Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 20-26 doi:10.4236/eng.2012.410B006 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Towards a Methodology for the Differential Analysis in Human Locomotion: A Pilot Study on t he Participation of Individuals with Multiple Sclerosis Huiying Y u, Thompson Sar kodie-Gyan Department of Electri cal Engineering, University of Texas at El Paso, Texas, USA. Email: hyu@utep.edu Received 2012 ABSTRACT Mult iple sclerosi s (MS) is an unpredi ctable di sease of the centr al nervous syste m that can range fro m relativel y benign to somewhat disabling to devastating, as communication between the brain and other parts of the body is disrupted. Scientists have learned a great deal ab out MS in recent years; yet still , its cau se remains el usive. This p aper in tend s to investigate the h ypothes is that gait dynamics have meaning and may be useful in providing insight into the neural control of locomotion. It further seeks to explore the mutual interactions and influences of MS functions on gait, and vice versa, in a quantitative and robust fashion. Ground reaction forces (GRFs), muscle activities, and segmental accelerations within a gait cycle were analyzed in this study. Patterns of the signals from six relapsing-remitting multiple sclerosis (RRMS) patients were compared with the healthy subjects. This quantitative gait analysis aids to illuminate a better understanding of the mobility-related di sease such as RRM S characteristi cs. An outcome of thi s study is a reproducible methodology for helping therapists make reliable and differentiable diagnosis, design a tailored therapeutic strategy, and comfortably evaluate the follow-ups on patient’s functional recovery. Keywords: Ground Reaction Forces; EMG Muscle Activities; Multiple Scelorsis; Segmental Accelerations; Kinetic and Kinematics Measurement; Fuzzy Relation Matrix; Fuzzy Similarity 1. Introduction Approximately 400,000 Americans have multiple sclerosis (MS), an d every week abo ut 200 new cases are diagno sed [20]. Worldwide, MS affects around 2.5 million people: it is one of the most common chronic neurological disorders and causes of disability in young adults between 20 and 40 years of age, especially in Europe and in N orth America [ 29]. Multiple sclerosis is a demyelinating disease of the central nervous system (CNS) that can result in severe morbidity and mortality. Mobility loss and walking impairment are among the direct impact on the outcomes and the quality of life of individuals with MS. A noticeable symptom of multiple sclerosis is tremor, a rhythmic, involuntary oscillatory movement of an area of the body. Tremor caused by MS can affect the head, neck, vocal cords, trunk, and limbs, but it is most common in the arms. The two most prevalent forms of tremor in MS are postural tremor (present when holding a position against gravity) and intention tremor (present during goal-directed movement, worsening as the individual approaches a target). Tremor can be incapacitating, seriously impairing functioning and ability to perform activities of daily living. Other common motor symptoms of multiple sclerosis include foot drop, muscle weakness and/or spasticity, and altered gait rhythm. Sutliff has evaluated that maintaining mobility was ranked as one of the highest priorities among patients with MS, regardless of disease duration or disability level [27]. The loss of mobility contributes to a substantial patient burden. The statistical techniques of path analysis has shown how difficult walking significantly affects physical activity in patients with MS. Impaired mobility is associated with reductions in quality of life and activities of daily living. Intrinsic to medical practice particularly in the evaluation of different therapeutic approaches to chronic disease, the health of the patient in association with the individual’s quality of life is extremely essential. In th e case of MS, a chronic debili tating disease, the preservation of the health-related quality of life becomes significant. Therefore, there is the need for measure- ment tools to accurately and consistently quantify the quality of life over the course of the disease. The reliable and efficient measurement of the level of impairment will assist in evaluating the variances from normal patterns through training and other compensatory strategies. To date, the evaluation of gait impairment in individuals with MS is typically performed through subjective examination by the clinician. The standardized clinical assessment tools for MS relating to gait include MS Functional Composite (MSFC) [4,12], Expanded Disability Status Scale (EDSS) [22], Disease Steps (DS) [9], MS walking scale -12 (MSWS-12) [8, 19], and other tests for balance and gait dysfunctions [1,10,15,17,18,21]. A significant problem with the subjective clinical examination is that it may be misleadi ng du e to an in ability to ob serve the smal l variance s from the unquantifiable impact of the observer. Therefore, clinical quantitative gait analysis must be used to provide accurate scientific evaluation through measurement of kinetic, kinematics and muscle activity. Current clinical methods of gait analysis including the optical motion capture system are time an d labor in tensi ve and invo lve extensive post-hoc data analysis [2,6,11]. These limitations 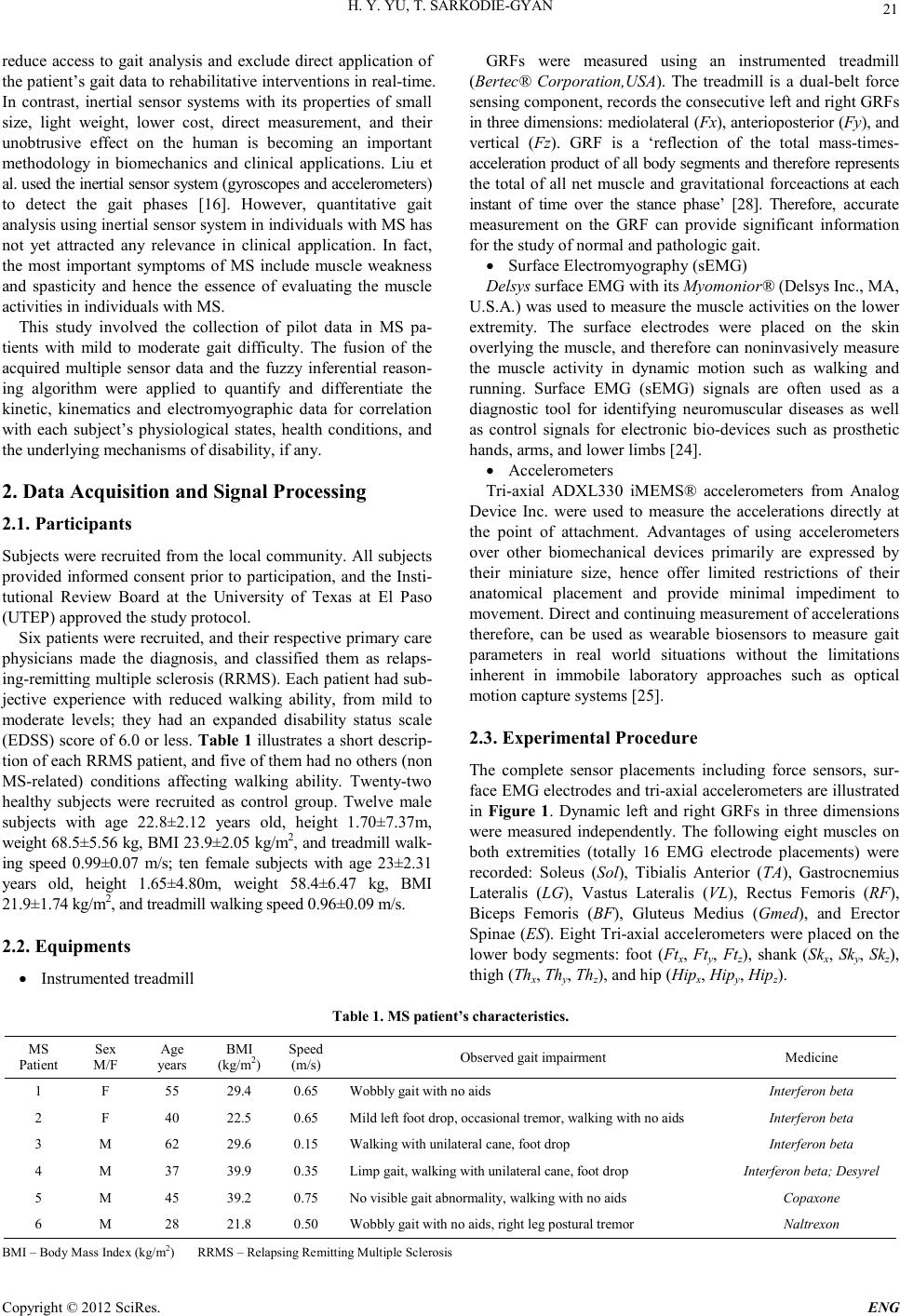 H. Y. YU, T. SARKODIE-GYAN Copyright © 2012 SciRes. E NG 21 reduce access to gait analysis and exclude direct application of the patien t’s gait dat a to rehabilitati ve interven tion s in real-time. In contrast, inertial sensor systems with its properties of small size, light weight, lower cost, direct measurement, and their unobtrusive effect on the human is becoming an important methodology in biomechanics and clinical applications. Liu et al. used the in ertial sensor system ( gyroscopes and accelerometers) to detect the gait phases [16]. However, quantitative gait analysis using inertial sensor system in individuals with MS has not yet attracted any relevance in clinical application. In fact, the most important symptoms of MS include muscle weakness and spasticity and hence the essence of evaluating the muscle activities in individuals with MS. This study involved the collection of pilot data in MS pa- tients with mild to moderate gait difficulty. The fusion of the acquired multiple sensor data and the fuzzy inferential reason- ing algorithm were applied to quantify and differentiate the kinetic, kinematics and electromyographic data for correlation with each subject’s physiological states, health conditions, and the underlying mechanisms of disability, if any. 2. Data Acquisition and Signal Processing 2.1. Participants Sub jects were recrui ted from th e local co mmunity. All subjects provided informed consent prior to participation, and the Insti- tutional Review Board at the University of Texas at El Paso (UTEP) approved the study protocol. Six pat ients were recrui ted, and their respective pr imary care physicians made the diagnosis, and classified them as relaps- ing-remitting multiple sclerosis (RRMS). Each patient had sub- jective experience with reduced walking ability, from mild to moderate levels; they had an expanded disability status scale (EDSS) score of 6.0 or less. Table 1 illustrates a short descrip- tion of each RRMS patient, and five of them had no others (non MS-related) conditions affecting walking ability. Twenty-two healthy subjects were recruited as control group. Twelve male subjects with age 22.8±2.12 years old, height 1.70±7.37m, weight 68.5±5.56 kg, BMI 23.9±2.05 kg/m2, and t readmill walk- ing speed 0.99±0.07 m/s; ten female subjects with age 23±2.31 years old, height 1.65±4.80m, weight 58.4±6.47 kg, BMI 21.9±1.7 4 k g /m 2, and t rea dm il l walk ing speed 0. 96± 0.09 m/s . 2.2. Equipments • Instrumented treadmill GRFs were measured using an instrumented treadmill (Bertec® Corporation,USA). The treadmill is a dual-belt force sensing component, records the consecutive left and right GRFs in three dimensions: mediolateral (Fx), anterioposterior (Fy), and vertical (Fz). GRF is a ‘reflection of the total mass-times- acceleration product of all body segments and therefore represents the total of all net muscle and gravitational forceactio ns at each instant of time over the stance phase’ [28]. Therefore, accurate measurement on the GRF can provide significant information for the study of normal and pathologic gait. • Surface Electr omyography (sEMG) Delsys surface EMG with its Myomonior® (Delsys Inc., MA, U.S.A.) was used to measur e the muscle activities o n the lo wer extremity. The surface electrodes were placed on the skin overl ying the muscle, and therefo re can nonin vasively measu re the muscle activity in dynamic motion such as walking and running. Surface EMG (sEMG) signals are often used as a diagnostic tool for identifying neuromuscular diseases as well as control signals for electronic bio-devices such as prosthetic hands, arms, and lower limbs [24]. • Accelero meters Tri-axial ADXL330 iMEMS® accelerometers from Analog Device Inc. were used to measure the accelerations directly at the point of attachment. Advantages of using accelerometers over other biomechanical devices primarily are expressed by their miniature size, hence offer limited restrictions of their anatomical placement and provide minimal impediment to movement. Direct and continuing measurement of accelerations therefore, can be used as wearable biosensors to measure gait parameters in real world situations without the limitations inherent in immobile laboratory approaches such as optical motion capture systems [25]. 2.3. Experimental Procedure The complete sensor placements including force sensors, sur- face EMG electro des an d tri-axial acceler ometers are i llust rated in Figure 1. Dynamic left and right GRFs in three dimensions were measured independently. The following eight muscles on both extremities (totally 16 EMG electrode placements) were recorded: Soleus (Sol), Tibialis Anterior (TA), Gastrocnemius Lateralis (LG), Vastus Lateralis (VL), Rectus Femoris (RF), Biceps Femoris (BF), Gluteus Medius (Gmed), and Erector Spin ae (ES). Eight Tri-axial accelero meters were placed on the lower body segments: foot (Ftx, Fty, Ftz), shank (Skx, Sky, Skz), thigh (Thx, Thy, Thz), and hip (Hipx, Hipy, Hipz). Tabl e 1. MS patient’s characteristics. MS Patient Sex M/F Age years BMI (kg/m2) Sp eed (m/s) Observed gait impairment Medicine 1 F 55 29.4 0.65 Wobbly gait with no aids Interfer o n beta 2 F 40 22.5 0.6 5 Mild left foot drop, oc casional tremor, walking with no aids Interfero n beta 3 M 62 29.6 0.1 5 Walkin g with unilateral cane, foot drop Int erferon beta 4 M 37 39.9 0.3 5 Limp gait, walking with unilateral cane, foot drop Interfer on be ta ; Desy rel 5 M 45 39.2 0.7 5 No visible gait abn ormality, walking with no aids Copaxone 6 M 28 21.8 0.5 0 Wobbly gai t with no aids, right leg postural tremor Naltrexon BMI – Body Mass Index (kg/m2) RRMS – Relapsing Remitting Mul tiple Sclerosis  H. Y. YU, T. SARKODIE-GYAN Copyright © 2012 SciRes. ENG 22 Figure 1. Multiple Sensors Placements for Data Acquisition. Each subject wore running shorts and comfortable T-shirt during the experiment. Subjects were allowed to become familiar with the walking track on the treadmill before conducting the experiment. For patients, partial body-weight support (harness) fixed on the instrumented treadmill was applied for safety purp os es. The sp eed of the tr eadmil l was in cre men tall y i nc rea sed or decr eased to determine the participant’s comfortable walking speed. Every subject was instructed to walk continuously for three minutes on the treadmill. The LabVi ew software was used for th e acquisition of accel- erations and GRFs data at sampling frequency of 100 Hz. EMG data was acquired at sampling frequency of 1000 Hz using Delsys EMGWorks acquisition software. The synchronization between the acquired data was achieved by Delsys EMG trigger module. Therefore, the GRFs, EMGs, and acceleration signals were coll ected simultaneously. 2.4. Data Processing White noise was observed in the force data due to the vibrations and the motion artifact during the walking process on the treadmill. The GRF data were smoothened using second order Butterworth low pass filter with cut-off frequency of 20 Hz. The second order Butterworth low pass filter with cutoff frequency of 6 Hz was applied to reduce the noise and improve the resolution of the acceleration data. Since unprocessed EMG data or raw EMG data cannot be used for quantitative comparison between subjects, the EMG raw data were filtered by: Band Pass Filtering (second order Butterworth band-pass filter with a frequency of 20-200 Hz), Full Wave Rectification, and Linear Envelope (second order Butterworth low pass digital filter with a cutoff frequency of 7 Hz). 2.5. Fuzzy Relation Matrix and Fuzzy Similarity In this study, a cluster of data relating to the kinematics, kinetics and muscle activities was acquired using an array of inertial sensors (accelerometers), instrumented treadmill and electromyographic device during normal walking tasks. These data were treated as aggregate information granules that enabled the eff ici e nt par tit ion of input spa ce a nd m ore rapid a na ly s is. Thi s means that we dealt with the relationships of the kinematic, kinetic and muscle activity functions. The relationships depict the attributive features of the human movement and are expressed in an implication table giving rise to a fuzzy relational matrix, established between the dynamic activities during the walking tasks. Therefore, we established granulated information between 1) the acquired kinetic and kinematic data and the gait phases; and 2) the acquired muscle activity data and the gait phases. Since data are usually represented in a tabular form, it is also easy to consider the table as a matrix. Hence, a fuzzy relational matrix was used as the expression of the strength of association or interaction amongst the elements of the gait functio ns, and it elucidates a ru le base th at is used to provide a model, a model of a featur e matri x. GRF data were normalized by individual body weight, whe- reas EMG and acceleration data were normalized by the maxi- mum averaged values. Normalized Data were then aver- aged from 100 strides (100 gait cycles). Averaged data within a gait cycle with s even fun ctional gait phas es were extracted using t he vertical GRFs [11]. The seven functional gait phases as illu- strated in Figure 2 are: Loading Response (LR), Mid- stance (MST), Terminal Stance (TST), P reswing (PSW), Initial Swing (ISW), Midswing (MSW), and Terminal Swing (TSW) [28]. The mean values of the data in the seven gait phases are represented in a matrix for m, fuzzy relational matrix. Three types of fuzzy relational matrices were constructed using the methods in [11]. The relatio nal matrix between GRF s { } ,,Fx FyFz and the 7 gait phases { } 1234567 ,,,,,,PPPPPPP ; EMG { } SolTALG VLRFBFGmedES and the seven gait p hases; the accelerati ons { } ,,,,,,,,, ,, xyzxyzxyz x y z FtFtFtSkSkSkTh ThThHipHipHip and the seven gait phases . Fuzzy similarity of each parameter was calculated using the equation (3) defined in [11]. This methodology involving the acquisition of the kinematics, kinetics and electromyographic data of human gait enable the understanding of the neurophysi- ology/biomechanics of gait for augmenting objective measure- ments of mobility and functional status. This methodology may be applied for the efficient and reliable analysis of human gait dynamics at a level that quantifies variations in MS from mor- phometrically adjusted normal. 3. Results The GRFs, the EMG muscle activities, and the accelerations within a gait cycle were analyzed in this study. Patterns of the signals from six RRMS patients were compared with the healthy subjects. The pathologic characteristics of multiple sclerosis were concluded and the intelligent algorithm provided a quantitative analysis for the clinical diagnosis for MS patients. In Figure 3(a), the normalizations of the 3-D GRFs (GRFs in % BW) with respect to the gait cycle were performed for both healthy and MS patients. Figure 3(b) illustrates the fuzzy simi- larities between the GRFs of the MS patients and the healthy  H. Y. YU, T. SARKODIE-GYAN Copyright © 2012 SciRes. E NG 23 Fi g ure 2. Seven gait phases using % gait cycle and transitional gait events. subjects in three dimensions. This similarity ranges between 0 and 1, and it represents the strength of the association between the GRFs of the healthy subjects and MS patients within the respecti ve gait p hase. Eight normalized EMG data within a gait cycle were illustrated in Figure 4(a). The Figure presents the gait pattern of the healthy subject as an asymptote to the variances in the gait patterns of the individual patients. Figure 4(b) illustrates the grade of similarities between the eight EMGs of the MS patients and the healthy subjects within the seven gait phases . Normalized 3-D acceleration data on four body segments (foot, shank, thigh, and hip) within a gait cycle are shown in Figure 5(a). Figure 5(b) represents the fuzzy similarities of accelerations of the MS patients within the seven gait phases. 4. Discussion The self-selected walking speeds of the MS patients were generall y lower as compared with th ose of the healthy subj ects (range 0.9~1.2 m/s). The effects can be observed from the GRFs in all dimensions, i.e. the increased stance phase/double stance phase, decreased swing phase. The GRF pattern has simply shown an estimated disease-severity of the individual, RRMS-3 subject for instance was found with more severe symptoms along all six MS patients. This may also be due to the age of this patient (62 years old) with other gait-related disorders such as diabetes (information not shown in methods). A previous research result suggested that the GRFs varied significantly between the MS patients and the healthy subjects in both vertical and anterior-posterior directions but showed no differences in the mediolateral direction [23, 30]. However this study found that the vertical GRF of the MS patients did not depi ct the referen ce M-shaped pattern, and the lower magnitude of the anterior-posterior forces appear in most of the MS patients. The result illustrated in Figure 3(a) suggests a common feature of MS patients in th e ver tical GRF, the magn itude of the first peak is excessi vel y high in t he earl y stance ph ase, wherea s that of the second peak is moderate in the terminal stance. In normal gait the magnitude of the two peaks are approximately (a) (b) Figure 3. Ground Reaction Force Pattern within a Gait Cycle and Fuzzy Similari tie s of the Seve n Gai t Phases.  H. Y. YU, T. SARKODIE-GYAN Copyright © 2012 SciRes. ENG 24 (a) (b) Figure 4. Ele ct romyogr ap hic a l P at tern within a G ait C y c le an d F u zzy Simi lari ties o f th e Seven Ga it Phases. (a) (b) Figure 5. Acceleration Pattern within a Gait Cycle and Fuzzy Similarities of the Seven Gait Phases. equal in which the M-shape force curve illustrates the weight transfer from the heel to the mid-foot and the mid-foot to the ball of the foot for push-off. The fact that the vertical GRF exceeds b ody weight in the terminal stan ce (i.e. seco nd peak of the vertical GRF) indicates that the body weight is being supported and some propulsion is generated successfully. On the other hand, the low propulsive force is generated in the MS patient, and the greater impairments (much more reduced second peak or sometimes even less than the body weight) indicated a more severely affected MS patient (RRMS-3). By comparing the magnitude of the anterior- posterior force of the healthy subjects, the MS subjects revealed much lower magnitu de of th e force. This study has also found different patterns in mediolateral GRF. Apart from the RRMS-3 patient, the higher magnitudes of the mediolateral force were found in all other five patients during the pre- and terminal stance phase (i.e. single support phase). The mediolateral force in normal gait is of lower magnitude in most situations and relates to balance during walking. The higher magnitude of the mediolateral force with MS patients indicates that more effort was applied to keep the balan ce, especial ly durin g t he single su pport phase. Figure 4(a) illustrates the eight muscle activities of both the healthy and MS subjects, respectively. The Soleus and Gastrocnemius muscles are ankle plantarflexors, and designed to stabilize the foot and knee during the stance phase of the gait. However, most of the MS subjects exhibited long-latency periods with increased activities on the Soleus and Gastrocnemius in the stance phase. The Tibialis Anterior (TA) is an ankle dorsiflexor and most active at heel strike and prevents “foot-slap”. The behavior of the TA in this study was significantly greater during the initial contact and during the toe-off. This increased EMG activity that was apparent in the ankle dorsiflexor muscles in the MS subj ects are co n sidered the mechan ism for co unt eract-balancing the deficits. The exhibited behavior may illustrate implications relating to fatigue and spasticity [13]. In addition, the TA activity of most of the MS patients was significantly greater during the pre-swing phase and the early swing phase. This activity is supposed to assist the toes in clearing the floor for the push-off into swing. Vastus Lateralis (VL) and Rectus Femoris (RF) muscles depict the knee extensors/hip flexors and the muscles of quadriceps group. Most of the MS subjects showed intense and prolonged action of the VL and RF from the midstance through to the initial swing. Extensor spasticity of the legs, particularly of the quadriceps, might be considered advantageous for standing, walking and particularly transferring, as it may compensate for muscular weakness. Clinical research has suggested that some structural changes of spastic muscles and of connective tissues develop over periods of weeks to months in MS patients, leading to changes in mechanical properties in the leg extensors. This finding may therefore be due to inadequate management of spasticity which leads to such structural changes of muscles with subsequent functional impairment [14]. Biceps Femoris (BF) muscle is the lateral hamstring, which is activated to accelerate the knee toward flexion and the hip toward extension during swing in normal gait. This was found in the prolonged and increased muscle activity of BF in the stance p hase, parti cul arly in the single-su p port phase, whereas a delay and reduced muscle activity of BF in the midswing through terminal swing phase. These findings suggest that the prolonged stance activity of the upper leg muscles, parti cularly tho se knee and hip ext ensors, could be r elated t o a more gen eral neuromuscular strategy that serves to supply additional support. Evidence for the use of this strategy was found in hemiparetic stroke fro m a recent muscle actuated simulation o f hemiparetic gait by Higginson, who showed that co-contraction of the upper leg muscles during midstance can be used to enhance knee stability [7]. It is suggested that this strategy is used to compensate BF weakness in the swing phase. These spastic upper leg extensors may in turn cause delayed Gluteus medius (Gmed) in the loading response. Gluteus medius is the hip abductor and pelvic stabilizer. Figure 4(a) shows the MS subjects with greater activity of Gmed in the midstance and midswing phases to support the body weight with single leg  H. Y. YU, T. SARKODIE-GYAN Copyright © 2012 SciRes. E NG 25 over the ground. Erector Spinae (ES) or lower back muscle monitors the paraspinal activity of the trunk movement and stabilization. The EMG activation level of the ES was found to be greater in the MS than in healthy subjects at the swing-stance and stance-swing transitions, which is likely to be a compensatory factor in the instability during phase transition, as well as serving to reduce the risk of falling. The use of a ccelero mete rs is an i mportant alt ernativ e appr oach for the acquisition of kinematic data in gait analysis techniques. Accelerometers placed on the foot, shank, thigh, and hip reveal both temporal aspects o f the g ait cycl e and ac celeratio n forc es at key events in the cycle, such as heel strike, toe-off, and events during swing phase. Study of the acceleration/deceleration patterns in MS patients can further confirm the muscle spasticity or muscle weakness and physiological tremor in the individual gait. There were significant different patterns of acceleration in MS sub jects as co mpared with the health y subjects, especi ally in the ant e ri opost e ri or a nd mediola t eral di re c t ions . In the medio lateral d irecti on, there was a greater a cceler ation occurring in the initial contact during the loading response (the fir st 10% of the gait cycle) in the MS subjects. It is thought to be the compensatory mechanism for the stability of the ankle and knee. The acceleration in the following gait cycle on that direction was generally reduced in most of the MS subjects, an expected occurrence with respect to the slow working speeds. In the anterior-posterior d irection, a sharp accelerati on in th e initial contact was found in most of the MS subjects, which depicts an ind ication of faster h eel-to-toe force tran sfers at that time, especially for patients with foot-drop. On the foot accelerat io n patt ern in this directio n, th ere were eith er mod erate deceleration at toe-off observed in RRMS-1 and RRMS-2, or faster deceleration followed by decreased acceleration at the beginning of the initial swing seen in RRMS-3 ~ RRMS-6. This is an indication of an inadequate toe clearance in the MS subjects, resulting in reduced forward progression of the limb with push off into swing. The histogram of Figure 3(b) illustrates a relational mapping between the GRFs of the subject with respect to the seven gait phases, for both male and female subjects. The normalized “1” is an asymptote of the GRFs of a healthy patient and is used as the reference p attern . The compar ative relati onsh ip between t he healthy subject and the MS patients illustrates a functional as- sociati on between t he elements o f the GRFs and th e gait phases. The same illustrations shown in Figures 4(b) and 5(b) for the muscles activities and acceleration respectively. The asymp- totes depicted in the histogram illustrate a direct grade of ag- gregation or association between the physiologic data of the MS patient with respect to those of the healthy subject. This strength of aggregation is a functional relationship that clearly illustrates an objective and differential assessment of the effi- cacy and outcomes of rehabilitation therapies and practices. This will also enable the further development of precise me- thods for measuring impairments and disabilities. 5. Conclusions This study offers a narrative description of the dynamic phasic patterns of GRFs, muscle activities, and accelerations in the body segments in the MS patients. The study enables the analy- sis of multiple components in three-dimensional plane for the reliable differentiation of pathological and/or compensatory functional impairments in MS and in normal gait patterns. The fuzzy inferential reasoning algorithm provides the understand- ing of the dynamic patterns of these components. In real-time data acquisition and pattern analysis, this intelligent methodol- ogy will serv e as a self-motivated tool for the individual subject during treatment. The technology may help therapists in choos- ing a tailored therapeutic strategy for the individual, predict responses to treatment, or determine if maximal recovery has occurr ed. 6. Acknowledgements The authors wish to thank the Stern Foundation for providing the funds for this research work. REFERENCES [1] Albrecht H., Wotzel C., Erasmus L.P., Kleinpeter M., Konig N., Pollmann W., “Day-to-day variability of maximum walking dis- tance in MS patients can mislead to relevant changes in th e Ex- pan d ed Di s abi li ty St a tu s Sca le (E DSS ): a vera g e walk i n g s p eed is a more constant parameter”, Mult Scler. 2001;7(2):105-109 [2] C appozzo A. , Cat ani F., Leard ini A., Benedet ti M.G. , Croce UD ., “Position and orientation in space of bones during movement: experimental artifacts.” Clin Biomech,1996; 11(2):90-100 [3] De Luca C.J. “The use of surface electromyography in biome- chani cs” Journal of Appli ed Biomechanics, 1997; 13(2): 135-163 [4] Fischer J.S., Rudick R.A., Cutter G.R., Reingold S.C., “The Mul- tiple Sclerosis Functional Composite measure (MSFC): an inte- grated approach to MS clinical outcome assessment”, Mult Scler August 1999; 5:244-250 [5] Giansanti D., Macellari V., and Maccioni G. (2005) “The devel- opment and tes t of a device for th e reconstruction of 3-D p osition and orientation by means of a kinematic sensor assembly with rate gyroscopes and accelerometers” IEEE Trans Biomed Eng, vol. 52, no. 7, pp . 1271-1277 [6] Hausdorff J.M., “Gait variability: methods, modeling and mean- ing” Journal of NeuroEngineering and Rehabilitation, 2005; 2:19-27 [7] Higginson J.S., Zajac F.E., Neptune R.R., Kautz S.A., Delp S.L., “Muscle contributions to support during gait in an individual with post-stroke hemiparesis”. J Biomech 2005 [8] Hobart J.C., Riazi A., Lamping D.L., Fitzpatrick R., Thompson A.J., “Measuring the impact of MS on walking ability. The 12-item MS Walking Scale (MSWS-12)”, Neurology. 2003;60(1):31-36 [9] Hohol M.J., Orav E.J., Weiner H.L., “Disease Steps in multiple sclerosis: A simple approach to evaluate disease progression”, Neurology February, 1995;45(2):251-255 [10] Hoogervorst E.L., van Winsen L.M., Eikelenboom M.J., Kalkers N.F., Untdehaag B.M., Polman C.H., “Comparisons of patient self-report, neurologic examination, and functional impairment in MS ”, Neurology. 2001 ; 56(7) : 934-937 [11] Huiying Yu, Murad Alaqtash, Eric Spier, Thompson Sarko- die-Gyan, “Analysis of muscle activity during gait cycle using fuzzy rule-based reasoning”, Elsevier Journal of Measurement, April, 2010; 43(9):1106-1114 [12] Kaufman M., Moyer D., Norton J., “The significant change for the Timed 25-Foot Walk in Multiple Sclerosis Functional Com- posite”, Mult Scler. 2000;6(4):286-290  H. Y. YU, T. SARKODIE-GYAN Copyright © 2012 SciRes. ENG 26 [13] Kelleher K.J., Spence W.D., Solomonidis S.E., Apatsidis D. “The characterization of gait patterns with multiple sclerosis”. Disabil Rehabil. 2010; 32(15): 1242-50 [14] Kesselring J., “Multiple Sclerosis”. Cambridge University Press, 1997 [15] Legters K., Whitney S.L., Porter R., Buczek F., “The relationship between the Activities-specific Balance Confidence Scale and the Dynamic Gait Index in peripheral vestibular dysfunction”, Physiotherapy Research International. 2005;10(1):10-22 [16] Liu T., Inoue Y., Shibata K., “Development of a wearable sensor system for quantitative gait analysis”, Measurement. 2009; 42(7):978-988 [17] Marchetti G.F., Whitney S.L., “Construction and Validation of the 4-Item Dynamic Gait Index”, Physical Therapy. 2006;86(12):1651-1660 [18] McConvey J., Bennett S.E., “Reliability of the Dynamic Gait Index in Individuals with multiple sclerosis”, Archives of Physi- cal Medicine and Rehabilitation. 2005; 86:130-133 [19] McGuigan C., Hutchinson M., “Confirming the validity and res- ponsiveness of the Multiple Sclerosis Walking Scale-12 (MSWS-12)”, Neurology. 2004;62(11):2103-2105 [20] National Institu te of Neurologi cal Di sorders and Stroke (NINDS). (2006a, last updated January). NINDS Multiple Sclerosis Infor- mation Page, 2006 [21] Ni euwen hu is M.M ., Ton geren H .V. , Soren sen P .S. , Ravnb org M ., “The Si x Spot Step Test : a new measu rement for walk ing abi lity in multiple sclerosis”, Mult Scler. 2006;12(4):495-500 [22] Noseworthy J.H., “Clinical scoring methods for multiple sclero- sis”, Ann Neurol. 1994; 36 Suppl:S80-5 [23] Pringle D., Seger A.M., and Ponichtera-Mulcare J., “Locomotive function in individuals with multiple sclerosis”, Biomedical En- gine ering Conference , 19 96; p p 43 3-436 [24] Pullman S.L., Goodin D.S., Marquinez A.I., Tabbal S., Rubin M., “Clinical utility of surface EMG” Report of the therapeutics and technolog y assessment subcommittee of th e American Academy of Neurology, Neurology, 2000; 55:171-177 [25] Rocon E., Moreno J.C., Ruiz A.F., Brunetti F., Miranda J.A., and Pons J.L., “Application of inertial sensors in rehabilitation ro- botics”, proceeding of the 2007 IEEE 10th International Confe- rence on Rehabilitation Robotics, June 12-15, 2007, Noordwijk, The Netherlands [26] Ruth E. Mayagoitaia, Anand V. Nene, Peter H. Veltink (2002) “Accelerometer and rate gyroscope measurement of kinematics: an inexpensive alternative to optical motion analysis systems” Elsevier Journa l of Bio mechanics, pp. 537-542 [27] Sutliff M.H., “Contribution of impaired mobility to patient burden in multiple sclerosis” Current Medical Research and Opinion, 2010; 26(1):109-119 [28] Winter D.A. (1991) “Biomechanics and Motor control of Human Gait: Normal, Elderly and Pathological”. Waterloo Biomechan- ics Press. Waterloo, Ontario [29] World Health Organization, “Neurological Disorders: Public heal t h c ha l le ng es”, 200 6 [30] Wurdeman S.R., Huisinga J.M., Filipi M., and Stergiou N., “Multiple sclerosis affects the frequency content in the vertical ground reaction forces during walking”, Clinical Biomechanics, 2010; 26(2):207-212. |

