Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 181-184 doi:10.4236/ampc.2012.24B047 Published Online December 2012 (htt p://www.Sc iRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Coacervation Microencapsulation of CaCO3 Particles with a Fluoropolymer by Pressure-Induced Phase Separation of Supercritical Carbon Dioxide Solutions Kenji Mishi ma1*, Haruo Yokota1, Takafumi Kato1 , Tadashi Suetsugu2, Xiuq i n Wei2, Keiichi Irie3, Ke nic hi Mishima3, Michihiro Fujiw ara3 1Department of Chemical Engi neering, Fukuoka Uni vers ity, Nanakuma Jonan-ku, Fukuoka, Ja pan 2Department of Electronics Engineering and Computer S cien ce, Fukuoka University, Nana kuma Jonan-ku, Fukuoka, Japan 3Department of N europharmacology, Fukuoka U niversity, Nanakuma Jonan-ku, Fukuoka, Japan Email: mishima@fukuoka-u.ac.jp Received 2012 ABSTRACT We report a method for the coacervation micro-encapsulation of several forms of CaCO3 microparticles with the fluoropolymer poly(heptadecafluorodecyl acrylate) (poly (HDFDA)) by pressure-induced phase separation of a supercritical CO2 solution. A sus- pension of CaCO3 in CO2 and dissolved poly(HDFDA) were mixed in supercritical CO2. After the system pressure was slowly de- creased to atmospheric p ressure, the microcap sules were obt ained. Coacer vation was achieved by the precipi tation of pol y(HDFD A) during the decrease in the pressure of CO2; the solubility of poly(HDFDA) in CO2 decreased with the pressure. The structure and morphology of the microparticles were investigated by using a scanning electron microscope (SEM) and an electron probe micro- analyzer (E PMA) eq uipped with a wavelength dispersive X-ray spectr os cope (WDX). Keywords: Component; Supercritical Carbon Dioxide; Microencapsulation ; Coacervation; Fluoropolymer; Calcium Carb onate 1. Introduction Polymer microcapsules containing inorganic materials are attracting much attention as the field of supercritical CO2 (scCO2) technology. ScCO2 is the solvent of choice because it is readily available, inexpensive, and environmentally benign. Many investigators have attempted the formation of polymer microcapsules using scCO2 [1-6]. Rapid expansion from supercritical solutions (RESS) is a well-known process, and a variety of polymer microcapsules have been produced with the help of this process by many investigators [2,3,5-9]. However, the RESS process is limited by the low polymer solubility in CO2, caused by its low dielectric constant. Relatively few polymers are soluble in CO2 without a cosolvent. RESS of fluoropolymers such as perfluoropolyether, poly(1,1,2,2-tetrahydroperfluorodecyl acrylate), and poly (heptadeca-fluorodecyl acrylate), which are highly soluble in CO2 at temperatures near the ambient temperatu re, prod uces coatin g materials [10-12] and submicron to several micron -s ized par ticles and fibers [12, 13]. In this work, we try to form microcapsules of CaCO3 and poly(heptadecafluorodecyl acrylate) (poly (HDFDA)) using scCO2. In a previous work[9], we proposed a production me- thod for the fluoropolymer microcapsules of talc particles by pressure-in duced phase sep aration of scCO2. Figure 1 provides a conceptual framework of our proposed process in comparison with the conventional RESS process. In RESS, a supercritical fluid solution is expanded across a nozzle, leading to rapid supersaturation and the production of small par ticles. After a susp ensio n o f CaCO3 in CO2 containing a dissolved fluoropolymer is sprayed through the nozzle at atmospheric pressure, microcapsules and small polymer particles are obtained as shown in Figure 1(a). For the industrial applica- tion s, we have to r estri ct the generat io n of polymer par ticl es no t containing CaCO3 bec ause th ey degrad e the prod ucts. Therefore, to prevent the nucleation and the precipitation of polymer particles not containing CaCO3, the pressure is decreased slowly, and microparticles are collected in the high-pressure cell as shown in Figure 1 (b). The objective of this work is to check the feasibility of the pressure-induced phase separation of the scCO2 solution to the formation of fluoropolymer microcapsules of several shapes of parti cles of Ca CO3 and to stu dy the effe ct of se veral exp eriment al conditions on particle morphology. nozzle RESS (a) atmospheric pressure fluoropolymer+ CO2 + CaCO3 at 20 M Pa slow depressurization atmospheric pressure coacervation of fluoropolymer phase separation (b) PIPS Figure 1. Principles of the formation of polymer microcapsules of CaCo3 by (a) RESS and (b) pressure-induced phase separation of scCO2 sol utions. 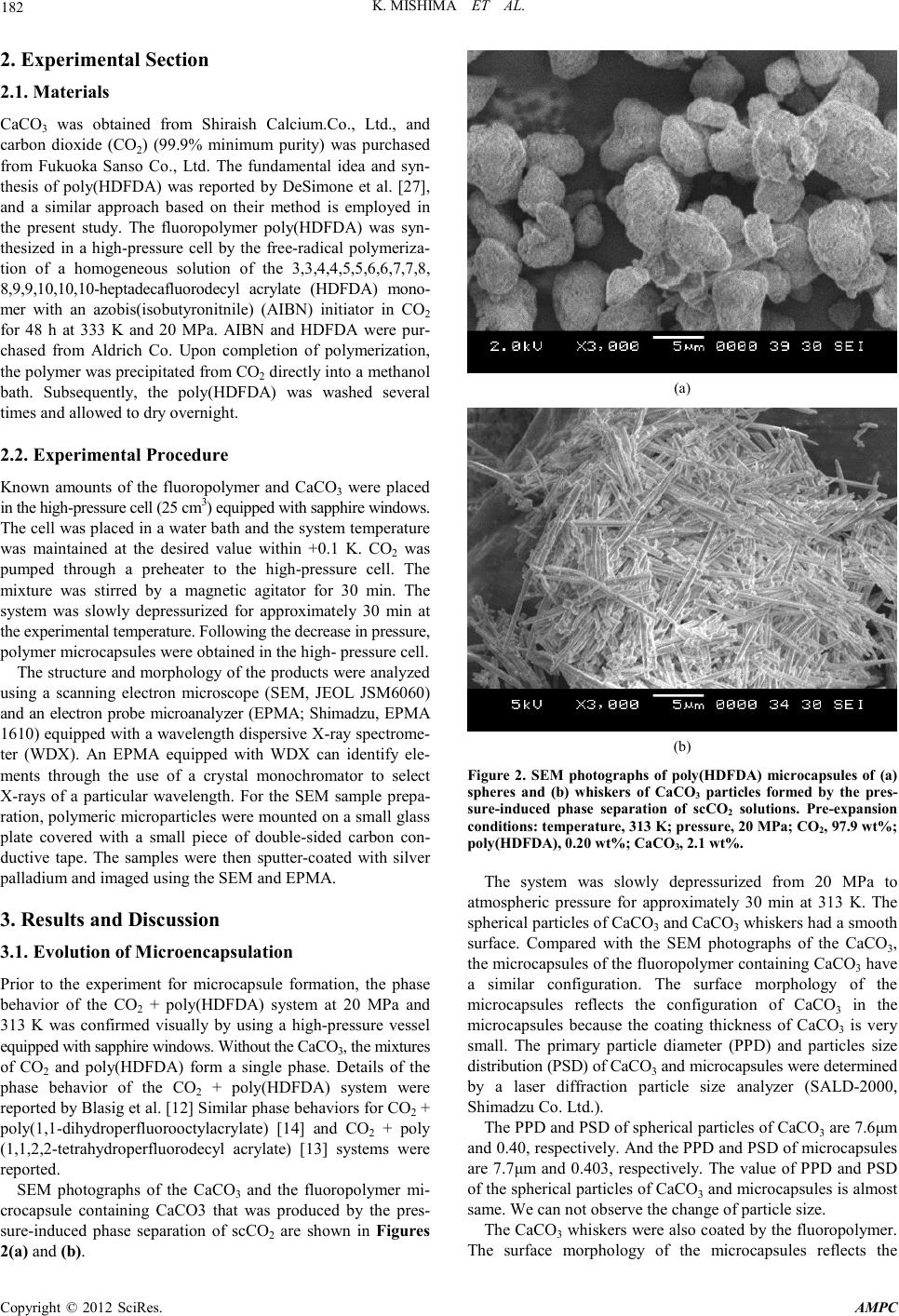 K. MISHIMA ET AL. Copyright © 2012 SciRes. AMPC 182 2. Experimental Section 2.1. Materials CaCO3 was obtained from Shiraish Calcium.Co., Ltd., and carbon dioxide (CO2) (99.9% minimum purity) was purchased from Fukuoka Sanso Co., Ltd. The fundamental idea and syn- thesis of poly(HDFDA) was reported by DeSimone et al. [27], and a similar approach based on their method is employed in the present study. The fluoropolymer poly(HDFDA) was syn- thesized in a high-pressure cell by the fre e-radical polymeriza- tion of a homogeneous solution of the 3,3,4,4,5,5,6,6,7,7,8, 8,9,9,10,10,10-heptadecafluorodecyl acrylate (HDFDA) mono- mer with an azobis(isobutyronitnile) (AIBN) initiator in CO2 for 48 h at 333 K and 20 MPa. AIBN and HDFDA were pur- chased from Aldrich Co. Upon completion of polymerization, the p olymer was p recip it ated from CO2 directly into a methanol bath. Subsequently, the poly(HDFDA) was washed several times and allowed to dry overnight. 2.2. Experimental Procedure Known amounts of the fluoropolymer and CaCO3 were placed in the high-pressure cell (25 cm 3) equipped w ith s a pphire w indows . The cell was placed in a water ba th an d the s ystem te mperature was maintained at the desired value within +0.1 K. CO2 was pumped through a preheater to the high-pressure cell. The mixture was stirred by a magnetic agitator for 30 min. The system was slowly depressurized for approximately 30 min at the expe r ime nta l tem pe rat ure . Follow i ng the dec r eas e in pr e ssur e , polymer microcapsules were obtained in the high- pressure cell. The structure and morphology of the products were analyzed using a scanning electron microscope (SEM, JEOL JSM6060) and an electron probe microanalyzer (EPMA; Shimadzu, EPMA 1610) equipped with a wavelength dispersive X-ray spectrome- ter (WDX). An EPMA equipped with WDX can identify ele- ments through the use of a crystal monochromator to select X-rays of a particular wavelength. For the SEM sample prepa- ration , pol ymeric micropar ticles were mou nted on a small glas s plate covered with a small piece of double-sided carbon con- ductive tape. The samples were then sputter-coated with silver palladium and imaged using the SEM and EPMA. 3. Results and Discussion 3.1. Evol ut ion of Microen capsul ati on Prior to the experiment for microcapsule formation, the phase behavior of the CO2 + poly(HDFDA) system at 20 MPa and 313 K was confirmed visually by using a high-pressure vessel equipped with sapphire windows. Without the CaCO3, the mixtures of CO2 and poly(HDFDA) form a single phase. Details of the phase behavior of the CO2 + poly(HDFDA) system were repor ted by Blasi g et al. [12] Similar phase b ehavi or s for CO2 + poly(1,1-dihydroperfluorooctylacrylate) [14] and CO2 + poly (1,1,2,2-tetrahydroperfluorodecyl acrylate) [13] systems were reported. SEM photographs of the CaCO3 and the fluoropolymer mi- crocapsule containing CaCO3 that was produced by the pres- sure-induced phase separation of scCO2 are shown in Figures 2(a) and (b). (a) (b) Figure 2. SEM photographs of poly(HDFDA) microcapsules of (a) spheres and (b) whiskers of CaCO3 particles formed by the pres- sure-induced phase separation of scCO2 solutions. Pre-expansion conditions: temperature, 313 K; pressure, 20 MPa; CO2, 97.9 wt%; poly(HDFDA), 0.20 wt%; CaCO3, 2.1 wt%. The system was slowly depressurized from 20 MPa to atmospheric pressure for approximately 30 min at 313 K. The spher ical p arti cles of CaCO3 and CaCO3 whiskers had a smooth surface. Compared with the SEM photographs of the CaCO3, the microcapsules of the fluoropolymer containing CaCO3 have a similar configuration. The surface morphology of the microcapsules reflects the configuration of CaCO3 in the microcapsules because the coating thickness of CaCO3 is very small. The primary particle diameter (PPD) and particles size distribution (PSD) o f C a C O 3 and microcap sules wer e dete rmined by a laser diffraction particle size analyzer (SALD-2000, Shimadzu Co. Ltd.). The PP D and PSD of spheri cal particles of CaCO3 are 7.6 μm and 0.40, respectively. And the PPD and PSD of microcapsules are 7.7μm and 0.403, respectively. The value of PPD and PSD of the sph erical parti cles of CaCO3 and microcap sules is almost same. We can not observe the change of particle size. The CaCO3 whiskers were also coated by the fluoropolymer. The surface morphology of the microcapsules reflects the  K. MISHIMA ET AL. Copyright © 2012 SciRes. AMPC 183 configuration of CaCO3 whiskers in the microcapsules because the coating thickness of CaCO3 is very small. But structure of CaCO3 whiskers coated by the fluoropolymer were more bulky than C aC O3 whiskers. Further evidence for the formation of fluoropolymer microcapsules of CaCO3 can be obtained using EPMA. The peak corresponding to F caused by the fluoropolymer can be observed for the microcapsules, it cannot be detected for CaCO3 because CaCO3 does not possess F. The surface distributions of F, O, and Ca were mapped in an EPMA image. Although the distribution of F in the microcapsules was fairl y sh arp, it was not detected on the CaCO3 surface. On the other hand, the distribution of Ca and O on the CaCO3 surface was sharper and broader. However, the distribution of Ca and O on the microcapsule surface was poorer than that on the CaCO3 surface. It can be considered that CaCO3 was completely encapsu lated by a thin fluoropolymer film. It was difficult to check the coating performance for all the collected microcapsules by using EPMA because in the proposed process, an extremely large number of microcapsules were produced. To evaluate the performance of the polymer coating, we examined the stability of the microcapsules in pure water. The CaCO3 particles or microcapsules were added to pure water (particle concentration: 1 wt%), and the suspended solution was shaken by a mechanical shaker. The stable conditions of t he spheri cal particles of C aCO3 and microcap sules in water were chec ked. Alth o ugh the CaC O3 was dispersed in pure water for more than 5 min, all the microcapsules floated on water because of the high water repellency of the fluoropolymer. The density of CaCO3 and microcap sules is almost same (ab out 2.8 g・cm-3), because microcapsules contain more than 90 % CaCO3. Although the density of microcapsules is higher than that of water, the microcapsules floated on the water. It is inferred that bulk density of microcapsules is lower than that of water. It is difficult to penetrate the water to the void between the microcapsules, becau se of the repellency of fluoropolymer. The CaCO3 was dispersed in water, because the CaCO3 has hydrophilic surfaces. To check the stability of the microcap- sules in pure water, a turbidity measurement was performed using an ultraviolet/visible (UV/VIS) spectrometer at 600 nm wavelength. The turbidity measurement was used to observe the stability of small particle dispersions [29]. We could not observe the dispersed particles through the stability analysis of microcap sules in water becau se as in the case of pur e water, no turbidity was observed. The stability analysis revealed that most of the CaCO3 particles were coated with the fluoropoly- mer and were p r es ent inside the produced microcapsules. 3.2. Formation M echanism of Microca psules To identify the advantage of the formation mechanism of mi- crocapsules by the pressure-induced phase separation of scCO2 as compared with RESS, the microcapsules were prepared by RESS. Because RESS is one of the promising methods for the formation of polymer microcapsules and/or composites by us- ing scCO2, several investigators have reported the formatio n of polymer microcapsules and/or composites by RESS [1,2]. The particle formation mechanism by RESS was analyzed thermo- dynamically [4]. In this work, we attempted the formation of microcapsules by RESS under the following experimental con- ditions. The pre-expansion pressure was 20 MPa, and the tem- peratu re was 313 K. Th e feed concen trations of the CaCO3 and the fluoropolymer were 2.1 wt% and 0.20 wt%, respectively. The feed composition in the RESS experiment was the same as that in the experiment on the formation of microcapsules by the pressure-induced phase separation of scCO2. The mixtures of scCO2, the fluoropolymer, and the CaCO3 were expanded across the capillary nozzle (L = 500 mm, D = 1.2 mm) to at- mospheric pressure. After the expansion, the microparticles were precipitated. SEM photographs of the fluoropolymer mi- crocapsules produced by RESS and containing CaCO3 were obtained. Compared with the morphology of microcapsules prepared by the pressure-induced phase separation of CO2 as shown in Figure 2, the polymer particles prepared by RESS were observed on the surfaces of the CaCO3 particles. The po- lymer does not form a smooth surface at the CaCO3 particles but is adhered as small particles at the s urface of the CaC O 3. To examine the coating performance of RESS, the obtained particl es were an al yzed by EP MA and b y per for ming a stab il it y test in water. F, Ca, and O wer e d etected in the WDX spectrum of the microcapsules. Furthermore, we examined the stability of the microcap sules in pure water to evalu ate the performance o f the polymer coating. The WDX spectrum and the stability test revealed that most of the CaCO3 was microencapsulated with the fluoropolymer. However, small polymer particles were precipitated on the surface through the RESS process. The for- mation mechanism of microcapsules and small polymer par- ticles in the RESS process may be considered as follows. Dur- ing rapid depressurization both the CaCO3 and the polymer precipitate from the solutions. And the CaCO3 particles are formed in the expanding jet. Some polymer coated on the Ca- CO3 particles, and some fine polymer particles are generated during the deposition. The evidence for the formation of fine polymer particles by RESS can be obtained by performing the RESS experiment without CaCO3. The mean particle diameter was Figure 3. Sta bility of micro capsules in pure water. (a) CaCO3 and (b) poly(HDFDA) microcapsules formed by the pressure-induced phase separation of scCO2 solutions. See Figure 2 for the pre-expansion conditions.  K. MISHIMA ET AL. Copyright © 2012 SciRes. AMPC 184 less than 1 μm. With regard to the RESS experiment for the formation of fluoropolymer particles, similar particle morphol- ogy was reported by Blasig et al.[12] and Mawson et al. [13] These fine po lymer particles p recipitated on an d adhered to th e CaCO3 surface by the supersaturation and homogeneous nuc- leation of the fluoropolymer that was caused by rapid depressu- rization. To prevent the formation of polymer particles, we have to inhibit the supersaturation of the solute and the homo- geneous nucleation caused by the rapid expansion of CO2. However, it is impossible to prevent the supersaturation in RESS. We can prevent the formation of polymer particles by the pressure-induced phase separation of CO2. Because the depressurizing rate is very slow compared with the convention- al RESS process, it is possible to inhibit the large supersatura- tion of the solute and the homogeneous nucleation of particles. During the slow depressurization, the coacervation was achieved. On the other hands, after the pressure in the high-pressure cell containing no CaCO3 decreased, polymer foams were obtained. With experimental setup, no pure fluoro- polymer were formed. It is inferred that the CaCO3 suspended in scCO2 act s as an accelerator for the precipit ation of polymer particles and the occurrence of coacervation on the CaCO3 surface. Furthermore, it is very difficult for the microcapsules to produce forms, because the microcapsules contain about 90 % CaCO3. In the conventional coacervation microencapsu- lation technique, coacervation i s induced by a ph ase separatio n caused due to a pH change and the addition of a nonsolvent or electrolyte [16]. In contrast, in the present experiment, coacer- vation was ind uced by a phase separatio n caused by a decreas e in pressure. 4. Conclusions The pressure-induced phase separation of scCO2 has been util- ized to produce fluoropolymer microcapsules of several shape particles of CaCO3. Prior to depressurization, the polymer and CaCO3 were mixed i n scCO2. Fluo ropo lymer co acervation was achieved during the slow decrease in the pressure. Following the coacervation, we obtained the fluoropolymer microcapsules of CaCO3. The products were analyzed by SEM and EPMA equipped with WDX. The CaCO3 was complet el y coated with a thin fluoropolymer film. Compared with the microcapsules formed by RESS, the obtained microcapsules had a smooth surface; fine polymer particles on the CaCO3 surface were not observed. REFERENCES [1] J. Jung and M. Perrut, “Particle design using supercritical fluids: Literature and patent survey,” The Journal of Supercritical Flu- ids, vol. 20, no. 3, pp .179-219, 2001. J. Clerk M axwell, A Trea- tise on Electricity and Magnetism, 3rd ed., vol. 2. Oxford: Cla- rendon, 1892, pp.68–73. [2] S. D. Yeo and E. Kiran, “Formation of polymerparticles with supercriticalfluids: A review,” The Journal of Supercritical Flu- ids, vol. 34, no. 3, pp. 287-308, 2005. [3] E. Reverchon and R. Adami, “Nanomaterials and supercritical- fluids,” The Journal of Supercritical Fluids, vol. 37, no. 1, pp. 1-22, 2006. [4] J.W.Tom, P.G. Debenedetti, R. Jerome, “ Precipitation of Po ly(L-lactic acid) and Composite Poly(L-lactic acid)-Pyrene Particles by Rapid Expansion of Supercritical Solutions.” J. Su- percritical Fluids vol.7, 9,1994. [5] K. Mishima, K. Matsuyama, D. Tanabe, S. Yamauchi, T. J. Young, and K. P. Johnston, “Microencapsulation of proteins by rapid expansion of supercritical solution with a nonsolvent,” AIChE Journal, vol. 46, no. 4, pp. 857-865, 2000. [6] K. Mi shima,” Biodegra dable part icle format ion for dru g and gene delivery using supercritical fluid and dense gas, “Advanced Drug Delivery Reviews, vol. 60, no. 3, pp. 411-432, 2008. [7] K. Matsuyama, K. Mishima, K. Hayashi, and H. Matsuyama, “Microencapsulation of TiO2 Nanoparticles with Polymer by Rapid Expansion of Supercritical Solution,” Journal of Nano- particle Research, vol. 5, no. 1-2, pp. 87-95, 2003. [8] K. Matsuyama, K. Mishima, K. I. Hayashi, H. Ishikawa, H. Mat suyama , and T. Harada, “Formation of microcapsules of me- dicines by the rapid expansion of a supercritical solution with a nonsolvent,” Journal of Applied Polymer Science, vol. 89, no. 3, pp. 742-752, 2003. [9] K. Matsuyama and K. Mishima, “Coacervation microencapsula- tion of talc particles with a fluoropolymer by pressure-induced phase separation of supercritical carbon dioxide solutions,” In- dustrial and Engineering Chemistry Research, vol, 45, no. 18, pp. 6462-6168, 2006. [10] DeSi mone, J. M. ; Guan, Z. ; Elsb ernd, C . S. Synth esis of Fluoro- polymers in Supercritical Carbon Dioxide. Science 257, 945-946,1992. [11] Chernyak, Y.; Henon, F.; Harris, R. B.; Gould, R. D.; Franklin, R. K.; Edwards, J. R.; DeSimone, J. M.; Carbonell, R. G. Formation of Perfluoropolyether Coatings by the Rapid Expansion of Su- percritical Solutions (RESS) Process. Part 1: Experimental Re- sults. Ind. Eng. Chem. Res. 2001, 40, 6118.. [12] Blasig, A.; Shi, C. M.; Enick, R. M.; Thies, M. C. Effect of Concentration and Degree of Saturation on RESS of a CO2-soluble Fluoropolymer. Ind. Eng. Chem. Res. 2002, 41, 4976. [13] Mawson, S.; Johnston, K. P.; Combes, J. R.; DeSimone, J. M. Formation of Poly(1,1,2,2-tetrahydroperfluorodecyl acrylate) Submicron Fibers and Particles from Supercritical Carbon Dio- xide Solutions. Macromolecules 1995, 28, 3182. [14] Luna-Barcenas, G.; Mawson, S.; Takishima, S.; DeSimone, J. M.; Sanchez, I. C.; Johnston, K. P. Phase Behavior of Po ly( 1,1-dihydroperfluorooctylacrylate) in Supercritical Carbon Dioxide. Fluid Phase Equilibria 1998, 146, 325. [15] Calvo, L.; Holmes, J. D.; Yates, M. Z.; Johnston, K. P. Steric Stabilization of Inorganic Suspensions in Carbon Dioxide. J. Sup ercritical Fluids 2000, 16, 247. [16] J. Lazko, Y. Popineau, J. Legrand, Soy Glycinin Microcapsules by Simple Coacervation Method. Colloids and Surfaces B: Bio- interfaces 2004, 37, 1. |

