Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 154-157 doi:10.4236/ampc.2012.24B040 Published Online December 2012 (htt p://www.SciRP.org/journal/ ampc) Copyright © 2012 SciRes. AMPC Aqueous Two Phase Extraction for the Recovery of 1,3-Propanediol from Its Aqueous Solutions Min Hee Chung1, Yeon Ki Ho ng1, Hyoung Wook Lee2, Sung-Jun Park3 1Department of Chemical and Biological Engineering, Korea National Univers ity of Tra nspor tation, Chungju, Chungbuk, Korea 2Department of Energy Syst em En g ineering , Korea National University of Transportation, Chungju, Chungbuk Korea 3Department of Mech anic al Engi neering, Korea National University of Transportation, Chungju, Chungbuk Korea Email: hongyk@ut .ac .kr, hwle e @ut.a c.kr, park@ut.ac.kr Received 2012 ABSTRACT As the biodiesel production is rapidly enhanced, the crude glycerol, which is by-product of biodiesel processes, is state of surplus. 1,3-PDO (1,3-propanediol), a valuable monomer of poly(trimethylene terephthalate) (PTT), can be produced from the fermentation process using crude glycerin as a carbon source. For the economic biological production of 1,3-PDO, the low cost and high efficient separation processes is essential. In this study, aqueous two-phase system composed of various hydrophilic alcohols and salt was used as a primary separation step for 1,3-PDO. It was found that the aqueous two-phas e systems are easi l y formed with d ecreasi ng o f the pol arit y of alcoho ls . The extr actio n efficienc y is proportional to the polarity of alcohols. In case of methanol or ethanol/K2HPO4, the extraction efficiency was more than 90%. It was concluded that the aqueous two-phase extraction using methanol or etha- nol/K2HPO4 can be applied for the primary separation of 1,3-PDO as an alternative to a conventional primary separation pro cesses. Keywords: 1,3 -Propanediol; Alcohols; Phase Separation; Extraction Efficiency 1. Introduction Over the last decade, biodiesel has emerged as an alternative fuel of fossil diesel. However, the production of biodiesel is not profitable without government subsidies due to its high produc- tion cost. For the economic production of biodiesel, finding new applications of glycerol which is a by-product from bio- diesel production is important. Generally, for every 9 kg of biodiesel produced, 1 kg of a crude glycerol by-product is pro- duced . There is a larg e amount of crude gl ycerol on t he market available fo r very low pri ce due to the rap id increase of biodie- sel prod uc t ion [1.2] . Glycerol can be u sed as a carb on source fo r the fer ment ative production of 1,3-PDO (1,3-propanediol) which is a monomer of PTT(Poly(trimethyleneterephthalate). PTT is a polyester with sup erior str etching and stretch r ecovery characteri stics an d has various usage for textile, carpets and upholstery manufac- turing [3]. Before two decades, 1,3-PDO had been produced by hydra- tion of acrolein or hydroformylation of ethylene oxide. In early 2003, DuPont developed a commercial biological process for 1,3-PDO based on the fermentation of glucose. Such a bio-based PDO made a petroleum-based PTT to be bioplastic. In spite of energy saving of glucose based fermentation by Du- Pont, the cost of 1,3-PDO has been still high and its price can depend on the price of glucose [4]. The low price of glycerol due to the rapid increasing of biodiesel production makes its usage of a promising alternative carbon source be possible. A biological production of 1,3-PDO has several advantages compared with the conventional chemical production because of low cost and more eco-friendly process. 1,3-PDO had been synthesized by various microbes such as Klebsiella pneumonia, Clostridium butyricum and Citrobactor freundii [5-7]. It was reported that the final concentration of 1,3-PDO in fermentation broth was low, ranging from 30 to 130 g/L. In addition, the fermentation broth contains a mixture of 1,3-PDO, 2,3-butanediol, glycerol, lactate, acetate, succinate and other impurities which make the downstream processing of 1,3-PDO fermentation broth be dif ficul t [8]. The downstream processes of biologically produced 1,3-PDO includes three main steps. In the first step, microbial cells are removed by using flocculation, filtration, and centrifugation. The secon d step contains the primary reco very processes fo r the removal of impurities such as acid salts and ethanol and the evaporation of water. In this step, evaporation, solvent extrac- tion, precipitation, electrodialysis and chromatography processes can be used. In the last step, the final purification of 1,3-PDO is carried out by vacuum distillation process [9]. +TPA Figu re 1. PTT synthes is from 1,3-PDO. 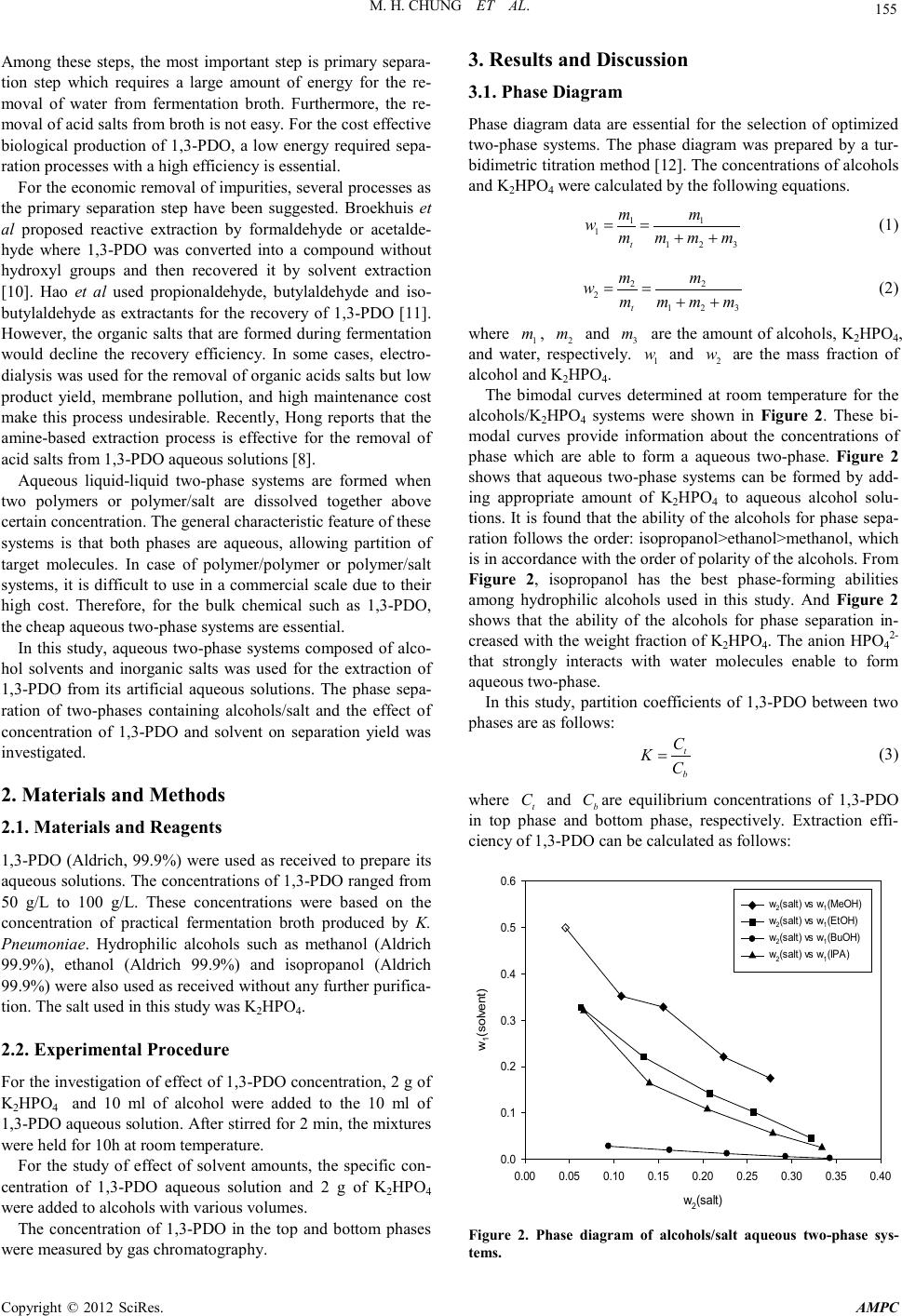 M. H. CHUNG ET AL. Copyright © 2012 SciRes. AMPC 155 Among these steps, the most important step is primary separa- tion step which requires a large amount of energy for the re- moval of water from fermentation broth. Furthermore, the re- moval of acid salts from broth is not easy. For the cost effective biological production of 1,3-PDO, a low energy required sepa- ration processes with a high efficiency is essential. For the economic removal of impurities, s everal pro cesses as the primary separation step have been suggested. Broekhuis et al proposed reactive extraction by formaldehyde or acetalde- hyde where 1,3-PDO was converted into a compound without hydroxyl groups and then recovered it by solvent extraction [10]. Hao et al used propionaldehyde, butylaldehyde and iso- butylaldehyde as extractants for the recovery of 1,3-PDO [11]. However, th e organic salts th at are for med d uring fermentatio n would decline the recovery efficiency. In some cases, electro- dialysis was used for the removal of organic acids salts but low product yield, membrane pollution, and high maintenance cost make this process undesirable. Recently, Hong reports that the amine-based extraction process is effective for the removal of acid salts from 1,3-PDO aque o us s ol utions [8 ]. Aqueous liquid-liquid two-phase systems are formed when two polymers or polymer/salt are dissolved together above certain concentration. The general characteristic feature of these systems is that both phases are aqueous, allowing partition of target molecules. In case of polymer/polymer or polymer/salt systems, it is difficult to use in a commercial scale due to their high cost. Therefore, for the bulk chemical such as 1,3-PDO, the ch eap aqueous two-phase s ystems are essenti al. In this study, aqueous two-phase systems composed of alco- hol solvents and inorganic salts was used for the extraction of 1,3-PDO from its artificial aqueous solutions. The phase sepa- ration of two-phases containing alcohols/salt and the effect of concentration of 1,3-PDO and solvent on separation yield was investigated. 2. Materials and Methods 2.1. Materials and Reagents 1,3-PDO (Aldrich, 99.9%) were used as received to prepare its aqueous solutions. The concentrations of 1,3-PDO ranged from 50 g/L to 100 g/L. These concentrations were based on the concentration of practical fermentation broth produced by K. Pneumoniae. Hydrophilic alcohols such as methanol (Aldrich 99.9%), ethanol (Aldrich 99.9%) and isopropanol (Aldrich 99.9%) were also used as received without any further purifica- tion. The salt used in this study was K2HPO4. 2.2. Experimental Procedure For the investigation of effect of 1,3-PDO concen tration, 2 g of K2HPO4 and 10 ml of alcohol were added to the 10 ml of 1,3-PDO aqueous solution. After stirred for 2 min, the mixtures were held for 10h at room temperature. For the study of effect of solvent amounts, the specific con- centration of 1,3-PDO aqueous solution and 2 g of K2HPO4 were added to alcohols with various volumes. The concentration of 1,3-PDO in the top and bottom phases were measured b y gas chromatography. 3. Results and Discussion 3.1. Phase Diagram Phase diagram data are essential for the selection of optimized two-phase systems. The phase diagram was prepared by a tur- bidimetric titration method [12]. The concentrations of alcohols and K2HPO4 were calculated by the following equations. 11 1 123t mm wm mmm = = ++ (1) 22 2 123t mm wm mmm = = ++ (2) where 1 m , 2 m and 3 m are the amount of alcohols, K2HPO4, and water, respectively. 1 w and 2 w are the mass fraction of alcohol and K2HPO4. The bimodal curves determined at room temperature for the alcohols/K2HPO4 systems were shown in Figure 2. These bi- modal curves provide information about the concentrations of phase which are able to form a aqueous two-phase. Figure 2 shows that aqueous two-phase systems can be formed by add- ing appropriate amount of K2HPO4 to aqueous alcohol solu- tions. It is found that the ability of the alcohols for phase sepa- ration follows the order: isopropanol>ethanol>methanol, which is in accordance with the order of polarity of the alcohols. From Figure 2, isopropanol has the best phase-forming abilities among hydrophilic alcohols used in this study. And Figure 2 shows that the ability of the alcohols for phase separation in- creased with the weight fracti on of K2HPO4. The anion HPO42- that strongly interacts with water molecules enable to form aqueous two-phase. In this study, partition coefficients of 1,3-PDO between two phases ar e as fo llows: t b C KC = (3) where t C and b C are equilibrium concentrations of 1,3-PDO in top phase and bottom phase, respectively. Extraction effi- ciency of 1,3-PDO can be calcu lated as follows: w 2 (s alt ) 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 w 1 (solvent) 0.0 0.1 0.2 0.3 0.4 0.5 0.6 w2(sal t) vs w1(MeOH) w2(sal t) vs w1(EtOH) w2(sal t) vs w1(BuOH) w2(sal t) vs w1(IPA) Figure 2. Phase diagram of alcohols/salt aqueous two-phase sys- te ms . 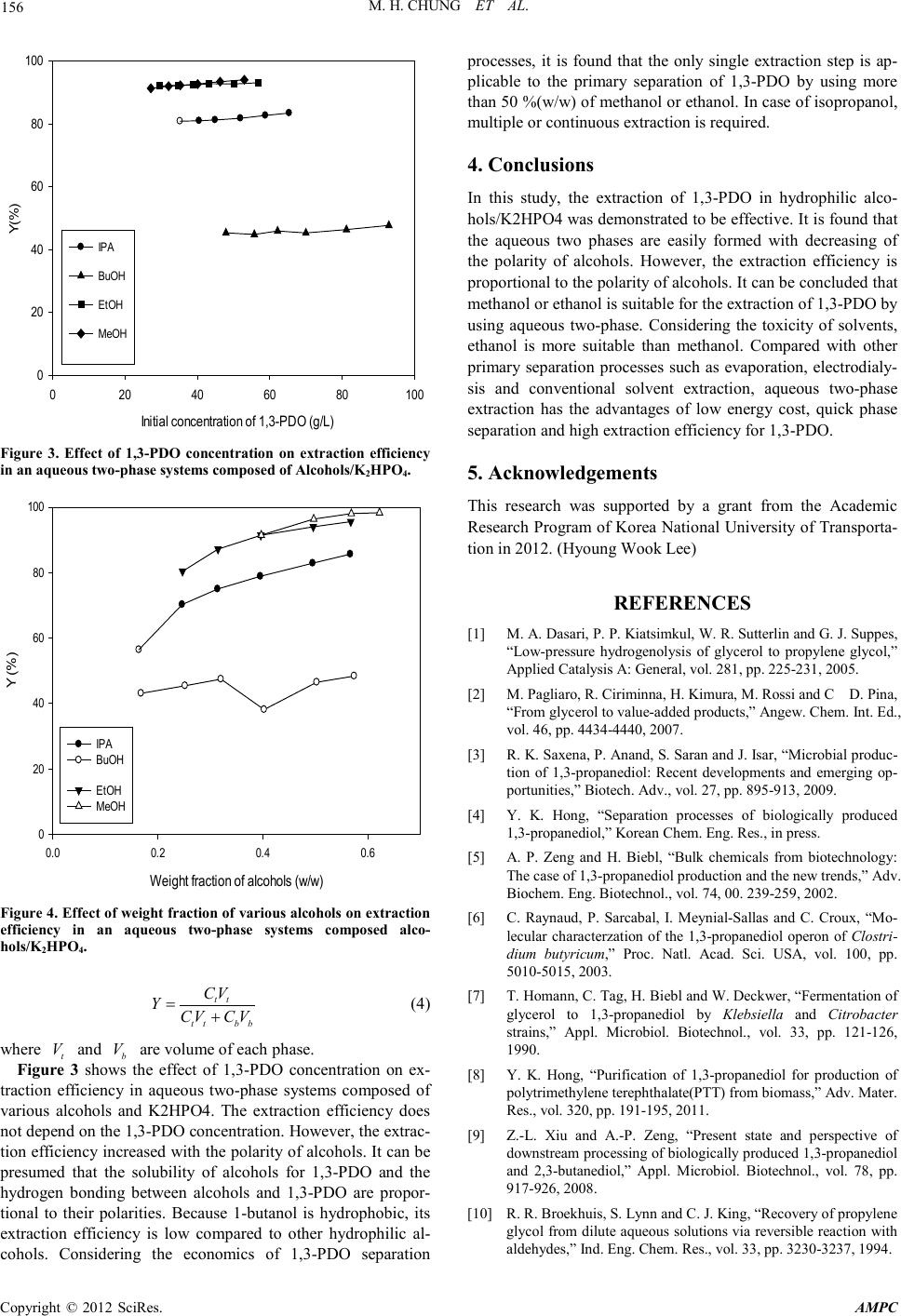 M. H. CHUNG ET AL. Copyright © 2012 SciRes. AMPC 156 In itial c onc entr at ion of 1, 3- PDO (g/ L) 020 40 60 80100 Y(%) 0 20 40 60 80 100 IPA BuOH E tOH MeOH Figure 3. Effect of 1,3-PDO concentration on extraction efficiency in an aqueous two-phase systems composed of Alcohols/K2HPO4. Weight fr action of alcohols ( w /w ) 0.0 0.2 0.4 0.6 Y (%) 0 20 40 60 80 100 IPA B uOH EtOH MeOH Figure 4. Effect of weight fraction of various alcohols on extraction efficiency in an aqueous two-phase systems composed alco- hols/K2HPO4. tt tt bb CV YCV CV = + (4) where t V and b V are volu me of each phase. Figure 3 shows the effect of 1,3-PDO concentration on ex- traction efficiency in aqueous two-phase systems composed of various alcohols and K2HPO4. The extraction efficiency does not depend on the 1,3-PDO concent rati on. However , th e extrac- tion efficien cy increased with the polar ity of alcohol s. It can be presumed that the solubility of alcohols for 1,3-PDO and the hydrogen bonding between alcohols and 1,3-PDO are propor- tional to their polarities. Because 1-butanol is hydrophobic, its extraction efficiency is low compared to other hydrophilic al- cohols. Considering the economics of 1,3-PDO separation proces ses, it is found that the only single extraction step is ap- plicable to the primary separation of 1,3-PDO by using more than 50 %(w/w) of methanol or ethanol. In case of isopropanol, multiple or continuous extraction is required. 4. Conclusions In this study, the extraction of 1,3-PDO in hydrophilic alco- hols/K2HPO4 was demonstrated to be effective. It is found that the aqueous two phases are easily formed with decreasing of the polarity of alcohols. However, the extraction efficiency is proportional to the polarity of alcohols. It can be concluded that methanol or ethanol is suitable for the extraction of 1,3-PDO b y using aqueous two-phase. Considering the toxicity of solvents, ethanol is more suitable than methanol. Compared with other primary separation processes such as evaporation, electrodialy- sis and conventional solvent extraction, aqueous two-phase extraction has the advantages of low energy cost, quick phase separati on and hi gh extraction efficiency for 1, 3-PDO. 5. Acknowledgements This research was supported by a grant from the Academic Research P rogram of Korea Nati onal University o f Transp orta- tion in 2012. (Hyoung Wook Lee) REFERENCES [1] M. A. Dasari, P. P. Kiatsimkul, W. R. Sutterlin and G. J. Suppes, “Low-pressure hydrogenolysis of glycerol to propylene glycol,” Applied Catalysis A: General, vol. 281, pp. 225-231, 2005. [2] M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C D. Pina, “From gl ycerol to value-added products,” Angew. Chem. Int. Ed., vol. 46, pp. 4434-4440, 2007. [3] R. K. Saxena, P. Anand, S. Saran and J. Isar, “Microbial produc- tion of 1,3-propanediol: Recent developments and emerging op- portuniti es,” Biotech . Adv., vol. 27, pp. 895-913, 200 9. [4] Y. K. Hong, “Separation processes of biologically produced 1,3-propanediol,” Korean Chem. Eng. Res., in press. [5] A. P. Zeng and H. Biebl, “Bulk chemicals from biotechnology: The case of 1,3 -propanediol production and the new trends,” Adv. Biochem. Eng. Biotechnol., vol. 74, 00. 239-259, 2002. [6] C. Raynaud, P. Sarcabal, I. Meynial-Sallas and C. Croux, “Mo- lecular characterzation of the 1,3-propanediol operon of Clostri- dium butyricum,” Proc. Natl. Acad. Sci. USA, vol. 100, pp. 5010-5015, 2003. [7] T. Homann, C. Tag, H. Biebl and W. Deckwer, “Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains,” Appl. Microbiol. Biotechnol., vol. 33, pp. 121-126, 1990. [8] Y. K. Hong, “Purification of 1,3-propanediol for production of polytrimethylene terephthalate(PTT) from biomass,” Adv. Mater. Res., vol. 320, pp. 191-195, 2011. [9] Z.-L. Xiu and A.-P. Zeng, “Present state and perspective of downst ream proc essing of bi ologica lly produc ed 1,3-propanediol and 2,3-butanediol,” Appl. Microbiol. Biotechnol., vol. 78, pp. 917-926, 2008. [10] R. R. Broekhuis, S. Lynn and C. J. King, “Recovery of propylene glycol from dilute aqueous solution s via reversib le reaction with aldehydes,” Ind. Eng. Chem. Res., vol. 33, pp. 3230-3237, 1994.  M. H. CHUNG ET AL. Copyright © 2012 SciRes. AMPC 157 [11] J. Hao, H. J. Liu and D . H. Liu , “Novel rout e of react ive extra c- tion to recover 1,3-propanediol from a dilute aqueous solution,” Ind. Eng. Chem . R e s . , vol. 44, pp. 4380-4385, 2005. |

