Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 165-168 doi:10.4236/ampc.2012.24B043 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Hydrogen as Carbon Gasifying Agent During Gly c erol Steam Reforming over Bimetallic Co-Ni Cataly st Chin Kui C he ng, Rwi Hau Lim, Anabil Ub il , Sim Yee C hin, Jolius Gimbun Faculty of Chemical & Natural Resources Engineering, Universiti Malaysia Pahang, Kuantan, Malaysia Email: chinkui@ump.edu.my Received 2012 ABSTRACT Alumina-supported bimetallic cobalt-nickel catal yst h as been pr epared an d employed in a fixed-b ed reactor for the dir ect production of synthesis gas from glycerol steam reforming. Physicochemical properties of the 5Co-10Ni/85Al2O3 catal yst were d eter mined fro m N2-physisorption, H2-chemisorption, CO2 and NH3-temperature-programmed desorption measurements as well as X-ray diffraction analysis. Both weak and s trong acid sit es are present on the catalyst sur face. The acidic: basic rat io is about 7. Carb on deposit ion was evident at 923 K; addition of H2 however has managed to reduce the carbon deposition. Significantly, this has resulted in the incre- ment of CH4 formation rate, consistent with the increased carbon gasification and methanation. Carbon deposition was almost non-existent, particularly at 1023 K. In addition, the inclusion of hydrogen also has contributed to the decrease of CO2 and increase of CO formation rates. This was attributed to the reverse water-gas-shift reaction. Overall, both the CO2:CO and CO2:CH4 ratios decrease with the hydrogen parti al pressure. Keywords: Carbon Deposition; Catalyst; Gasification; Glycerol; Steam Re forming 1. Introduction The use of renewable feedstock is fast gaining attention in lieu of the context of securing sustainable use of energy and pre- serving the environment for the future generations. Considera- ble effort has been devoted into applying green catalytic route to co nvert renewable fe edstock su ch as biomass into commodi- ty chemicals and clean bio-fuels. In particular, glycerol, also known as 1,2,3-propanetriol, is produced in excess quantity in the form of by-product during biodiesel synthesis. It constitutes an approximately 10wt% of the total product. In an effort to add value to glycerol as precursor for renewable and clean energy production, it was steam-reformed at temperatures greater than 773 K to produce H2, CO and CO2 which are important ingre- dients for the manufacture of a variety of industrial chemicals [1,2]. The relevant r eactions are: C3H8O3 + 3H2O → 3CO2 + 7H2 (1) C3H8O3 → 3CO + 4H2 (2) Carbon deposition is a perennial issue during glycerol steam reforming. Cheng et al. [3] have reported a kinetic study of carbon deposition during glycerol steam reforming over alumi- na supported bimetallic Co-Ni catalyst. At least two types of carbonaceo us sp ecies were depo sited on the cat alyst surface [ 3]. Sanchez and Comelli [4] published a paper on deactivation process and regeneration technique during glycerol steam re- forming over a Ni-alumina catalyst. A TPO characterized by a main peak centered at 963 K was o btained [ 4] . As a continuation to the previous works, this paper reports on the adoption of H2 as co-reactant during glycerol steam re- forming to encourage simultaneous carbon gasification. Results on the effects of H2 addition towards carbon deposition and product variation will be presented and elucidated in detailed. 2. Methodology 2.1. Catalyst Sy nthesis and Phy sic oche mical Characterizat io n Bimetallic 5Co-10Ni/85Al2O3 catalyst was prepared via co- impregnation of cobalt and nickel nitrate solutions on γ-alumina which has been preheated at 873 K for 6 h. Subsequently, the slurry catalyst was oven-dried at 403 K for overnight and then calcined at 873 K for 6 h to obtain oxide metals. For the physi- cochemical char acteri zatio n , BE T surface area an d po re volume were obtained from liquid N2 physisorption on the Quantach- rome Autosorb-1 unit. The metal catalyst dispersion and sur- face area were determined from Micromeritics ASAP 2000 via H2-chemisorption technique. The crystallography of catalyst was examined via XRD techni que via s can rate o f 4 o min-1 from 10o to 80o. Carbon content of collected samples post-reforming reaction, was determined using a Shimadzu Solid Sample Module (SSM-5000A) based on combustion at 1173 K. 2.2. Reaction Studies Figure 1 shows the experimental set-up for the current experi- mental work. Reaction runs were conducted in a stainless-steel fixed bed reactor under minimal influence from physical trans- port limitations. 60 wt% aqueous glycerol solution was pre- pared and directly injected into a 10-mm diameter fixed-bed reactor system using 50 mL syringe pump reacting at tempera- tures between 923 and 1023 K. Prior to reaction, catalyst was reduced in-situ using 50 ml min-1 of H2 for 2 h. Subsequently, H2 was co-added to the reactor as gasifying reactant. Total GHSV for the experiment was co ntro lled at 5×104 mL g-1 h-1. For fixed-bed reactor operation with no recycle stream, Levenspiel [5] s hows that the reaction rate is go verned by: 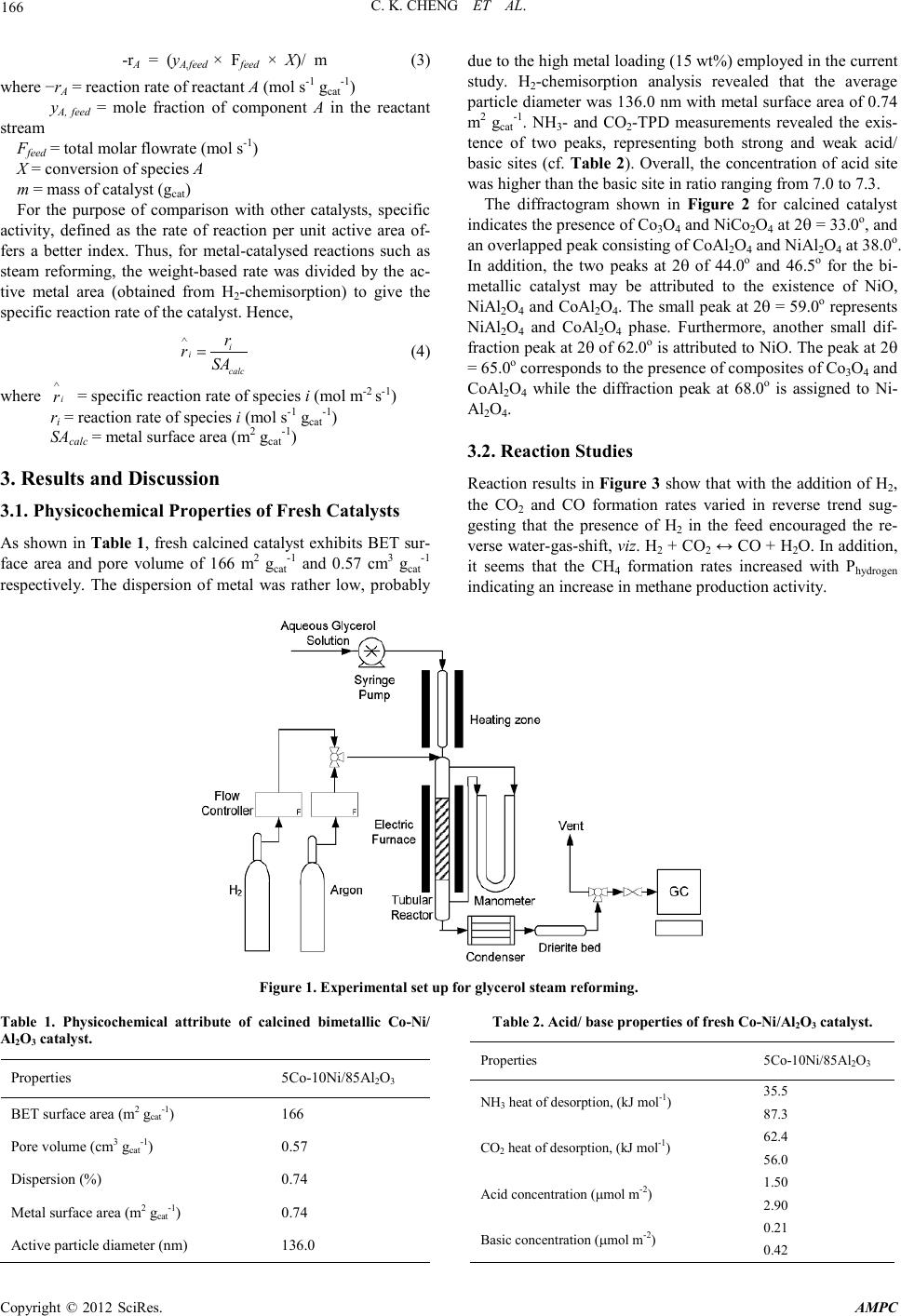 C. K. CHENG ET AL. Copyright © 2012 SciRes. AMPC 166 -rA = (yA,feed × Ffeed × X)/ m (3) where −rA = reaction rate of reactant A (mol s-1 gcat-1) yA, feed = mole fraction of component A in the reactant stream Ffeed = tot al molar flowrat e (mol s-1) X = conversi on of species A m = mass of catalyst (gcat) For the purpose of comparison with other catalysts, specific activity, defined as the rate of reaction per unit active area of- fers a better index. Thus, for metal-catalysed reactions such as steam reforming, the weight-based rate was divided by the ac- tive metal area (obtained from H2-chemisorption) to give the specific reaction rate of the catalyst. H ence, i i calc r rSA ∧ = (4) where i r ∧ = specific reactio n rate of species i (mol m-2 s-1) ri = reaction rate of sp ecies i (mol s-1 gcat-1) SAcalc = metal surface area (m2 gcat-1) 3. Results and Discussion 3.1. Physicochemical Properties of Fresh Catalysts As shown in Table 1, fresh cal cined catalyst exhibits BET sur- face area and pore volume of 166 m2 gcat-1 and 0.57 cm3 gcat-1 respectively. The dispersion of metal was rather low, probably due to the high metal loading (15 wt%) employed in the current study. H2-chemisorption analysis revealed that the average particl e diameter was 1 36.0 nm with metal sur face area of 0 .74 m2 gcat-1. NH3- and CO2-TPD measurements revealed the exis- tence of two peaks, representing both strong and weak acid/ basic sites (cf. Table 2). Overall, the concentration of acid site was higher than the basic site in ratio ranging from 7.0 to 7.3. The diffractogram shown in Figure 2 for calcined catalyst ind icates t he pres ence o f Co3O4 and NiCo2O4 at 2θ = 33 .0o, and an overlapped peak consisting of CoAl2O4 and NiAl2O4 at 38.0o. In addition, the two peaks at 2θ of 44.0o and 46.5o for the bi- metallic catalyst may be attributed to the existence of NiO, NiAl2O4 and CoAl2O4. The small peak at 2θ = 59.0o represents NiAl2O4 and CoAl2O4 phase. Furthermore, another small dif- fractio n peak at 2θ of 62.0o is attributed to NiO. The peak at 2θ = 65.0o corresponds to the presence of composites of Co3O4 and CoAl2O4 while the diffraction peak at 68.0o is assigned to Ni- Al2O4. 3.2. Reaction Studies Reaction results in Figure 3 show that with the addition of H2, the CO2 and CO formation rates varied in reverse trend sug- gesting that the presence of H2 in the feed encouraged the re- verse water-gas-shift, viz. H2 + CO2 ↔ CO + H2O. In addition, it seems that the CH4 formation rates increased with Phydrogen ind icating an increase in methane p r oduction activit y. Figure 1. Experimental set up for glycerol steam reforming. Table 1. Physicochemical attribute of calcined bimetallic Co-Ni/ Al2O3 catalyst. Properties 5Co-10Ni/85Al2O3 BET surface area (m2 gcat-1) 166 Pore v o l ume (cm3 gcat-1) 0.57 Dispersion (%) 0.74 Metal surface area (m2 gcat-1) 0.74 Active part i cle di ameter (nm) 136.0 Tabl e 2. Acid/ base properties of fresh Co-Ni/Al2O3 catalyst. Properti es 5Co-10Ni/85Al2O3 NH3 heat of desorption, (kJ mol-1) 35.5 87.3 CO2 heat of desorption, (kJ mol-1) 62.4 56.0 Acid concentration (µmol m-2) 1.50 2.90 Basic concentration (µmol m-2) 0.21 0.42  C. K. CHENG ET AL. Copyright © 2012 SciRes. AMPC 167 Subsequently, the product ratios (CO2:CO, CO2:CH4 and CO:CH4) as a function of Phydrogen showed that the CO2:CO ratio is practically constant with Phydrogen as a result of re- verse-water-gas-shift reaction (cf. Figure 4). The ratio was lower than unity indicating that CO formation rate was higher than CO2 as the latter was being consumed to produce the for- mer. CO2:CH4 decr eas ed with P hydrogen du e to th e increased CH4 formation rate, whilst the ratio CO:CH4 decreased because the increase in CH4 formation rate was higher than the increase recorded for CO formation rate. 3.3. Carbon Deposition Figure 5 suggests a decr ease in carb on deposition rate with H2 partial pressure, in part icular at 923 K. This observation is con- sistent with the increase in CH4 formation rate indicating that both methanation and carbon gasification contributed to the increase i n CH4 formatio n. However, t emperatu re see ms to play an increasingly dominant role in reducing carbon laydown than adding H2 gasifyi ng reactan t at temperatu res ≥ 973 K. At 1023 K, deposition of carbon was essentially zero. Figure 2. X RD patt ern of cal cined Co-Ni/Al2O3 catalyst. Figure 3. Effect of Phydrogen on product formation rates at 973 K. Figure 4. Effect of Phydrogen on pro duct ratios at 973 K. Figure 5. Effect of Phydrogen on carbon laydown as function of tem- perature. 4. Conclusions The effects of adding H2 gasifying reactant during the glycerol steam reforming have been examined. Reaction data revealed that H2 addition led to the increased CH4 formation which can be attributed to the gasification of carbon and methanation. In addition, the product formation rate of CO2 decreased due to reverse wat er-gas-shift. 5. Acknowledgements Authors would like to thank Universiti Malaysia Pahan g for the provision of short-term grant to fund this project. REFERENCES [1] S. Adhikari, S. Fernando, and A. Haryanto, “A comparative thermodynamic and experimental analysis on hydrogen produc- tion by steam reforming of glycerin,” Energy Fuels, vol. 21, pp. 2306 – 2310. [2] C. K. Cheng, S. Y. Foo, and A. A. Adesina, “Glycerol steam  C. K. CHENG ET AL. Copyright © 2012 SciRes. AMPC 168 reofmring over bi metallic Co-Ni/Al2O3,” Ind. Eng. Chem. Res., vol. 49, pp. 10804–10817. [3] C. K. Cheng, S. Y. Foo, and A. A. Adesina, “Carbon deposition on bimetallic Co-Ni/Al2O3 catalyst during steam reforning of glycerol,” Catal. Today, vol. 164, pp. 268–274. [4] E. A. Sanchez, and R. A. Comelli, “Hydrogen by glycerol steam reforming on a nickel-alumina catalyst: Deactivation processes and regeneration,” Int. J. Hydrogen Energy, in press. [5] O. Levenspiel, Chemical Reaction Engineering. New York: John Wily & Sons, 1999 . |

