Paper Menu >>
Journal Menu >>
 Kinetic Study of Sulfur Dioxide Elimination by Limestone through the Lab Scale Circulating Fluidized Bed Combustor Dowon Shun*, Dal-Hee Bae *, In-Kyu Jang**, Keon-Hee Park** and Seung Kyu Park**,† *Greenhouse Gas Research Center, Korea Institute of Energy Research, 71-2 Daejon 305-343, Korea **Department of Chemical Engineering, Hoseo University, Asan 336-795, Korea Email : skpark@hoseo.edu Abstract—Characteristics of sulfur dioxide emission from coal and petroleum coke combustion were examined in a lab scale circulating fluidized bed (CFB) combustor. The rate constant of the first order rate expression for the absorption SO2 on the CaO surface was similar regardless of the origin of the limestone, the particle size and the initial SO2 concentration. However, the total SO2 absorption capacity was different depending on the origin of the limestone. The breakability of the particle which provides new surface for the reaction seems to play a major role in the absorption characteristics. Keywords-Sulfur Dioxide (SOx); Limestone; Circulating Fluidized Bed Combustor; Emission; Kinetics; First order reaction 1. Introduction Sulfur oxides (SOx) gases form when the coal and heavy oil are burned. The SO2 readily dissolves in water vapor and leads to the formation of acid and interacts with other gases and particles in the air to form sulfates and other products that can be harmful to people and environment [1,2]. SO2 pollution is thought to promote wheezing, bronchial constriction, shortness of breath, and exacerbation of asthma [2]. Nitrogen oxides (NOx) are highly reactive gases that contain nitrogen and oxygen in varying molecular combinations. The major source of NOx is the combustion of fossil fuels such as coke in electric power plants or petroleum in vehicle engines [3]. Many of the nitrogen oxides are colorless, but nitrogen dioxide (NO2) combined with particles in the air can cause a reddish-brown haze. The presence of NOx leads to a variety of environmental problems such as ground level ozone, acid rain and the deforestation by acid rain. They are poisonous for the respiratory system, provoking both lung infection and respiratory allergies. Both sulfur dioxide and nitrogen oxides contribute to acid rain [4]. Just a few decades ago, SOx and NOx pollution had gotten so bad that acid rain had damaged countless buildings, monuments, car finishes [5]. One member of the NOx family, nitrous oxide (N2O), is a potent greenhouse gas [6]. Air pollution caused by SOx and NOx, which are largely the result of industrial processes, may also produce environmental impacts. For these reasons, SOx and NOx emissions should be eliminated. In the field of fluidized bed combustion with high thermal capacities, circulating fluidized bed (CFB) reactors are considered to be the most efficient commercial utility which burns various solid fuels, including coals, with a minimum operation and maintenance cost [7-9]. The reactors are usually lack of sulfur capture facilities, such as flue gas treatment, since the in-situ SO2 capture by the limestone injection into the combustor itself is sufficient to comply with the regulation. Although the best operation parameters for the boilers could be collected from the actual experience of the commercial scale boilers [10], the information is not always intuitive since the parameters of the commercial boilers in operation are normally interrelated. It is quite difficult to understand the actual effects of specific variables on the performance of the commercial boiler in operation. On the other hand, many experimental results from previous researches were carried out with packed bed units in small scale and turned out to be impractical to be applied to the commercial CFB boilers [3]. To investigate the emission characteristics of CFB systematically, a CFB combustor was built in-house grade. Many researchers have been performed in conventional air-combustion fluidized bed boilers to eliminate the SOx by limestone flux, and much information has been accumulated through the kinetic and spectroscopic studies, many basic questions still remain to be answered clearly. The emission of SOx and NOx gases during the combustion of coal or petroleum pitch in the CFB has been studied. The aim of this study is to elucidate the absorption of SO2 by three kinds of limestone produced from Tanyang, Samchuk and Jinsan in Korea. Emission of SOx and NOx in the CFB and reaction of them with limestone have been investigated. 2. Experimental A. Circulated fluidized bed The circulated fluidized bed (CFB) combustor was prepared with quarts tubes [8-10]. The CFB combustor consists of a riser (combustion chamber) and a cyclone, a loop seal and an ash classifier. The silica sand in the riser was heated to more than 900 ºC and was blown upward by the combustion air from Advances in Materials Physics and Chemistry Supplement: 2012 world Con g ress on En g ineerin g and Technolo gy Cop y ri g ht © 2012 SciRes.189 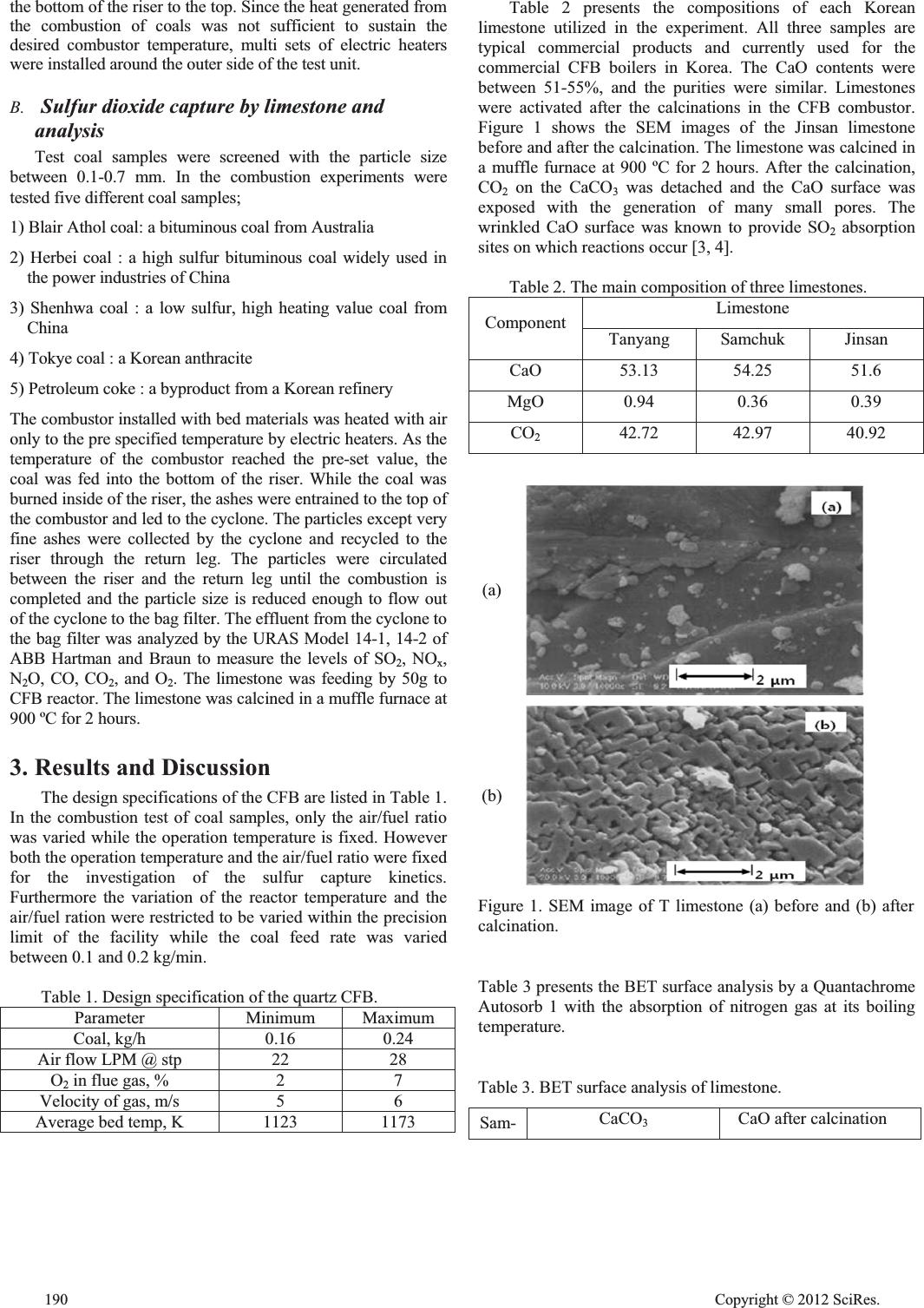 the bottom of the riser to the top. Since the heat generated from the combustion of coals was not sufficient to sustain the desired combustor temperature, multi sets of electric heaters were installed around the outer side of the test unit. B. Sulfur dioxide capture by limestone and analysis Test coal samples were screened with the particle size between 0.1-0.7 mm. In the combustion experiments were tested five different coal samples; 1) Blair Athol coal: a bituminous coal from Australia 2) Herbei coal : a high sulfur bituminous coal widely used in the power industries of China 3) Shenhwa coal : a low sulfur, high heating value coal from China 4) Tokye coal : a Korean anthracite 5) Petroleum coke : a byproduct from a Korean refinery The combustor installed with bed materials was heated with air only to the pre specified temperature by electric heaters. As the temperature of the combustor reached the pre-set value, the coal was fed into the bottom of the riser. While the coal was burned inside of the riser, the ashes were entrained to the top of the combustor and led to the cyclone. The particles except very fine ashes were collected by the cyclone and recycled to the riser through the return leg. The particles were circulated between the riser and the return leg until the combustion is completed and the particle size is reduced enough to flow out of the cyclone to the bag filter. The effluent from the cyclone to the bag filter was analyzed by the URAS Model 14-1, 14-2 of ABB Hartman and Braun to measure the levels of SO2, NOx, N2O, CO, CO2, and O2. The limestone was feeding by 50g to CFB reactor. The limestone was calcined in a muffle furnace at 900 ºC for 2 hours. 3.Results and Discussion The design specifications of the CFB are listed in Table 1. In the combustion test of coal samples, only the air/fuel ratio was varied while the operation temperature is fixed. However both the operation temperature and the air/fuel ratio were fixed for the investigation of the sulfur capture kinetics. Furthermore the variation of the reactor temperature and the air/fuel ration were restricted to be varied within the precision limit of the facility while the coal feed rate was varied between 0.1 and 0.2 kg/min. Table 1. Design specification of the quartz CFB. Parameter Minimum Maximum Coal, kg/h 0.16 0.24 Air flow LPM @ stp 22 28 O2 in flue gas, % 2 7 Velocity of gas, m/s 5 6 Average bed temp, K 1123 1173 Table 2 presents the compositions of each Korean limestone utilized in the experiment. All three samples are typical commercial products and currently used for the commercial CFB boilers in Korea. The CaO contents were between 51-55%, and the purities were similar. Limestones were activated after the calcinations in the CFB combustor. Figure 1 shows the SEM images of the Jinsan limestone before and after the calcination. The limestone was calcined in a muffle furnace at 900 ºC for 2 hours. After the calcination, CO2 on the CaCO3 was detached and the CaO surface was exposed with the generation of many small pores. The wrinkled CaO surface was known to provide SO2 absorption sites on which reactions occur [3, 4]. Table 2. The main composition of three limestones. Limestone Component Tanyang Samchuk Jinsan CaO 53.13 54.25 51.6 MgO 0.94 0.36 0.39 CO2 42.72 42.97 40.92 (a) (b) Figure 1. SEM image of T limestone (a) before and (b) after calcination. Table 3 presents the BET surface analysis by a Quantachrome Autosorb 1 with the absorption of nitrogen gas at its boiling temperature. Table 3. BET surface analysis of limestone. Sam- CaCO3 CaO after calcination 190 Cop y ri g ht © 2012 SciRes.  ple Area (m2/g) Vol. (cm3/ g) Pore dia. (ȝm) Area (m2/g) Vol. (cm3/ g) Pore dia. (ȝm) Tan- yang 0.53 0.15 1.13 6.77 2.21 1.30 Sam- chuk 0.28 0.09 1.30 11.24 3.66 1.30 Jin- san 0.41 0.12 1.17 1.85 0.59 1.28 (a) 0 100 200 300 400 500 01234567 O2[%] E m i s i s o n [ p p m @ 3 . 5 % O 2 ] NOx N2O SO2 (b) 0 100 200 300 400 500 600 0123456 O2[%] E m i s i s o n [ p p m @ 3 . 5 % O 2 ] 7 N2O NOx SO2 Figure 2. The effects of the aeration on the emissions of SO2, NOx and N2O from the combustions of (a) the Blair Athol coal and (b) the Tokye coal. In the Blair coal combustion, about 280 ppm of SO2 emission was slightly decreased to 200 ppm as the oxygen content increases. Meanwhile, the N2O emission was slightly increased from 110 ppm to 310 ppm as the oxygen feeding was increased. In the Tokye coal combustion, the SO2 emission gradually was decreased from 500 ppm to 400 ppm as the oxygen feeding increases. Figure 3 shows the variation of CaO conversion rate of Tanyang limestone as the reaction goes by. The comparison was made among different average particle sizes. The maximum conversion rates were observed when CaO conversion, XCaO, was 0 - 0.1. After that it decayed rapidly. 0 0.5x10-5 1.0x10-5 1.5x10-5 2.0x10-5 2.5x10-5 00 .0 50 .10 .1 50 .20 .2 5 X C a O 1.4-0.2 0.2-0.08 0.08-0 d F S O [ m o l / s ] 2 Figure 3. Variation of CaO conversion rate with respect to the particle size of Tanyang limestone. CaSO4 layer is formed after SO2 gas is absorbed on the CaO surface, the surface larger of CaSO4 molecules will block further absorption of SO2 to inner CaO site. So the ash diffusion model is excluded. The reaction must be either controlled by the gas film diffusion or chemical kinetics. In this experiment the large mass of CaCO3 is introduced to provide infinite reaction surface. The external transport rate will be same for all the experiment and will not affect the reaction rate. The gas film diffusion control mechanism and the chemical reaction control mechanism were compared and the chemical reaction control showed better fit to the data. A second order absorption kinetics was proposed to analyze the experimental data. CaOSO CkCr 2 Where, r ; SO2 absorption rate [gmol SO2/gmol limestone-s] ; 2nd order rate constant [liter/gmol CaO-s] k ; Concentration of CaO CaO C [gmol CaO/ gmol limestone] ; Concentration of SO2 at the combustor exit [gmol SO2/liter] 2 SO C The kinetic equations can be restated as; CaOSOSO FkCdF 22 Where CaOSO dFdF 2 [gmol SO2/s] Since the excess amount of CaO was injected in the reactor. The concentration of CaO in the circulating fluidized bed combustor is considered to be constant and the reactor is considered as a mixed reactor regarding CaO. The reactor equation is the first order only to the concentration of SO2. 2 'SO Ckr Where, ; 1st order rate constant [1/s]. 'k When the SO2 absorption rate of limestone with different origin is compared, the Jinsan limestone showed the highest rate constant and that of Samchuk showed the lowest constant. Cop y ri g ht © 2012 SciRes.191  Sulfur elimination by limestone under CFB combustion conditions is the net effect of a competition between sulfur capture and sulfur release during which the composition of the Ca surface changes continuously between CaO, CaS and CaSO4. Therefore, we conclude that the limestone feeding is enough to capture all SO2 gases, so the reactions become depend on the concentration of SO2. And the reaction can be disrupted by the formation of CaSO4 after reaction goes by. For understanding of SO2 gas adsorption onto limestone, the chemical reaction model should be studied [5, 12]. For the comparison of the rate controlling step, we postulate that the particle is a globular shape at the shrinking core model during the adsorption of gas onto the particle. G At the reaction : A (gas) + B (soild) -> Solid Products 4. Conclusion The CaO contents of three kinds of limestone were between 51-55%. The BET surface area of Samchuk limestone was increased about 40 times after calcinations. The surface area of Samchuk CaCO3 was increased from 0.28 m2/g to 11.24 m2/g after the calcinations to CaO at 900 oC for 2 hours at the CFB combustor. Based on the kinetic study of SO2 gas adsorption to CaO solid, the CaO conversion curve fits with respect to the reaction time. Since the slope of time versus is linear and close to 1, it follows gas film diffusion control. We suggest that the reaction is the first order. Since the limestone feeding is enough to capture all SO2 gases, the reaction become depend on the concentration of SO2. And the reaction can be disrupted by the formation of CaSO4 after reaction goes by. In the case of kinetic control, REFERENCES [1]F. Garcia-Labiano, A. Rufas, L. F. de Diego, M. de las Obras- Loscertales, P. Gayan, and J. Adanez, Fuel, 90, 3100 (2011). Therefore, [2]G. Ozkan, and G. Dogu, Chem Engineering and Processing, 41, 11 (2002) [3]S. K. Park, V. Kurshev, Z. Luan, C. W. Lee, and L. Kevan, Micropor. Mesopor. Mater., 38, 255 (2000). [4]D. R. M. Wright, F. Pinto, L. Armesto, M. A. Caballero, M. P. Aznar, A. Cabanillas, Y. Huang, C. Franco, I. Gulyurtle, and J. T. McMullan, Fuel Processing Technol. 87, 793 (2006). Figure 4 shows the CaO conversion curve fitting with respect to the reaction time. It is clearly seen that the slope of time versus is linear and close to 1, the reaction follows gas film diffusion control. [5]M. Higashi, S. Uchida, N. Suzuki, and K. I. Fujii, IEEE Trans. on Plasma Sci., 20, 1 (1992) [6]D. Shun, D. H. Bae, J. Y. Paek, and Y. S. Park, Korean J. Chem. Eng., 21, 890 (2004). [7]D. Shun, D. H. Bae, K. H. Han, S. H. Cho, and S. Y. Lee, Hwahak Konghak 40, 345 (2002). G [8]D. R. M. Wright, F. Pinto, L. Armesto, M. A. Caballero, M. P. Aznar, A. Cabanillas, Y. Huang, C. Franco, I. Gulyurtle, and J. T. McMullan, Fuel Processing Technol. 87, 793 (2006). [9]K. Redemann, E. U. Hartge, and J. Werther, Fuel, 87, 3669 (2008). [10]D. Shun, H. S. Chang, T. S. Park, D. H. Bae, and G. T. Jin, Korean J. Chem. Eng., 18, 630 (2001). [11]P. Gayan, J. Adanez, L. F. de Diego, F. Garcia-Labiano, A. Cabanillas, A. Bahillo, M. Aho, and K. Veijonen, Fuel, 83, 277 (2004). [12] L. Jia, J. Wang, and E. J. Anthony, Chem. Eng. J., 94, 147 (2003). Figure 4. CaO conversion curve fitting to the shrinking core model. 192 Cop y ri g ht © 2012 SciRes. |

