Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 1-3 doi:10.4236/eng.2012.410B001 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Rapid Determination of Sexually Transmitted Infections by Real-time Polymerase Chain Reaction Using Microchip Analyzer O. Suvorova1, A. Perchik1, M. Slyadnev1, O. Suvorova2, A. Perchik2, M. Slyadnev2, D. Navolotskii2, N. Mushnikov2 1Department of chemistry, Saint Petersburg State University, Saint Petersburg, Russia 2Lumex Ltd., Saint Petersburg, Russia Received 2012 ABSTRACT Development of non expensive and time-savin g techniqu es based on the po lymerase chain react ion (PCR) is of great importance for modern diagnostics. We considered a new approach for PCR determination of a variety of sexually transmitted infections using mi- crochi p analyzer “Aria DNA”, whi ch had been tested usi ng clinical samples in sever al medical i nstituti ons of St. Pet ersburg (Ru ssia). The use of microchips containing lyophilized PCR reagents allows reducing significantly time of analysis and the number of mani- pulations thus preventing possible sample contamination. Keywords: Polymeras e C hain R eaction; Sexually Transmitted Infections; Microch ip 1. Introduction Sexually transmitted diseases (STDs) are common for modern society. According to the World Health Organization, 448 mil- lion new cases of di fferent sexual l y trans mitted in fection s (STIs) are registered each year. Alth ou gh the number of laboratories to perfor m STIs analyzes in creases signi ficantly, it i s challenge o f modern diagnostics to develop new approaches for rapid screening of samples. The development of molecular biology methods has allowed significant progress in diagnostics of STDs. A polymerase chain reaction (PCR) method is usually used to detect and identify pathogens (e.g. pathogenic microor- ganisms, fungi and viruses). Compared to cultural methods of analysis, PCR has many advantages and allows to solve many diagnostic tasks quickly and accurat el y [1] . Curr ent ly, ther e is a wide range of commercial kits designed to detect a variety of STIs by real time PCR (rt-PCR). P CR system’s miniaturi zation is one of the attractive directions for medical device develop- ment, which decrease analysis time, consumption of expensive reagents and improve the sensitivity. In the last decade many devices that use microchip technology were extensively inves- tigated [2]. We propose a new approach to miniaturize rt-PCR system, which allows to simplify automation, reduce analysis time and the number of manual operations. Thus, in the de- signed s ystem, all necessary reagents for PCR are lyophilized in microreact or cells of the microchip. 2. Development of Microchips with Lyophilized PCR Reagents for STI Determination 2.1. The Lyophilization of PCR Reagents A microchip consisted of a silicon plate with 30 cells (Lumex, Russia) has been used as a substrate for lyophilization (see Figure 1). To stabilize dried PCR reagents and to avoid degradation of polymerase or other components of PCR mixture we have pre- viously developed a stabilizer solution [3]. Using of this solu- tion prevents aging of reagents and allows to achieve the PCR efficiency for lyophilized reagents similar to that of liquid PCR mixture. A solution containing stabilizers, dNTPs, Taq-polymerase, and primers corresponding to STI’s DNA was put into each microreactor. As a positive control (K+), several microreactors included the synthetic plasmid DNA with insertion of particular bacteria sequence, while as a negative control (K-) Deionized water was used. It should be noted that particular microchip may include different quantities and combinations of micro- reactors for identifying different STIs, depending on the prob- lem being solved. Figure 1. Microchip for CT, TV and MG identification in eight samples (each digits represent different samples). Microchip size:35 x 35 mm, microreactor size 1.6 x 1.6 mm 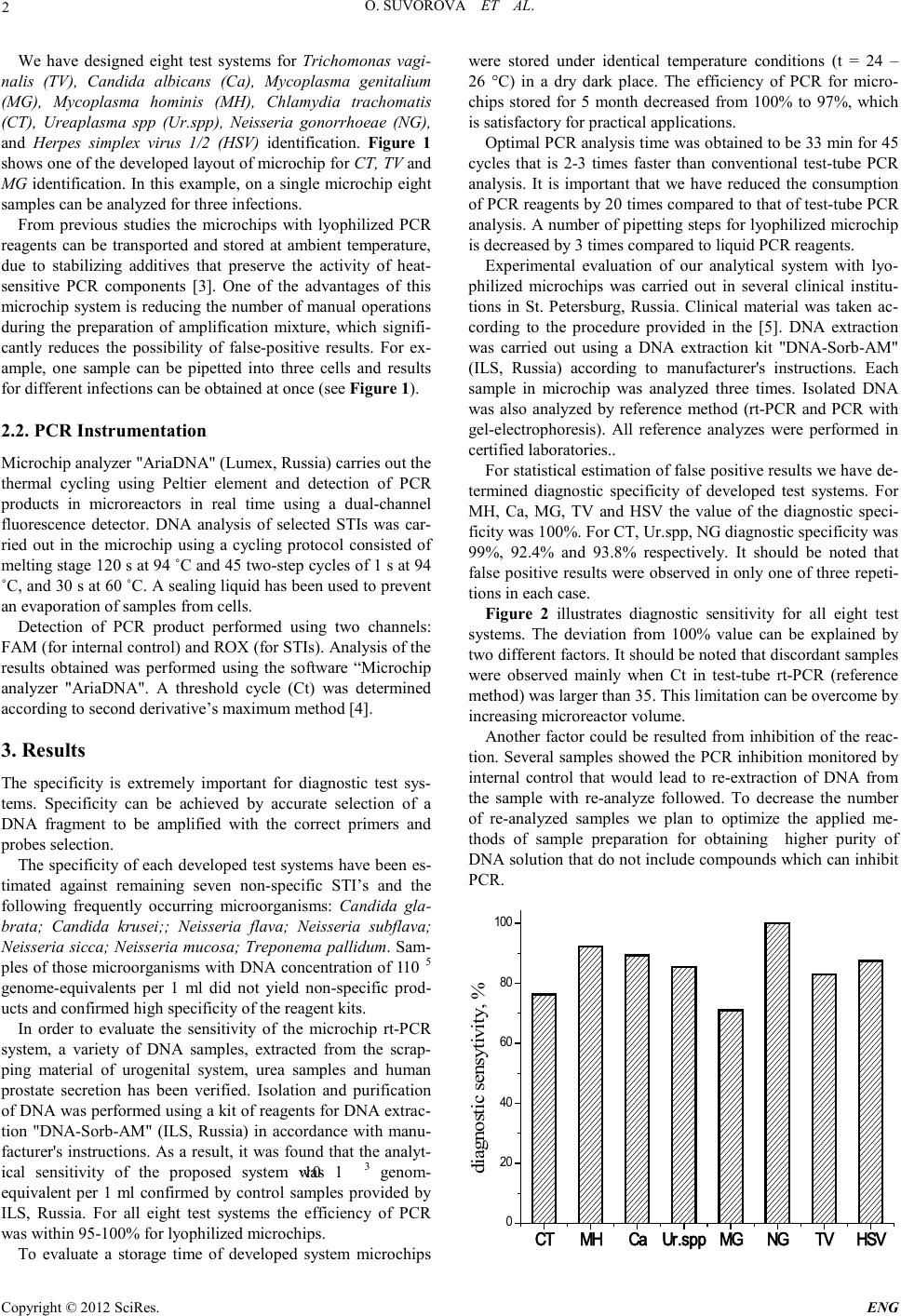 O. SUVOROVA ET AL. Copyright © 2012 SciRes. ENG 2 We have designed eight test systems for Trichomonas vagi- nalis (TV), Candida albicans (Ca), Mycoplasma genitalium (MG), Mycoplasma hominis (MH), Chlamydia trachomatis (CT), Ureaplasma spp (Ur.spp), Neisseria gonorrhoeae (NG), and Herpes simplex virus 1/2 (HSV) identification. Figure 1 shows one of the developed layout of microchip for CT, TV and MG identification. In this example, on a single microchip eight samples can be anal yzed for th ree infections. From previous studies the microchips with lyophilized PCR reagents can be transported and stored at ambient temperature, due to stabilizing additives that preserve the activity of heat- sensitive PCR components [3]. One of the advantages of this microchip system is reducing the number of manual operations during the preparation of amplification mixture, which signifi- cantly reduces the possibility of false-positive results. For ex- ample, one sample can be pipetted into three cells and results for different infecti ons can be obtained at on ce (see Figure 1). 2.2. PCR Instrumentation Micro chip anal yzer "AriaDNA" (Lumex, Russia) c ar ries out the thermal cycling using Peltier element and detection of PCR products in microreactors in real time using a dual-channel fluorescence detector. DNA analysis of selected STIs was car- ried out in the microchip using a cycling protocol consisted of meltin g stage 120 s at 94 ˚C and 45 two-step cycles of 1 s at 94 ˚C, and 30 s at 60 ˚C. A sealing liquid has been used to prevent an evapo ration of samples from cells. Detection of PCR product performed using two channels: FAM (for internal control) and ROX (for STIs). Analysis of the results obtained was performed using the software “Microchip analyzer "AriaDNA". A threshold cycle (Ct) was determined according to second derivat ive’s maximum metho d [4]. 3. Results The specificity is extremely important for diagnostic test sys- tems. Specificity can be achieved by accurate selection of a DNA fragment to be amplified with the correct primers and probes selection. The specificit y of each develo ped test systems have b een es- timated against remaining seven non-specific STI’s and the following frequently occurring microorganisms: Candida gla- brata; Candida krusei;; Neisseria flava; Neisseria subflava; Neisseria sicca; Neisseria mucosa; Treponema pallidum. Sam- ples of those microorganisms with DNA concentration of 1∙10 5 genome-equivalents per 1 ml did not yield non-specific prod- ucts and confirmed high specificity of the reagent kits. In order to evaluate the sensitivity of the microchip rt-PCR system, a variety of DNA samples, extracted from the scrap- ping material of urogenital system, urea samples and human prostate secretion has been verified. Isolation and purification of DNA was performed using a kit of reagents for DNA extrac- tion "DNA-Sorb-AM" (ILS, Russia) in accordance with manu- facturer's instructions. As a result, it was found that the analyt- ical sensitivity of the proposed system was 1∙10 3 genom- equivalent per 1 ml confirmed by control samples provided by ILS, Russia. For all eight test systems the efficiency of PCR was within 95-100% for lyophilized microchips. To evaluate a storage time of developed system microchips were stored under identical temperature conditions (t = 24 – 26 °C) in a dry dark place. The efficiency of PCR for micro- chips stored for 5 month decreased from 100% to 97%, which is satisfactory for practical applications. Optimal PCR analysis time was obtained to be 33 min for 45 cycles that is 2-3 times faster than conventional test-tube PCR analysis. It is important that we have reduced the consumption of PCR reagents b y 20 times co mpared t o that of test-tube PCR analysis. A number of pipetting steps for lyophilized microchip is decreased by 3 times compared to l iq ui d PCR reagent s . Experimental evaluation of our analytical system with lyo- philized microchips was carried out in several clinical institu- tions in St. Petersburg, Russia. Clinical material was taken ac- cording to the procedure provided in the [5]. DNA extraction was carried out using a DNA extraction kit "DNA-Sorb-AM" (ILS, Russia) according to manufacturer's instructions. Each sample in microchip was analyzed three times. Isolated DNA was also analyzed by reference method (rt-PCR and PCR with gel-electrophoresis). All reference analyzes were performed in certified l aborat ories. . For statistical estimation of false positive results we have de- termined diagnostic specificity of developed test systems. For MH, Ca, MG, TV and HSV the value of the diagnostic speci- ficity was 100%. For CT, Ur.spp, NG diagnostic specificity was 99%, 92.4% and 93.8% respectively. It should be noted that false po sitive results were observed in only one of three repeti- tions in each case. Figure 2 illustrates diagnostic sensitivity for all eight test systems. The deviation from 100% value can be explained by two different factors. It should be noted that discordant samples were observed mainly when Ct in test-tube rt-PCR (reference method) was larger than 35. This limitation can be overcome by increasi ng microrea ctor volume. Another factor could be resulted from inhibition of the reac- tion. Several samples showed the PCR inhibition monitored by internal control that would lead to re-extraction of DNA from the sample with re-analyze followed. To decrease the number of re-analyzed samples we plan to optimize the applied me- thods of sample preparation for obtaining higher purity of DNA solution that do not include compounds which can inhibit PCR. CT MH CaUr.spp MG NG TV HSV 0 20 40 60 80 100 diagnostic sensytivity, %  O. SUVOROVA ET AL. Copyright © 2012 SciRes. ENG 3 Figure 2. Diagnosti c s ens itiv ity for des i gn ed test sys tems . 4. Acknowledgements The authors gratefully acknowledge The D.O. Ott Research Institute of Obstetrics and Gynecology and The Pavlov State Medical University of Saint Petersburg for providing the sam- ples an d their analysis. REFERENCES [1] Vicky Jasson, Liesbeth Jacxsens, Pieternel Luning, Andreja Rajkovic, Mieke Uyttendaele, “Alternative microbial methods: An overvie w and select ion criteria”, Food Mic robiology, vol. 27 (6), p p . 710-730, 2010 [2] Zhang C., Xing D. “Miniaturized PCR Chips for Nucleic Acid Amplification and Analysis: Latest Advances and Future Trends”, Nuclei c Acids R es ea r ch, vol. 35, pp. 4223–4237, 2007 [3] Navolotskii, D.; Perchik, A.; Mark'yanov, I.; Ganeev, A.; Slyad- nev, M., “Microchip analytic system for multiplex analysis by real-time polymerase chain reaction with reagents immobilized in microreactors”, Applied Biochemistry & Microbiology, vol. 47(2), pp . 2 21-229, 2011 [4] Tichopad A, Dilger M, Schwarz G, Pfaffl MW: “Standardized det ermin ation of real-time PCR efficiency from a single reaction setup”. Nucleic Acids Research, vol. 31 issue 20, pages e122, 2003 [5] Official publication, “Organization of laboratories using nucleic acid amplification methods for the work with materials contain- ing microorganisms I – IV pathogenicity groups”, MU 1.3. 2569-09, Moscow, 2009. |

