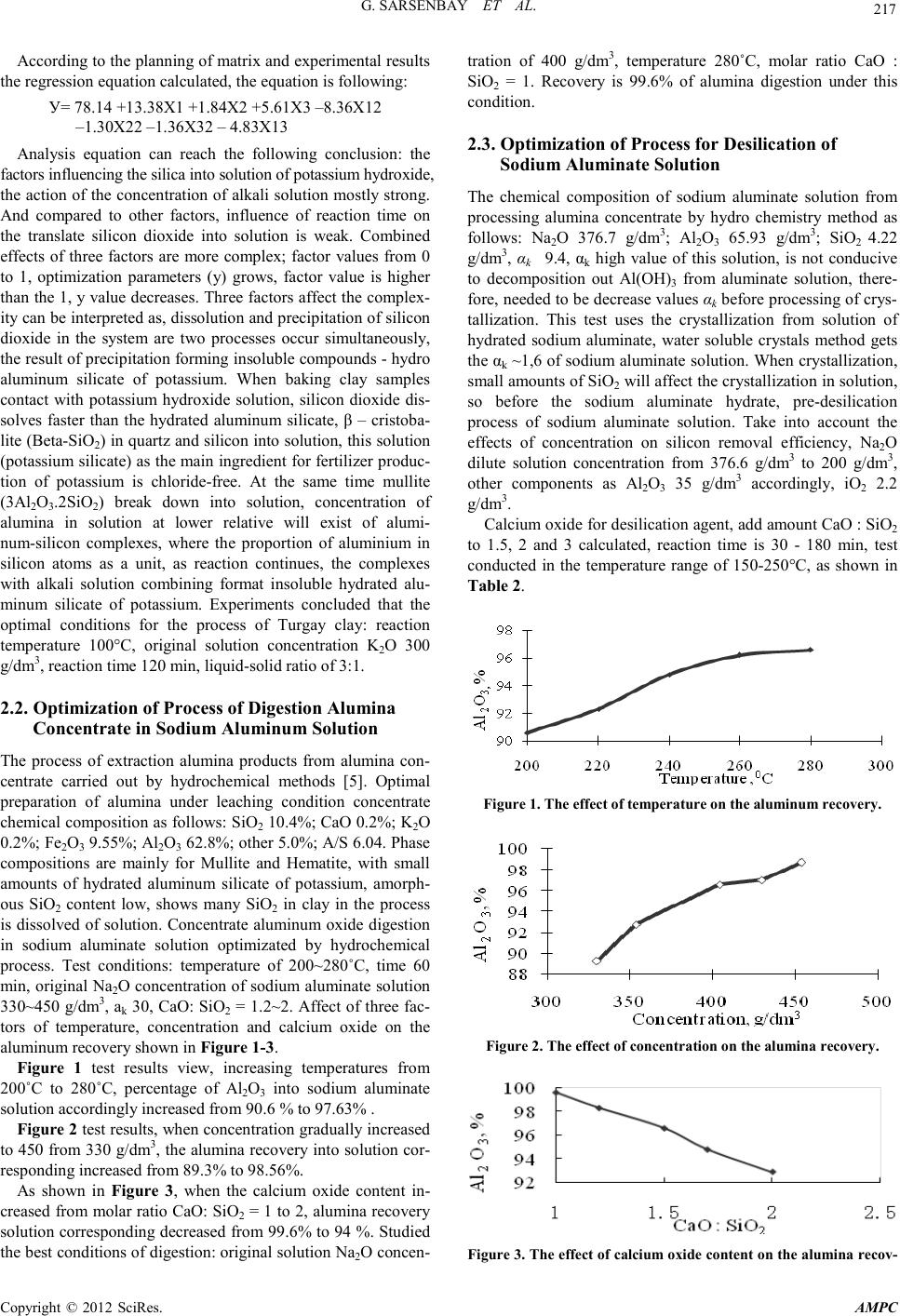 G. SARSENB AY ET AL. Copyright © 2012 SciRes. AMPC 217 According to the planning of matrix and experimental results the regression equatio n cal culated, t he equati on is following: У= 78.14 +13.38Х1 +1.84Х2 +5.61Х3 –8.36Х 12 –1.30Х22 –1.36Х32 – 4.8 3Х 13 Analysis equation can reach the following conclusion: the factors influencing the silica into solutio n of potassium hydroxide, the action of the concentration of alkali solution mostly strong. And compared to other factors, influence of reaction time on the translate silicon dioxide into solution is weak. Combined effects of three factors are more complex; factor values from 0 to 1, optimization parameters (у) grows, factor value is higher than the 1, у value decreases. Three f actors affect t he complex- ity can be interpreted as, dissolution and precipitation of silicon dioxide in the system are two processes occur simultaneously, the result of precipitation forming insoluble compounds - hydro aluminum silicate of potassium. When baking clay samples contact with potassium hydroxide solution, silicon dioxide dis- solves faster than the hydrated aluminum silicate, β – cristoba- lite (Beta-SiO2) in quartz and silicon into solution, this solution (potassium silicate) as the main ingredient for fertilizer produc- tion of potassium is chloride-free. At the same time mullite (3Al2O3.2 S iO2) break down into solution, concentration of alumina in solution at lower relative will exist of alumi- num-silicon complexes, where the proportion of aluminium in silicon atoms as a unit, as reaction continues, the complexes with alkali solution combining format insoluble hydrated alu- minum silicate of potassium. Experiments concluded that the optimal conditions for the process of Turgay clay: reaction temperature 100°C, original solution concentration K2O 300 g/dm3, reaction time 120 min, liquid-solid ratio of 3:1. 2.2. Optimization of Process of Digestion Alumina Concentrate in Sodium Aluminum Solution The process of extraction alumina products from alumina con- centrate carried out by hydrochemical methods [5]. Optimal preparation of alumina under leaching condition concentrate chemical composition as follows: SiO2 10.4%; CaO 0.2%; K2O 0.2%; Fe2O3 9.55%; Al2O3 62.8%; other 5.0%; A/S 6.04. Phase compositions are mainly for Mullite and Hematite, with small amounts of hydrated aluminum silicate of potassium, amorph- ous SiO2 content low, shows many SiO2 in clay in the process is dissolved of solution. Concentrate aluminum oxide digestion in sodium aluminate solution optimizated by hydrochemical proces s. Test conditions: temperature of 200~280˚C, time 60 min, original Na2O concentration of sodium aluminate solution 330~450 g/dm3, аk 30, CaO: SiO2 = 1.2~ 2. Affect of three fac- tors of temperature, concentration and calcium oxide on the aluminum recovery shown in Figure 1-3. Figure 1 test results view, increasing temperatures from 200˚C to 280˚C, percentage of Al2O3 into sodium aluminate solution accordingly increased from 90.6 % to 97.63% . Figure 2 test resu lts, when concen trati on gradu ally increased to 450 from 330 g/dm3, the alumina recovery into solution co r- responding increased from 89.3% to 98.56%. As shown in Figure 3, when the calcium oxide content in- creased from molar rati o CaO: Si O2 = 1 to 2 , alumina reco very solution corresponding decreased from 99.6% to 94 %. Studied the best conditions of d igestion: original solution Na2O concen- tration of 400 g/dm3, temperature 280˚C, molar ratio CaO : SiO2 = 1. Recovery is 99.6% of alumina digestion under this condition. 2.3. Optimization of Process for Desilication of Sodium Aluminate Solution The chemical composition of sodium aluminate solution from processing alumina concentrate by hydro chemistry method as follows: Na2O 376.7 g/dm3; Al2O3 65.93 g/dm3; SiO2 4.22 g/dm3, αk 9.4, αk high value of this solution, is not conducive to decomposition out Al(OH)3 from aluminate solution, there- fore, need ed to be decrease valu es αk before processing of crys- tallization. This test uses the crystallization from solution of hydrated sodium aluminate, water soluble crystals method gets the αk ~1,6 of sodium aluminate solution. When crystallization, small amounts o f SiO2 will affect t he cr ystall ization in solution, so before the sodium aluminate hydrate, pre-desilication process of sodium aluminate solution. Take into account the effects of concentration on silicon removal efficiency, Na2O dilute solution concentration from 376.6 g/dm3 to 200 g/dm3, other components as Al2O3 35 g/dm3 accordingly, iO2 2.2 g/dm3. Calcium oxide for desilication agent, add amount CaO : SiO2 to 1.5, 2 and 3 calculated, reaction time is 30 - 180 min, test conducted in the temperature range of 150-250°C, as shown in Table 2. Figure 1. The effect of temperature on the aluminum recovery. Figure 2. The effect of concentration on the alumina recovery. Figure 3. The effect of calcium oxide content on the al umina recov- 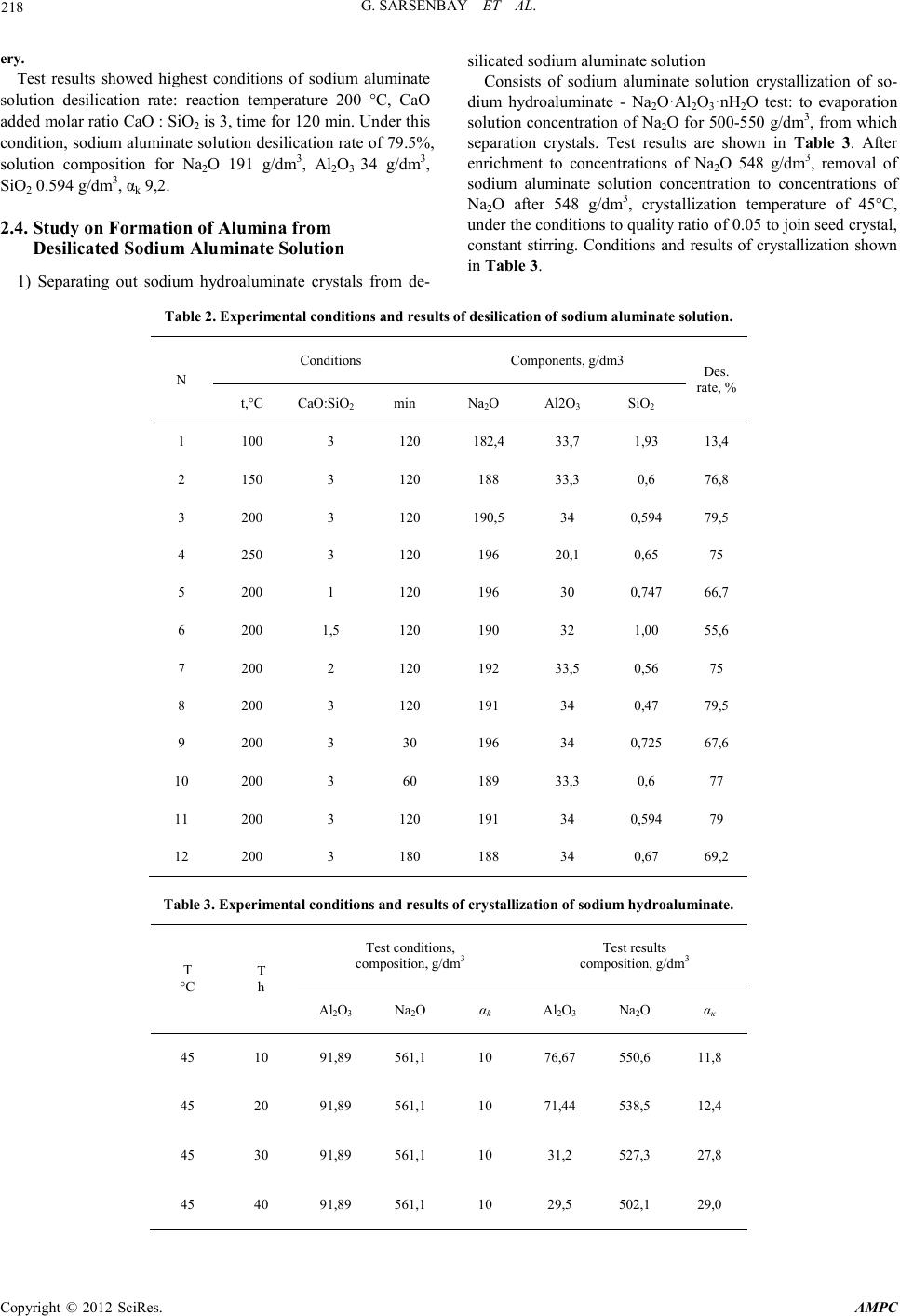 G. SARSENB AY ET AL. Copyright © 2012 SciRes. AMPC 218 ery. Test results showed highest conditions of sodium aluminate solution desilication rate: reaction temperature 200 °C, CaO added molar rat io CaO : SiO2 is 3, time for 120 min. Under this condition, sodium aluminate solution desilication rate of 79.5%, solution composition for Na2O 191 g/dm3, Al2O3 34 g/dm3, SiO2 0.594 g/dm3, αk 9,2. 2.4. Study on Formation of Alumina from Desilicated Sodium Aluminate Solution 1) Separating out sodium hydroaluminate crystals from de- silicated sodium aluminate solutio n Consists of sodium aluminate solution crystallization of so- dium hydroaluminate - Na2O·Al2O3·nH2O test: to evaporation solution concentration of Na2O for 500-550 g/dm3, from which separation crystals. Test results are shown in Table 3. After enrichment to concentrations of Na2O 548 g/dm3, removal of sodium aluminate solution concentration to concentrations of Na2O after 548 g/dm3, crystallization temperature of 45°C, under the conditions to quality ratio of 0.05 to join seed crystal, constant stirring. Conditions and results of crystallization shown in Table 3. Table 2. Experimental conditio ns and res ults of desilication of sodium aluminate solutio n. N Conditions Components, g/dm3 Des . rate, % t,°C CaO:SiO2 min Na2O Al2O3 SiO2 1 100 3 120 182,4 33,7 1,93 13,4 2 150 3 120 188 33,3 0,6 76 ,8 3 200 3 120 190,5 34 0,594 79,5 4 250 3 120 196 20,1 0,65 75 5 200 1 120 196 30 0,747 66,7 6 200 1 ,5 120 190 32 1,0 0 55,6 7 200 2 120 192 33,5 0,56 75 8 200 3 120 191 34 0,47 79,5 9 200 3 30 196 34 0,725 67,6 10 200 3 60 189 3 3 ,3 0,6 77 11 200 3 120 191 34 0,594 79 12 200 3 180 188 34 0,6 7 69,2 Table 3. Experimental conditio ns and res ults of crystallization of sodium hydroaluminate. T °С T h Test conditions, composition, g/dm3 Test results composition, g/dm3 Al2O3 Na2O αk Al2O3 Na2O ακ 45 10 91,89 561,1 10 76,67 550,6 11,8 45 20 91,89 561,1 10 71,44 538,5 12,4 45 30 91,89 561,1 10 31 ,2 527,3 27,8 45 40 91,89 561,1 10 29 ,5 502,1 29,0 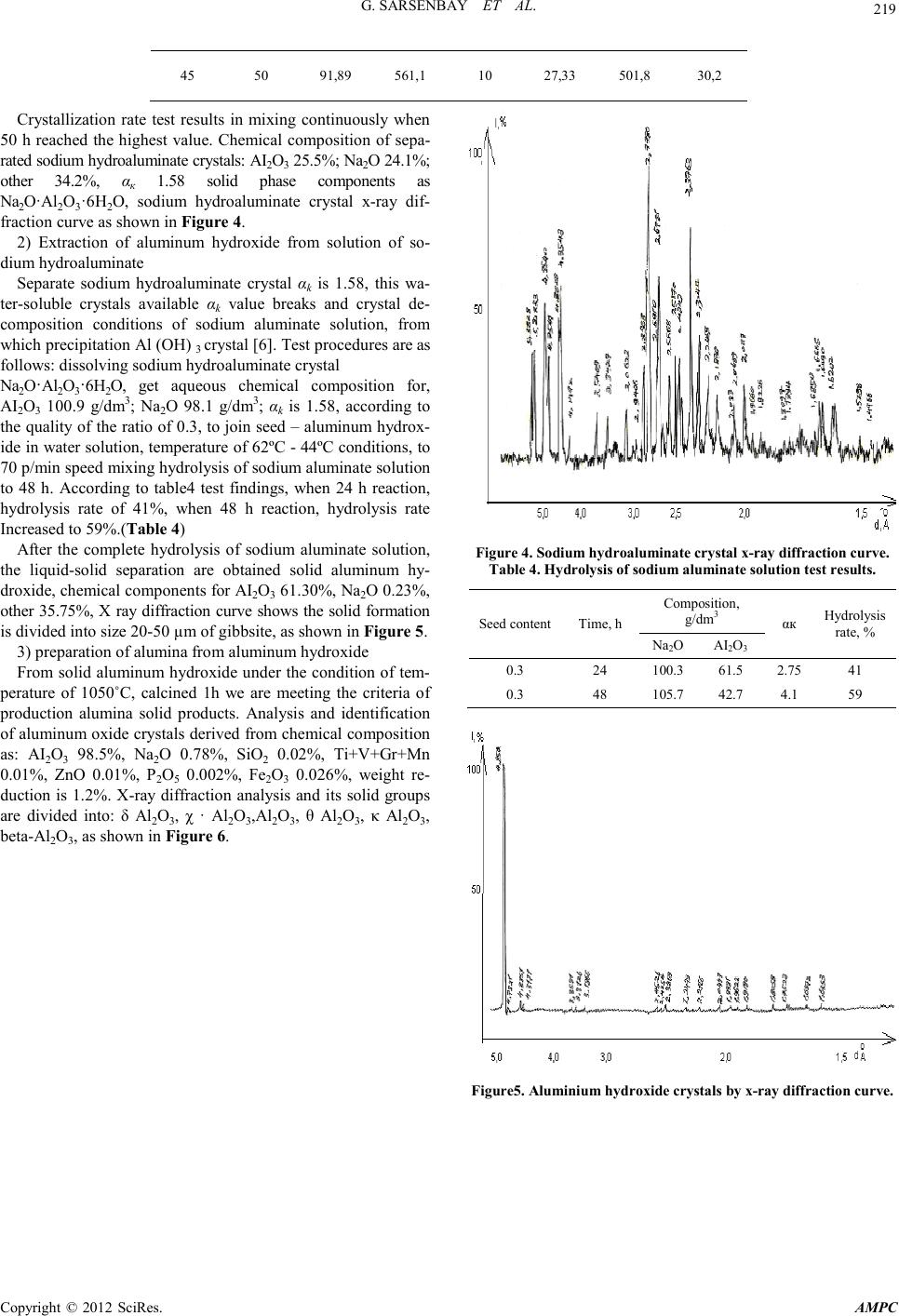 G. SARSENB AY ET AL. Copyright © 2012 SciRes. AMPC 219 45 50 91,89 561,1 10 27,33 501,8 30,2 Crystallization rate test results in mixing continuously when 50 h reached the highest value. Chemical composition of sepa- rated sodiu m h ydroa lu minat e cryst als: AI2O3 25.5%; Na2O 24.1%; other 34.2%, αк 1.58 solid phase components as Na2O·Al2O3·6H2O, sodium hydroaluminate crystal x-ray dif- fraction curve as shown in Figure 4 . 2) Extraction of aluminum hydroxide from solution of so- dium hydroaluminate Separate sodium hydroaluminate crystal αk is 1.58, this wa- ter-soluble crystals available αk value breaks and crystal de- composition conditions of sodium aluminate solution, from which precipitation Al (OH) 3 crystal [6]. Test pr oced u res are as follows: dissolving sodium hydroaluminate crystal Na2O·Al2O3·6H2O, get aqueous chemical composition for, AI2O3 100.9 g/dm3; Na2O 98.1 g/dm3; αk is 1.58, according to the quality of the ratio of 0.3, to join seed – aluminum hydrox- ide in water solution, temperature of 62ºC - 44 ºC cond itions, to 70 p/min speed mixing hydrolysis of sodium aluminate solution to 48 h. According to table4 test findings, when 24 h reaction, hydrolysis rate of 41%, when 48 h reaction, hydrolysis rate Increased to 59 %. (Table 4) After the complete hydrolysis of sodium aluminate solution, the liquid-solid separation are obtained solid aluminum hy- droxide, chemical components for AI2O3 61.30%, Na2O 0.23%, other 35.75%, X ray diffraction curve shows the solid formation is divided into size 20-50 µm of gibbsite, as shown in Figure 5. 3) prepara t ion of alumina from aluminum hydroxide From solid aluminum hydroxide under the condition of tem- perature of 1050˚C, calcined 1h we are meeting the criteria of production alumina solid products. Analysis and identification of aluminum oxide crystals derived from chemical co mposition as: AI2O3 98.5%, Na2O 0.78%, SiO2 0.02%, Ti+V+Gr+Mn 0.01%, ZnO 0.01%, P2O5 0.002%, Fe2O3 0.026%, weight re- duction is 1.2%. X-ray diffraction analysis and its solid groups are divided into: δ Al2O3, χ · Al 2O3,Al2O3, θ Al 2O3, κ Al2O3, beta-Al2O3, as shown in Figure 6. Figure 4. Sodium hydroaluminate crystal x-ray diffraction curve. Table 4. Hydrolysis of sodium aluminate solution test results. Seed content Tim e, h Composition, g/dm3 αк Hydrolysis rate, % Na2O AI2O3 0.3 24 100. 3 61.5 2.7 5 41 0.3 48 105. 7 42.7 4.1 59 Figure5. Aluminium hydroxide crystals by x-ray diffraction curve. 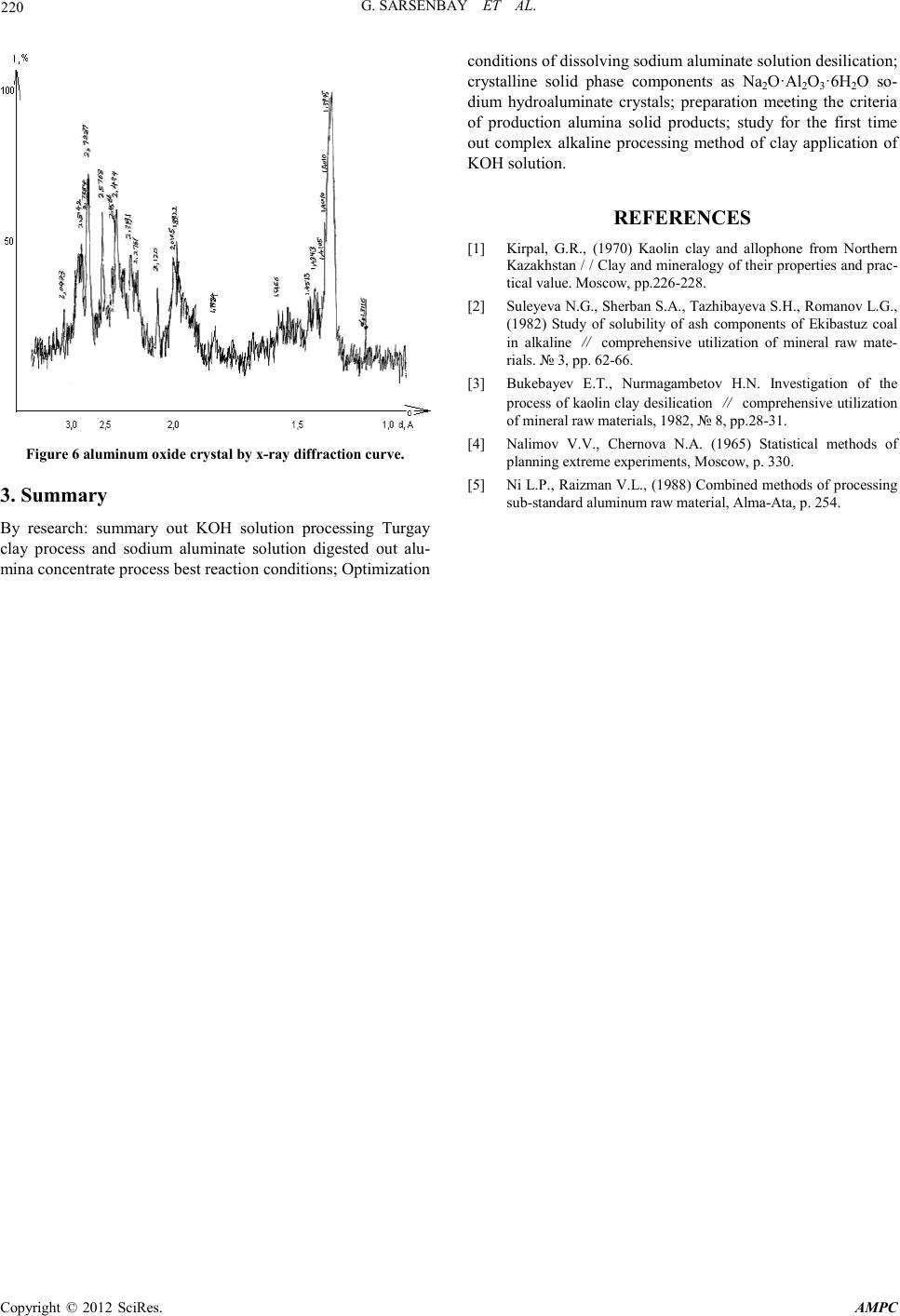 G. SARSENB AY ET AL. Copyright © 2012 SciRes. AMPC 220 Figure 6 aluminum oxide crystal by x-ray diffraction curve. 3. Summary By research: summary out KOH solution processing Turgay clay process and sodium aluminate solution digested out alu- mina concentrate process best reaction conditions ; Optimizatio n conditions of dissolving sodium aluminate solution desilication; crystalline solid phase components as Na2O·Al2O3·6H2O so- dium hydroaluminate crystals; preparation meeting the criteria of production alumina solid products; study for the first time out complex alkaline processing method of clay application of KOH solution. REFERENCES [1] Kirpal, G.R., (1970) Kaolin clay and allophone from Northern Kazakhstan / / Clay and mineralogy of their properties and prac- tica l value. Moscow, pp.226-228. [2] Suleyeva N.G., Sherban S.A., Tazhibayeva S.H., Romanov L.G., (1982) Study of solubility of ash components of Ekibastuz coal in alkaline ∥ comprehensive utilization of mineral raw mate- rials. № 3, pp. 62-66. [3] Bukebayev E.T., Nurmagambetov H.N. Investigation of the process of kaolin clay desili cation ∥ comprehensive utilization of mineral raw materials, 1982, № 8, pp.28-31. [4] Nalimov V.V., Chernova N.A. (1965) Statistical methods of planning extreme experiments, Moscow, p. 330. [5] Ni L.P. , Raizman V. L., (1988) Combin ed methods of p rocessing sub-standard aluminum raw material, Alma-Ata, p. 254. |

