Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 126-129 doi:10.4236/ampc.2012.24B033 Published Online December 2012 (http://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Fabrication of Sn Coatings on Alumina Balls by Mechanical Coating Technique and Relevant Process Analysis Liang Hao, Yun Lu, Hiromasa Sato, Kazuki Chiba School & Faculty of Engineering, Chiba University, Chiba, Jap an Email: hl.resaca.jcu@gmail.com, luyun@faculty.chiba-u.jp Received 2012 ABSTRACT Sn co atings wer e fabricated by mech anical coatin g technique for the first time. Th e coatings were ch aracterized b y XRD an d SEM, among others. The SEM showed that the coatings had an irregular and uneven morphology. The influence of the rotation speed of planetary ball mill on the evolution and formation of the coatings was also investigated. The results indicated that continuous Sn coatings can be formed under a moderate rotation speed. In other words, the coatings cannot be formed when rotation speed was too high or too low. The evolution of the coatings was examined and discussed. The results showed that it followed the universal evolu- tion law of metal coatings which included four stages. However, the exfoliation of the coatings was not seen even the milling time rea c he d 30 h. Keywords: Sn Coatings; Mechanical Coating Technique; Adhesion; Cold Welding; Film Evolution 1. Introduction To develop nickel-based superalloy for gas turbine application, Benjamin and his colleagues at the International Nickel Com- pany (INCO) developed mechanical alloying, well known as ball milling around 1966. Since then, ball milling has been developed to prepare a variety of equilibrium and non-equilibrium alloy phases that cannot or were difficult to b e prepared by traditional techniques [1]. The non-equilibrium phases synthesized include supersaturated solid solutions, me- tastable crystalline and quasicrystalline phases, nanostructures and amorphous alloys, among others. Our group developed a novel coating technique derived from ball milling to prepare metal (alloy) coatings and TiO2/metal composite photocatalyst coatings and named it mechanical coating technique (MCT) [2,3]. MCT is based on the principle of collision, friction and abrasion among grinding mediums and milled powders during ball milling. By this technique, Ti-Al coatings [4], Fe-Si coat- ings [5], Cu-Ni solid solution coatings [6], were prepared by other researchers. We also fabricated Cu coatings [7], Fe coat- ings [8] and Zn coatings [9] on alumina balls by MCT and in- vestigated th e evo lu tion o f these co ati ngs. However, a u ni versal law for evolution of metal coatings during the process of me- chanical coating is still unknown. The influence of a series of proces sin g parameters i ncluding rotation speed on the evolution and formation of metal coatings still needs to clarify. The in- fluence of the intrinsic properties of metal powder on the evo- lution and formation of the coatings are also important not only for mechanical coating technique but also for mechanical al- loying and mechanical pulverization. The aim of this paper is to try preparing continuous Sn coat- ings by mechanical coating technique. In addition, we will try to summarize the universal law for the evolution of metal coat- ings during mechanical coating process. The influence of rota- tion speed on the evolution and formation of the coatings will be studied. 2. Experimental 2.1. Fabrication of Sn Coatings 35 g Sn powder (apparent density: 3.72 g/cm3) and 30 g Al2O3 balls (average diameter: 1 mm) were used as the coating ma- terial and the substrates r espectively. They were ch arged into a bowl made of alumina with a dimension of Φ75 mm × 70 mm (250 ml). The mechanical coating was then performed by a planetary ball mill (Pulverisette 6, Fritsch). To investigate the influence of the rotation speed, the rotation speed of the plane- tary ball mill was set between 100 and 300 rpm. The milling time was from 4 to 40 h. To ensure the safety of mechanical milling process, a 10-minute milling operation was followed by a 2-minute cooling interval in order to avoid an excessive heat- ing of the bowl although a temperature rise may promote the welding between metal powder particles. The schematic dia- gram of mechanical coating can be seen in our early work [8] . 2.2. Characterization Before the characterization of all the Sn-coated Al2O3 balls, they were treated by ultrason ic cleaning (frequency: 28 kHz) in acetone to remove the Sn particles that did not strongly adhere to Al2O3 balls. The phase composition and the change of the surface coverage of Al2O3 balls with Sn was examined by a XRD analyzer (JDX-3530, JEOL) with Cu Kα radiation at 30 kV and 20 mA. The surface morphologies and the microstruc- tures of the cross sections of the Sn-coated Al2O3 balls were observed by SEM (JSM-6510, JEOL). 3. Results and Discussion 3.1. Fabrication and Evolution of Sn Coatings The XRD patterns of the Sn-coated Al2O3 balls after the me- chanical coating at 150 rpm for different milling times are given 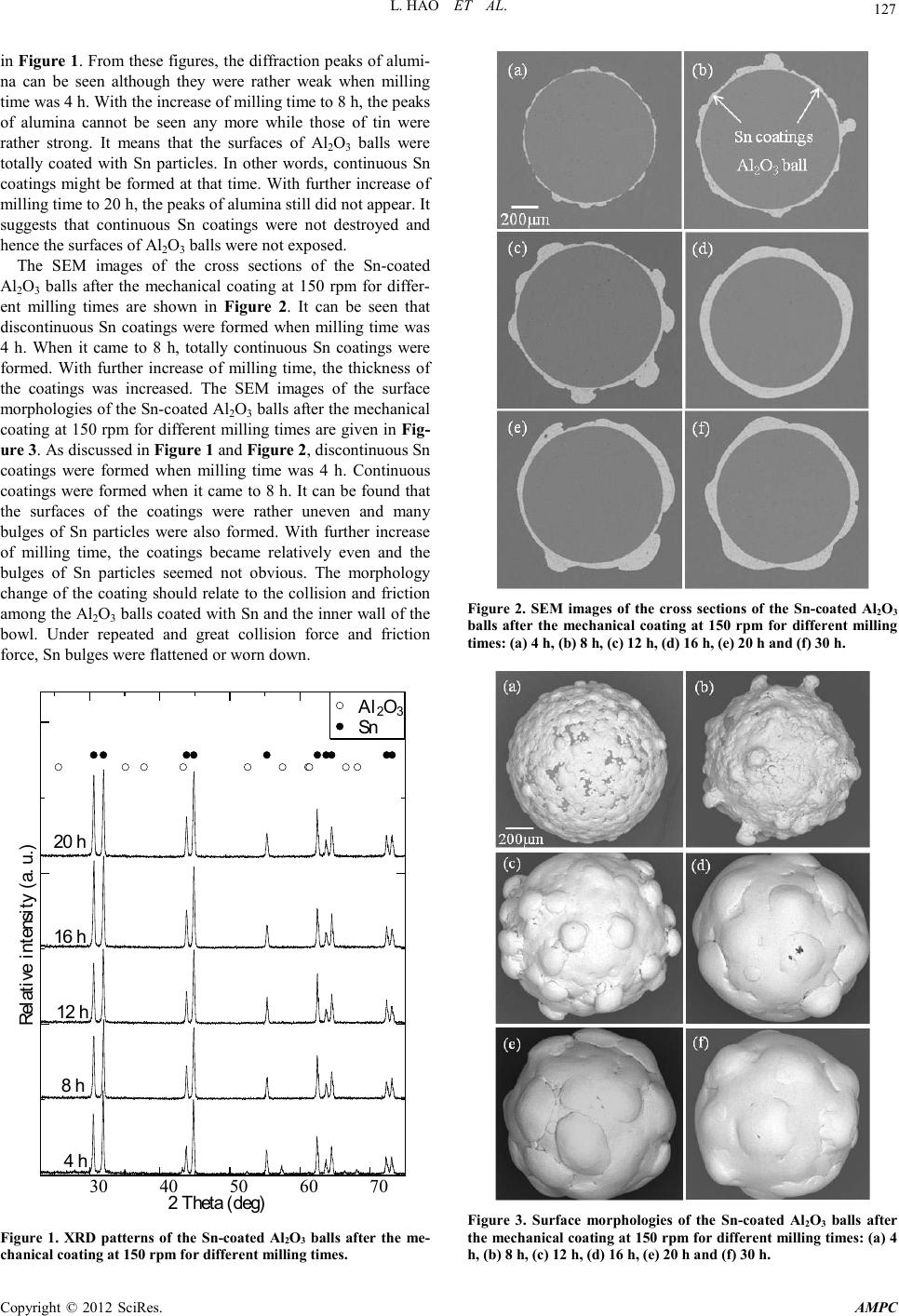 L. HAO ET AL. Copyright © 2012 SciRes. AM PC 127 in Figure 1. F rom these figures, t he diffractio n peaks of alumi- na can be seen although they were rather weak when milling time was 4 h. With the increase of milling time to 8 h, the peaks of alumina cannot be seen any more while those of tin were rather strong. It means that the surfaces of Al2O3 balls were totally coated with Sn particles. In other words, continuous Sn coatings might be formed at that time. With further increase of milling time to 20 h, the peaks of alumina still did not appear. It suggests that continuous Sn coatings were not destroyed and hence t he surfaces o f Al2O3 balls were not exposed. The SEM images of the cross sections of the Sn-coated Al2O3 balls after the mechanical coating at 150 rpm for differ- ent milling times are shown in Figure 2. It can be seen that discontinuous Sn coatings were formed when milling time was 4 h. When it came to 8 h, totally continuous Sn coatings were fo rmed. With further increase of milling time, the thickness of the coatings was increased. The SEM images of the surface morphologies of the Sn-coated Al2O3 balls after the mechan ical coating at 150 rpm for different milling times are given in Fig- ure 3. As d iscussed in Figure 1 and Figure 2, discontinuous Sn coatings were formed when milling time was 4 h. Continuous coatings were formed when it came to 8 h. It can be found that the surfaces of the coatings were rather uneven and many bulges of Sn particles were also formed. With further increase of milling time, the coatings became relatively even and the bulges of Sn particles seemed not obvious. The morphology change of the coating should relate to the collision and friction among the Al2O3 balls co ated with Sn and the inner wall of the bowl. Under repeated and great collision force and friction force, Sn bulges were flattened or worn down. 30 40 50 60 70 Al 2 O 3 Sn Relative intensity (a. u.) 2 Theta (deg) 4 h 8 h 12 h 16 h 20 h Figure 1. XRD patterns of the Sn-coated Al2O3 balls after the me- chanica l co ating at 150 rpm for different m ill in g times. Figure 2. SEM images of the cross sections of the Sn-coated Al2O3 balls after the mechanical coating at 150 rpm for different milling times: (a) 4 h, (b) 8 h, (c) 12 h, (d) 16 h, (e) 20 h and (f) 30 h. Figure 3. Surface morphologies of the Sn-coated Al2O3 balls after the mechanical coating at 150 rpm for different milling times: (a) 4 h, (b) 8 h, (c) 12 h, (d) 16 h, (e) 20 h and (f) 30 h. 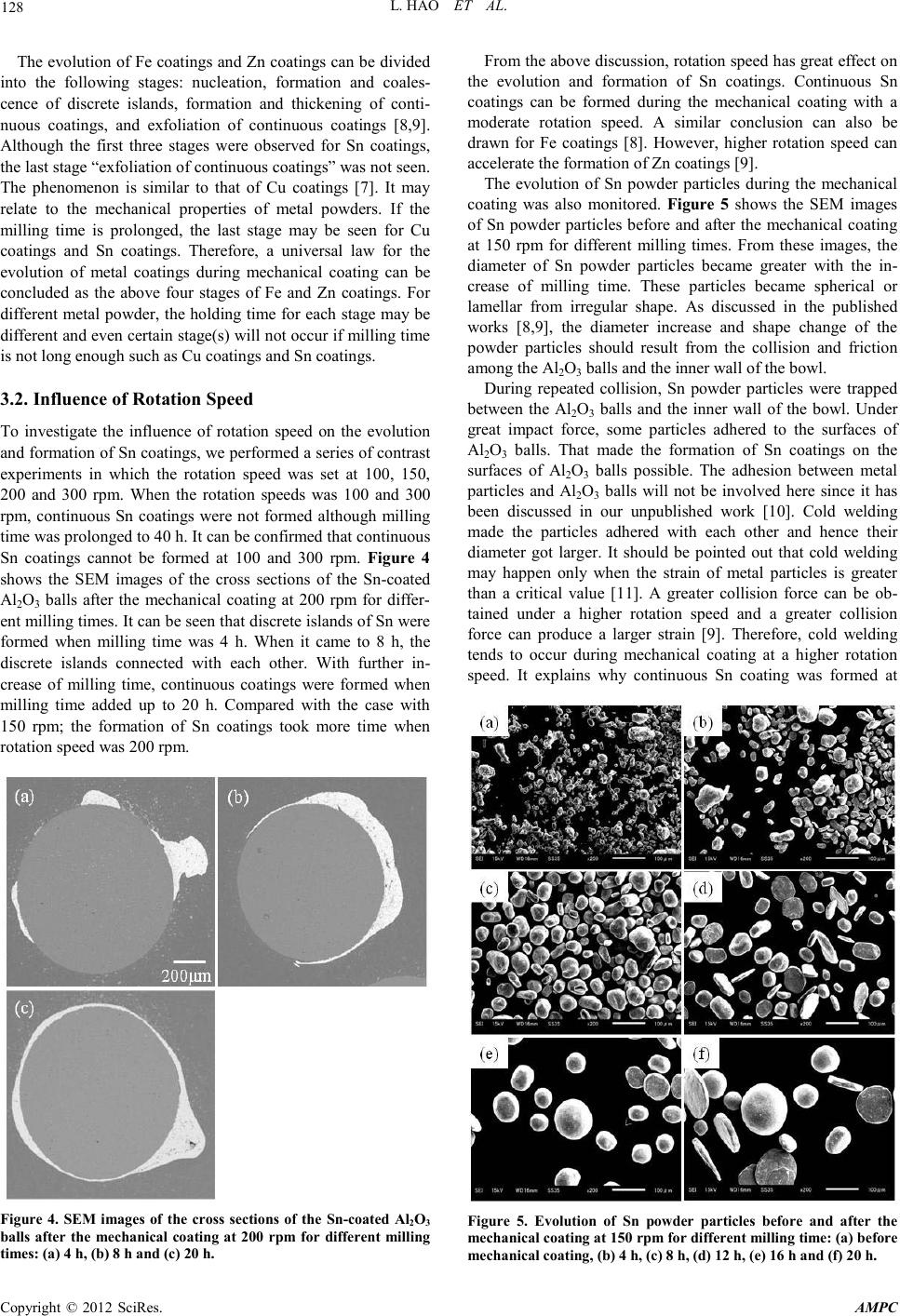 L. HAO ET AL. Copyright © 2012 SciRes. AM PC 128 The evolution of Fe coatings and Zn coatings can be divided into the following stages: nucleation, formation and coales- cence of discrete islands, formation and thickening of conti- nuous coatings, and exfoliation of continuous coatings [8,9]. Although the first three stages were observed for Sn coatings, the last stage “exfoliation of continuous coatings” was not seen. The phenomenon is similar to that of Cu coatings [7]. It may relate to the mechanical properties of metal powders. If the milling time is prolonged, the last stage may be seen for Cu coatings and Sn coatings. Therefore, a universal law for the evolution of metal coatings during mechanical coating can be concluded as the above four stages of Fe and Zn coatings. For different metal po wder, the holding time for each st age may be different and even cert ain stage(s) will not occur if milling time is not long enough such as Cu coatings and Sn coatings. 3.2. Influence of R otation Spe e d To investigate the influence of rotation speed on the evolution and formation of Sn coatings, we performed a series of contrast experiments in which the rotation speed was set at 100, 150, 200 and 300 rpm. When the rotation speeds was 100 and 300 rpm, continuous Sn coatings were not formed although milling time was prolonged to 40 h. It can be confirmed that continuous Sn coatings cannot be formed at 100 and 300 rpm. Figure 4 shows the SEM images of the cross sections of the Sn-coated Al2O3 balls after the mechanical coating at 200 rpm for differ- ent milling ti mes. I t can be seen th at d iscr ete isl and s o f Sn were formed when milling time was 4 h. When it came to 8 h, the discrete islands connected with each other. With further in- crease of milling time, continuous coatings were formed when milling time added up to 20 h. Compared with the case with 150 rpm; the formation of Sn coatings took more time when rotation speed was 200 rpm. Figure 4. SEM images of the cross sections of the Sn-coated Al2O3 balls after the mechanical coating at 200 rpm for different milling times: (a) 4 h, (b) 8 h and (c) 20 h. From the above discus sion, rot ation speed has great effect o n the evolution and formation of Sn coatings. Continuous Sn coatings can be formed during the mechanical coating with a moderate rotation speed. A similar conclusion can also be drawn for Fe coatings [8]. However, higher rotation speed can accelerat e the formation of Zn coatings [9]. The evolution of Sn powder particles during the mechanical coating was also monitored. Figure 5 shows the SEM images of Sn powder particles before and after the mechanical coating at 150 rpm for different milling times. From these images, the diameter of Sn powder particles became greater with the in- crease of milling time. These particles became spherical or lamellar from irregular shape. As discussed in the published works [8,9], the diameter increase and shape change of the powder particles should result from the collision and friction among the Al2O3 balls and the inner wall of the bowl. During repeated collision, Sn powder particles were trapped between the Al2O3 balls and the inner wall of the bowl. Under great impact force, some particles adhered to the surfaces of Al2O3 balls. That made the formation of Sn coatings on the surfaces of Al2O3 balls possible. The adhesion between metal particles and Al2O3 balls will not be involved here since it has been discussed in our unpublished work [10]. Cold welding made the particles adhered with each other and hence their diameter got larger. It should be pointed out that cold welding may happen only when the strain of metal particles is greater than a critical value [11]. A greater collision force can be ob- tained under a higher rotation speed and a greater collision force can produce a larger strain [9]. Therefore, cold welding tends to occur during mechanical coating at a higher rotation speed. It explains why continuous Sn coating was formed at Figure 5. Evolution of Sn powder particles before and after the mechanical coating at 150 rpm for different milling time: (a) before mechanical coating, (b) 4 h, (c) 8 h, (d) 12 h, (e) 16 h and (f) 20 h.  L. HAO ET AL. Copyright © 2012 SciRes. AM PC 129 150 and 200 rpm but did not form at 100 rpm. However, cold welding among Sn powder particles was greatly accelerated when the rotation speed was increased to 300 rpm. It can be confirmed fro m the si gnificantly in crease of Sn parti cle diame- ter after mech anical co atin g at 300 rp m. However, t he adhesion of Sn particles to the surfaces of Al2O3 balls was not accele- rated due to the increase of rotation speed. Therefore, Sn par- ticles had grown up before the adhesion of the particles to Al2O3 balls. It is the reason why the diameter of Sn particles was largely increased but continuous Sn coatings on Al2O3 balls was not formed when rotation speed was 300 rpm. The de formation of Sn particles between Al2O3 balls and the inner wall of the bowl can be regarded as the forging between two parallel plates because the volume of the trapped Sn particles was much smal ler t han th e co llid ing bod ies. Therefore, la mellar particles were formed. Under collision force and friction force, the sharp corners of the particles were eliminated and hence large spherical p ar ticles ap peared. From the above analysis about the influence of rotation speed, the formation of metal coatings on the surfaces of Al2O3 balls includes two kinds of interaction: the adhesion of metal par- ticles to the surfaces of Al2O3 balls and then the cold welding between metal particles. If we want to prepare metal coatings on Al2O3 balls, we have to find out the metals which tend to adhere to the surfaces of Al2O3 and easy to weld with each other. 4. Collisions Continuous Sn coatings were fabricated on Al2O3 balls by me- chanical coating technique. The results of XRD and SEM indi- cate that the evolution of the coatings follows a universal law for the evolution of metal coatings which includes the follow- ing stages: nucleation, formation and coalescence of discrete islands, formation and thickening of continuous coatings, and exfoliation of continuous coatings. Continuous Sn coatings can be prepared during the mechanical coating at a moderate rota- tion speed such as 150 and 200 rpm but cannot be formed at higher or lower ones such as 300 and 100 rpm. The influence of rotation speed should relate to the adhesion of metal powder particles to the surfaces of Al2O3 balls and the cold welding among metal powder particles. 5. Acknowledgements The authors gratefully acknowledge the financial support from Denshi-Jisso Company in Japan. The authors would like to thank Fukuda metal foil & powder Co., Ltd. of Japan for pro- viding us with the Sn powder used in the work. REFERENCES [1] C. Suryanarayana, “Mechanical alloying and milling,” Prog. Mater. Sci. vol. 46, pp. 1-184, January 2001. [2] H. Yoshida, Y. Lu, H. Nakayama and M. Hirohashi, “Formation of Ti O2 film by mech anical coati ng techniqu e and its photocat a- lyst activity,” J. Alloy. Compd., vol. 475, pp. 383-386, May 2009. [3] Y. Lu, H. Yoshida, H. Nakayama, L. Hao and M. Hirohashi, “Formation of TiO2/Ti composite photocatalyst film by 2-step mechanical coating technique,” Mater. Sci. Forum, vol. 675-677, pp. 12 29-1232, February 2011 [4] S. Romankov, W. Sha, S.D. Kaloshkin and K. Kaevitser, “For- mation of Ti-Al coatings by mechancial alloying method,” Surf. Coat . Tech., vol. 201, pp. 3235-324 5, D ecem ber 2006. [5] G. Gupta, K. Mondal and R. Balasubramaniam, “In situ nano- crystalline Fe-Si coating by mechanical alloying,” J. Alloy. Compd., vol. 482, pp. 118-122, August 2009. [6] I. Farahbakhsh, A. Zakeri, P. Manikandan and K. Hokamoto, “Evaluation of nanostructured coating layers form ed on Ni balls durin g mechani cal allo ying of Cu powder,” Appl. Surf. Sci., vol. 257, pp. 2830-2837, January 2011. [7] Y. Lu, L. Hao, K. Toh and H. Yoshida, “Fabrication of TiO2/Cu composite photocatalyst thin film by 2-step mechanical coating technique and its photocatalytic activity,” Adv. Mat. Res., vol. 415-417, pp. 1942-1948, February 2012. [8] L. Hao, Y. Lu, H. Asanuma and J. Guo, “The influence of the processing parameters on the formation of iron thin films on alumina balls by mechanical coating technique,” J. Mater. Process. Tech., vol. 212, pp. 1169-1176, May 2012. [9] L. Hao, Y. Lu, H. Asanuma and J. Guo, “Fabrication of zinc coatings on alumina balls from zinc powder by mechanical coat- ing technique and the process analysis,” Powder Technol., vol. 228, pp. 377-384, September 2012. [10] L. Hao, Y. Lu, H. Sato, H. Asanuma and J. Guo, “Influence of metallic properties on formation and evolution of mechanical coatings during mechanical coating technique,” unpublished. [11] L. Lü, M.O. Lai and S. Zhang, “Modeling of the mechani- cal-alloying process,” J. Mater. Process. Tech., vol. 52, pp. 539-546, June- July 1995. |

