Paper Menu >>
Journal Menu >>
 Advances in Ma terials Ph ysics and Ch emistry, 2012, 2, 96-98 doi:10.4236/ampc.2012.24B026 Published Online December 2012 (http://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. A MPC Development and Characte r i zation of Ultra Low Ce ment Castable Cordierites by Thixotropic Properties Mixtures Ana M. Paniagua-Mercado1, Arturo Méndez-Sánc he z 1, Elvia Díaz Valdés1, Concepción Mejía García1, Paulino Estrada Díaz2 1Depto. de Fí sica, Escuela Superior de Física y Matemáticas, Instituto Politécni co Nacional, Col. Lind avista, México 2Sector-Ceramics an d Re f r acto ries, Manuchar International, México Email: ampani@esfm.ipn.mx, pestrada@manuchar.com.mx Received 2012 ABSTRACT The main target in this investigation was to take advantage of the reology properties of the tixotropic mixes in Ultra Low Cement Castabl es (ULCC). The cor di erit e ph ase in refracto ry mix can be o b tain ed usin g raw materials with magnesi u m oxid e in it s composi- tion, such as, Mg(OH)2 or H2Mg3(SiO3)4 (Talc mineral), with a content of 63.5% SiO2, 31.7% MgO and 4.8% H2O. In this investiga- tion, as magnesium sou rce, a commercial c alcined magnesi te with 90% M gO was u sed. This mineral was selected in stead of Talc mineral, b ecause this last contains more impurities in its composition that tend to form more amounts of liquid phases with low fu- sion points. For this work two different ULCC mixes were designed. These were fired at 1260ºC, the cordierite phase was quantified in each mix. Keywords: Reology; Thixotropic Mixtures; Ultra Low Cement Castabl es ; Cordierite 1. Introduction There are in the market, several industrial methods and a wide of conventional raw materials to produce cordierite mixes (MgO・Al2O3・SiO2). The complete phase transformation is around 1300ºC depending on the raw material used in the mix [1]. The industrial application is mainly focus where high ther- mal shock resistant, low thermal expansion and corrosion resis- tance are demanded. Such is the case o f the cord ierite refractory plates used for the conventional firing of sanitary and tableware, or recently used as substrate material in microelectronics [2]. The main manufacturing processes to develop cordierite phase are the sol-Gel method [3], co-precipitation [4], solid-state re- action [5] and by slurry. Aluminosilicate based ultra low ce- ment castab les (ULCC) ar e widely used mainly in t he Steel and cement industries due to improved refractory properties at high temperatures. The bonding system in ultra low cement castables is achieved by using high alumina calcium alu min ate cement . Increasing the cement content in the concrete mix also in- creases the amount of liquid phases as the anorthita (CaAl2Si2 O8) and gelenite (Ca2Al2SiO7). These both tend to reduce the amount of free silica and decrease, in an important way, its chemical corrosion resistance. This last affects negatively their mechanical properties at high temperatures and in consequence the thermal shock resistant comes down. The lime/silica ratio is very important in the formation of liquid phases and its viscosity at high temperatures, because it affects the strength and corro- sion resistance [6]. The use of very low amounts of high alu- mina cement in ULCC is principally to avoid the liquid phases formatio n ins ide the refractor y matrix. O ther impo rtant variab le is the particle size distribution because it has a major impact in the reology of ULCC and in the final physical properties [7]. 2. Experimental Procedure Table 1 shows the chemical formulation of the two concrete mixes tested in this investigation. For each one of the designed mixes the preparation was as follows. First, the raw materials were dry mixed for at l east 1 min u te, after t hat , th e defloccu lan t (sodium tripolifosfate); the polypropylene fibers and the high alumina ce ment were add ed to the final mix. At once, th e water was added slowly and mixed 10 second more. After the mix was placed in a vibratory table (3000 cps), for no more than 30 seconds up to the mix, it got a thixotropic behavior. After the vibratory step, the mix was allowed to dry and it was set for 12 hours at room temperature (25ºC). Afterwards the mix was dried 24 h in a laboratory stove at 110ºC. Finally, the mix was calcin ed b etween 1 26 0°C - 1280°C during 5 h, in a gas furnace. In order to determine the cordierite forming and main phases present, the calcined samples were analyzed by X-ray diffraction in a Siemens D-500 diffractometer, with Kα of Cu in the Bragg-Brentano configuration, in a 2 range of 10º- 120º. Micrographs of each one of the two calcined mixes were obtained with secondary electrons, at 800X of magnification, in a scanni ng electro n microscopy JEOL-6300. Tabl e 1. Chemical Formulation of Mixtures. Compound MIX I ( %) MIX II (%) Al 2 O 3 47.92 47.49 SiO2 20.86 41.02 CaO 0.80 1.08 MgO 27.15 7.37 Fe 2 O 3 1.08 0.82 TiO 1.22 1.19 K2O 0.65 0.66 Na 2 O 0.29 0.31 *Work supported by Instituto Politécnico Nacional through project SIP- IPN 20120167. 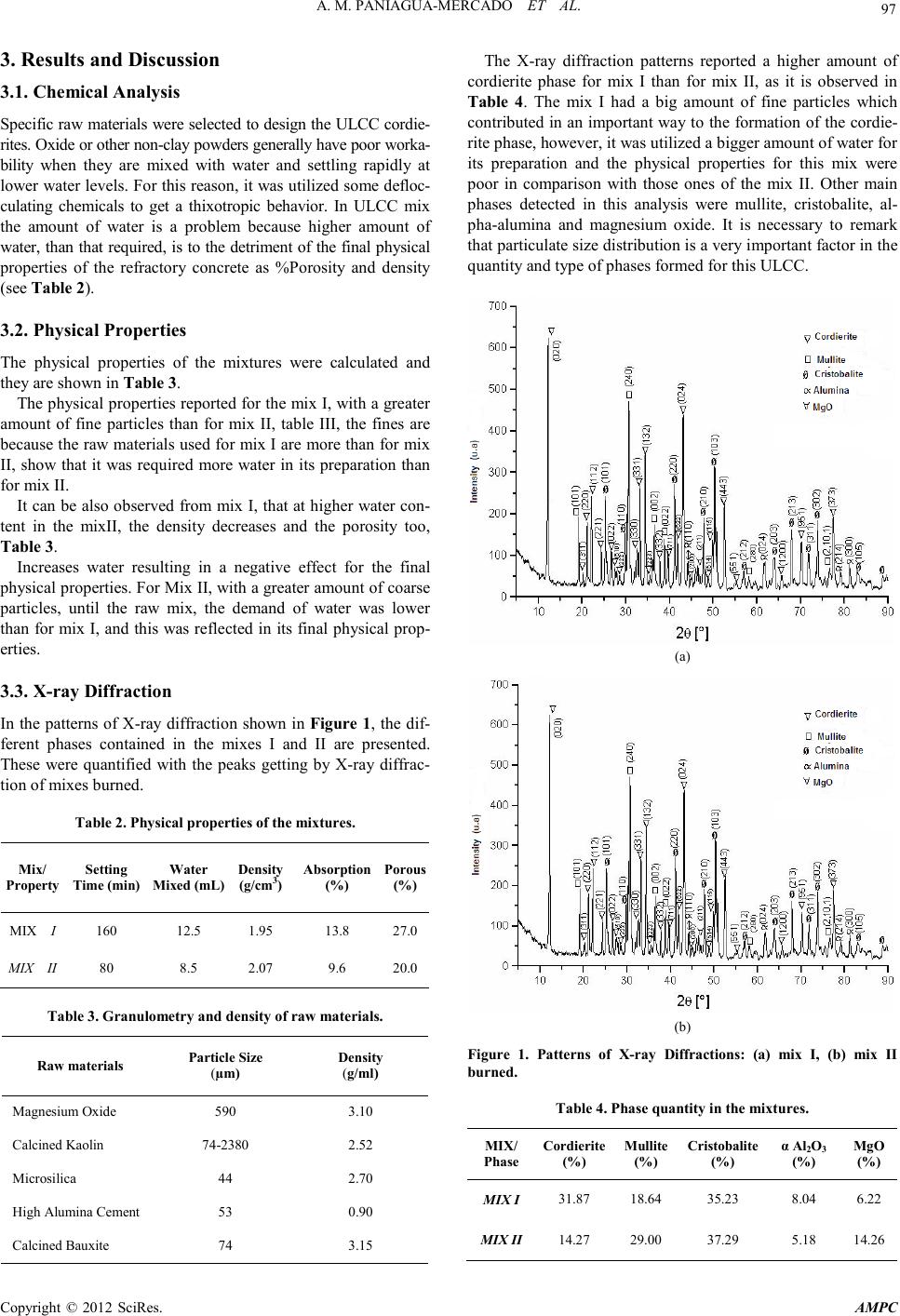 A. M. PANIAGUA-MERCADO ET AL. Copyright © 2012 SciRes. AMPC 97 3. Results and Discussion 3.1. Chemical Analysis Speci fic raw materi als were sel ected t o design the U LCC cordie- rites. Oxide or other non-clay powders generally have poor worka- bility when they are mixed with water and settling rapidly at lower water levels. For this reason, it was utilized so me defl o c- culating chemicals to get a thixotropic behavior. In ULCC mix the amount of water is a problem because higher amount of water, than that required, is to the detriment of the final physical properties of the refractory concrete as %Porosity and density (see Table 2). 3.2. Physical Properties The physical properties of the mixtures were calculated and they are shown in Table 3. The physical properties reported for the mix I, with a greater amount of fine particles than for mix II, table III, the fines are because t he raw materials used for mix I are more than fo r mix II, show that it was required more water in its preparation than for mix II. It can b e also observed from mix I, that at h igher water con- tent in the mixII, the density decreases and the porosity too, Table 3. Increases water resulting in a negative effect for the final physical properties. For Mix II, with a greater amount of coarse particles, until the raw mix, the demand of water was lower than for mix I, and this was reflected in its final physical prop- erties. 3.3. X-ray Diffraction In the patterns of X-ray diffraction shown in Figure 1, the dif- ferent phases contained in the mixes I and II are presented. These were quantified with the peaks getting b y X-ray diffrac- tion of mixes burned. Tabl e 2. Physical properties of the mixtures. Mix/ Proper ty Setting Time (min) Water Mixe d (mL) Density (g/cm3) Absorption (%) Porous (%) MIX I 160 1 2 .5 1.95 13.8 27 .0 MIX II 80 8.5 2.07 9 .6 20.0 Tabl e 3. Granulometry and density of raw materials. Raw materials Particle Size (µm) Density (g/ml) Magnesium Oxide 5 90 3.10 Calcined Kaolin 74-2380 2.52 Microsilica 44 2.70 High Alumina Cement 53 0 .90 Calcined Bauxite 74 3.15 The X-ray diffraction patterns reported a higher amount of cordierite phase for mix I than for mix II, as it is observed in Table 4. The mix I had a big amount of fine particles which contributed in an important way to the formation of the cordie- rite phase, however, it was utilized a bigger amount of water for its preparation and the physical properties for this mix were poor in comparison with those ones of the mix II. Other main phases detected in this analysis were mullite, cristobalite, al- pha-alumina and magnesium oxide. It is necessary to remark that particulate size distribution is a very important factor in the quantity and type of phases formed for this ULCC. (a) (b) Figure 1. Patterns of X-ray Diffractions: (a) mix I, (b) mix II burned. Tabl e 4. Phase quanti ty i n the mixture s. MIX/ Phase Cordierite (%) Mullite (%) Cristobalite (%) α Al2O3 (%) MgO (%) MIX I 31.87 18.64 35.23 8 .0 4 6.22 MIX II 14.27 29.00 37.29 5.18 14.26  A. M. PANIAGUA-MERCADO ET AL. Copyright © 2012 SciRes. AMPC 98 (a) (b) Figure 2. Micrographs: (a) mix I and (b) mix II. 3.4. Scanning Electron Microscopy In the micrographs shown in Figure 2 we can o bserve material like flakes corresponding to cordierite, and liquid phases with composition of Fe2O3, Na2O, K2O and GeO formed with the presen ce of SiO2 and detected b y Micro anal yses of SEM, all of these h ave low fusion points. 4. Conclusions 1. It was processed a refractory Cordierite ULCC mix, with level of cordierite phase commercially acceptable and with thixotropic properties . 2. The main physical properties of this type of ULCC are subj ected to a very restricted particle sizes distribution to obtain the b etter physical propert ies after fired . 3. Further research work must be doing to improve in a better way the cordierite performance phase and also the final physi- cal properties of the castable. REFERENCES [1] R.S. Lamar, M.F. Warner, Reaction and fired property studies of cordierite compositions J. Am. Ceram. Soc., 37, 12, pp. 602–610, 1954. [2] L. A. Radzikhovskii, Cordierite bodies wity improved refractoriness. Keramika No. 6 p.p.21-22, June 1980. [3] C.A. Bertran, N.T. da Silva, G.P. Thim Citric acid effect on aqueous sol–gel cordierite synthesis, J. Non-Cryst. Solids, 273, pp. 140–144, 2000. [4] M. Awano, H. Takagi, Y. Kuwahara Grinding effects on the synthesis and sintering of cordierite J. Am. Ceram. Soc., 75, 9, pp. 2535–2540, 1992. [5] Ö. Çakır, Production of cordierite from domestic raw materials, M.Sc. thesis, 1981. Middle East Technical University, Ankara. [6] H. Sarpoolaky, K. Ahari, W. Lee, Ceram. Int., 28, , 487-493, 2002. [7] E. A.Firoozjaei, A. Saidi, A.Monshi, P. Koshi, The effect of microsilica and refractory cement content on the properties of andalusite based Low cement castables used in aluminium casthouse, Cerami c 56 , 411-421, 2010. |

