Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 84-88 doi:10.4236/ampc.2012.24B023 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Laser Deposition of Tetrasulfonated Phthalocyanine Layers for Gas Sensors Premysl Fitl1, Martin Vrnata1, Dusan Kopecky1, Jan Vlcek1, Jitka Skodova1, Jaroslav Hofmann1, Vladimir Myslik2 1De par t ment of Physics a n d Measurement, Institute of Chemical Technology, Tech ni cka 5, P rague 6, CZ - 166 28, Czech Republ ic 2Department of Solid-St ate Eng in eer in g , Institute of Chemical Technology, Technicka 5, Prague 6, CZ - 166 2 8, C z ech Republic Email: martin.vrnata@vscht.cz Received 2012 ABSTRACT Thin layers of nickel and copper tetrasulfonated phthalocyanines (NiPcTS and CuPcTS) were prepared by Matrix Assisted Pulsed Laser Evapo rat io n method . Th e depo sit ions were carr ied ou t with KrF exci mer laser (en er g y densit y of laser r adiation EL = 0.1 t o 0.5 J.cm-2) from dimethylsulfoxide matrix. For both materials the ablation threshold EL-th was determined. The following properties of deposited layers were characterized: a) chemical composition (FTIR spectra); b) morphology (SEM and AFM portraits); c) imped- ance of gas senso rs b ased on NiPcTS and CuP cTS layers i n the pres ence o f two anal ytes - hydrogen and ozone. The prepared sensors exhibit response to 1000 ppm of hydrogen and 100 ppb of ozone even at laboratory temperatur e. Keywords: Matrix Assisted Pulsed Laser Evaporation; Substituted Phthalocyanines; Gas S ensors; Impedance Measurement 1. Introduction Matrix Assisted Pulsed Laser Evaporation (MAPLE), intro- duced by Piqué et al. in 1999, is one of the experimental laser deposition methods used for deposition of thin uniform films o f organic [1] and even biological materials [2]. MAPLE is ch aracterized b y indirect con tact o f the laser radi- ation with depo sited material. Th e target us ed for MAPLE con- sists of two substances; each has a different function during the deposition process. The first one is the deposited material itself, the second is a matrix, which has majority representation (ap- proximately 95% of target) and has usually a character of low molecular weight volatile solvent of the deposited material. Both substances are mixed together and frozen to liquid nitro- gen temperature to suppress sublimation of the matrix at low temperature. In case of correct setting of the deposition condi- tions, all the energy of laser pulse is absorbed by matrix. The matrix has two functions: i) it protects the environment of a fragile deposited material from high-energy laser radiation and ii) serv es as an en erg y trans mitter fro m ele ctro magneti c radiation to kinetic energy of the molecular oscillation motion, which causes abl ation of a depo sited material to a plasma state and it s subsequent deposition. Selection of a suitable matrix is there- fore essential for successful and non-destructive deposition of a material. The mechanism of MAPLE deposition is shown in Figure 1. Frozen target is placed i n a vacuum and impinged by pulses of laser radiation, whose energy is absorbed preferentially by the matrix, which leads to local overheating of the frozen target, followed by abrupt release of the matrix – so-called surface ablation of target. Through collective collisions, matrix mole- cules pull the molecules of the deposited material and impart them sufficient kinetic energy required to overcome the tar- get-substrate distance. Large molecules of the deposited ma- terial have lower vapor pressure than volatile small molecules of matrix and therefore they are less often pumped away by vacuum system; so the substrate is gradually covered with a thin layer of deposited material with minimum content of ma- trix molecules. The most significant group of chemical gas sensors operates on the basis of ability of thin semiconductive layers (=sensitive layers) to chemisorb various gaseous analytes on their surfaces with subsequent exchange of electrons between analyte and sensitive layer [ 3]. Wh ile red u cing gas es po sses ab ility to act as electron donors, the oxidizing ones are electron acceptors, i.e. they extract el ectron s from the sen sitive layer. At present , some classes of organic substances (conducting polymers [4], com- plexes of organic ligands with metallic cation [5] et c.) are inten- sively invest igated as prosp ective materials for sensitive layers. In general , the s uitab le materials can be ch aracterized as o rgan- ic molecules containing conjugated system of double bonds, Figure 1. Principle of MAPLE method.  P. FITL ET AL. Copyright © 2012 SciRes. AMPC 85 where π-electrons form delocalised and highly polarizable sys- tem which exhibits ability to enter reversible interaction with the an alyte. Among organocomplexes, both phthalocyanines and substi- tuted phthalocyanines are known to be excellent materials for gas sensing [ 6,7]. However, when one selects proper method for depositing sensitive layer, there is a significant difference between them: while phthalocyanines are almost insoluble in all solvents, some of their substituted derivatives exhibit a good solubility in low molecular solvents. Due to this fact substituted phthalocyanines (unlike non-substituted ones) can be deposited by MAPLE method. The presented paper deals with preparation of gas sensor sensitive layers based on tetrasulfonated phthalocyanines (NiPcTS and CuPcTS). The depositions were carried out from dimethyl- sulfoxide matrix by MAPLE method, providing tool for gentle, non-destructive and easily adjustable grown of organic mate- rials. Responses of prepared sensors to hydrogen and ozone are also p resented. 2. Experimental 2.1. Deposition of NiPcTS a nd C uPcTS thin Layers by MAPLE Method In our experiments MAPLE instrumentation was carried out as follows: Powder of NiPcTS or CuPcTS (Sigma-Aldrich) was diluted in dimethylsulfoxide matrix to obtain solution containing 0.2 weight%. Dimethylsulfoxide matrix was recently proved to be proper for depositions with KrF excimer laser [8]. After sonification the resulting solution was filtered and then frozen by liquid nitrogen. The freezing process proceeded in a tubular mould so as to produce targets for MAPLE in the form of tab- lets (approx. 40 mm in diameter and 10 mm thick). Then the deposition conditions were set (KrF excimer laser operating at 248 nm; energy density of laser radiation EL ranging from 0.1 to 0.5 J.cm-2; repetition rate of laser pulses frep = 10 Hz, pulse duration 15 ns; residual pressure in the deposition chamber 10-4 Pa; working atmosphere during depositions was 3 Pa of nitro- gen; target -subst r ate dist ance 35 mm). 2.2. Characterization of Chemical Composition and Morphology of the Layers Chemical composition of the deposited layers was analyzed from the IR sp ectra scanned b y the Attenu ated Total Reflecti on Fourier Transform Infrared spectroscopy (ATR FTIR). The spectra were scanned using a BRUKER IFS 66 V device (di- amond crystal) in the interval of wavenumbers from 600 to 1800 cm-1covering finger-print of MePcTS molecules. The surface morphology of the samples deposited on po- lished silicon wafer was acquired using Atomic Force Micro- scopy (AFM). The AFM images were taken on Veeco Digital Instruments CP II apparatus. For sample characterization, ‘Tap- ping mode’ rather than ‘Contact mode’ was chosen to minimize damage to the sample surfaces. A Veeco oxide-sharpened sili- con probe RTESPA-CP attached to a flexible microcantilever was used at its resonant frequency of 300 kHz. The image res- olution was 256×256 pixels. Layers morphology was further characterized by Scanning Electron Microscopy (SEM) with JEOL JSM-7500F instrument. 2.3. Measuring of Gas Sensor Response In ord er to test gas sensing propert ies the layers were depo sited onto alumina sensors substrates (2.0 x 2.5 mm2) equipped with interdigital electrodes (Figure 2). The sensor impedance was measured in “pure” synthetic air (as a reference atmosphere) and in synthetic air containing 1000 ppm of hydrogen or 100 ppb of ozone respectively. The impedance measurements were performed with a HP4192LF impedance analyser with fre- quency of testing signal from 15 Hz to 10 MHz and amplitude remained constant at 100 mV. The obtained data were represented as Nyquist diagrams - i.e. they are plotted as im- aginary part of complex impedance vs. real part of complex impedance with frequency of testing signal as a parameter. From Nyquist diagrams the so called phase-angle sensitivity Spa [deg.] o f sensors was evaluated. The detailed definiton of Spa is in [9,10] and s ee also F igure 3. Figure 2. Sensor substrate - front side with interdigital electrodes (lef t); back side with resis tance hea ting (right). Figure 3. A typical Nyquist diagram of gas sensor with MePcTS sensitive layer in synthetic air (reference) and measured gas (1000 ppm of hydrogen). In this example phase-angle sensitivity Spa is evaluated as a difference of sensor impedance arguments (θ angle) for 1 MHz frequency of testing signal.  P. FITL ET AL. Copyright © 2012 SciRes. AMPC 86 3. Results and Discussion 3.1. Determination of Ablation Threshold The depositions made by KrF laser in the range of energy den- sity of laser radiation from 0.1 to 0.6 J∙cm -2 can be assembled into a dependence of growth rate on laser fluence, known as the ablati on cu rve (Figure 4). On basis of these curves, the ablation thresholds EL-th were determined to be EL-th ~ 0.2 J∙cm -2 for NiPcTS and EL-th ~ 0.3 J∙cm -2 for CuPcTS. Ablation threshold is a parameter important from the practical point of view, as it corresponds to energy density sufficient for effective layer grown on one side, while there is no excessive photo- and heat - stress of deposited material on the other side. 3.2. FTIR Spectra of Source Substances and Deposited Layers Infrared spectra o f sou r ce s ubstances were compared to thos e of deposited materials (Figures 5(a),(b) and Figures 6(a), (b) in order to evaluate the degree of material decomposition during MAPLE pr ocess. Bo th mater ials wer e deposit ed at ener gy den si- ties corresponding to their ablation threshold. Analyzing spectra of sou rce material s (Figures 5(a) and 6(a)) one may notice that absorbtion bands are rather wide. This phenomenon can be attributed to occurrence of traces of mono-, di- and tri- sulfo- nated phthalocyanines in commercially distributed metal tetra- sulfonated phthalocyanines (MePcTS). Nevertheless, it is ap- parent, that in both cases the transfer of material by MAPLE method was nondestructive, as the positions and in most cases also amplitudes of absorbtion maxima are retained when com- paring Figure 5(a) with (b) or Figures 6(a) with (b). An over- view of absorption bands of phthalocyanines is summarized in [11]. Figure 4. Ablation curves for NiPcTS (top) and CuPcTS (botto m). 3.3. Layers Morphology The layers deposited at ablation thresholds were also studied by SEM (Figure 7) and AFM (Figure 8) methods. The portraits resulting from these methods reveal that the structure of MAPLE deposit ed layer is rath er segmented with lar ge relative surface; these properties are favourable for applications in gas sensing (the detection process is localized on the surface of sensitive layer). Figure 5. FTIR spectrum of NiPcTS: source material (a); layer deposited at EL-th = 0. 2 J∙cm-2 (b). Figure 6. FTIR spectrum of CuPcTS: source material (a); layer deposited at EL-th = 0. 3 J∙cm-2 (b). Figure 7. SEM portraits of NiPcTS (left) and CuPcTS (right). 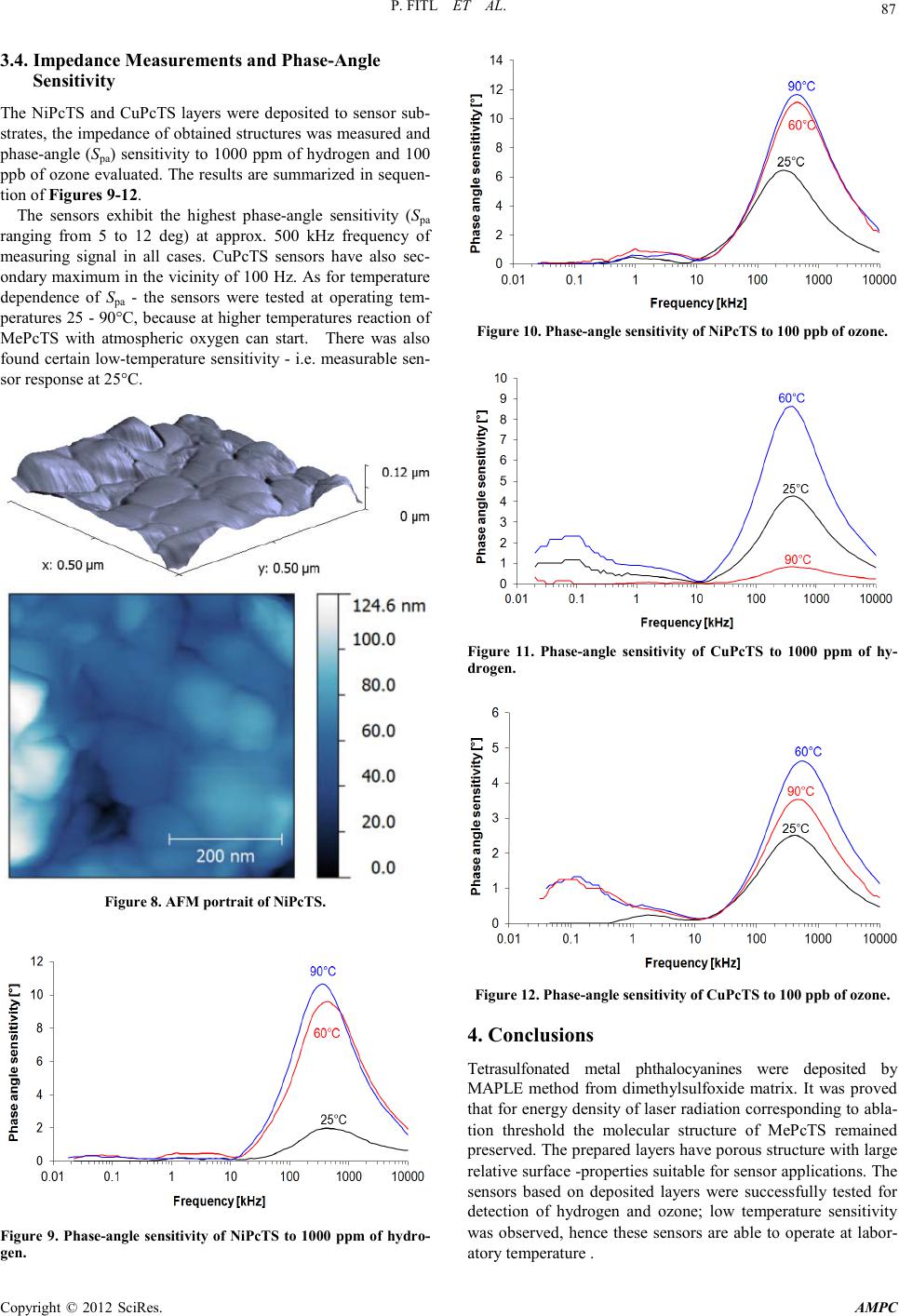 P. FITL ET AL. Copyright © 2012 SciRes. AMPC 87 3.4. Impedance Measurements and Phase-Angle Sensitivity The NiPcTS and CuPcTS layers were deposited to sensor sub- strates, t he impedance o f obtained structures was measu red and phase-angle (Spa) sensitivity to 1000 ppm of hydrogen and 100 ppb of ozone evaluat ed. The results are summarized i n sequen- tion of Figures 9-12. The sensors exhibit the highest phase-angle sensitivity (Spa ranging from 5 to 12 deg) at approx. 500 kHz frequency of measuring signal in all cases. CuPcTS sensors have also sec- ondary maximum in the vicinity of 100 Hz. As for temperature dependence of Spa - the sensors were tested at operating tem- peratu res 25 - 90°C, becau se at higher temperatu res reaction o f MePcTS with atmospheric oxygen can start. There was also found certain low-temperature sensitivity - i. e. measu rable sen- sor response at 25°C. Figure 8. AFM portrait of NiPcTS. Figure 9. Phase-angle sensitivity of NiPcTS to 1000 ppm of hydro- gen. Figure 1 0. Phase-an gl e se n s it ivity of NiPc TS to 100 ppb o f ozone . Figure 11. Phase-angle sensitivity of CuPcTS to 1000 ppm of hy- drogen. Figure 1 2. Phase-angle sensitivity of CuPcT S to 100 pp b of ozone. 4. Conclusions Tetrasulfonated metal phthalocyanines were deposited by MAPLE method from dimethylsulfoxide matrix. It was proved that for energy density of laser radiation corresponding to abla- tion threshold the molecular structure of MePcTS remained preserved. The pr epared l ayers have po rous str ucture with large relative su rface -properties suitable for sensor applications. The sensors based on deposited layers were successfully tested for detection of hydrogen and ozone; low temperature sensitivity was observed, hence these sensors are able to operate at labor- atory temperature .  P. FITL ET AL. Copyright © 2012 SciRes. AMPC 88 5. Acknowledgements This work was supported by Grant Agency of the Czech Re- public (GAČR) projects No. P108/11/1298 and P108/12/P802 and also financial support from specific university research (MSMT No. 21/2012) . REFERENCES [1] A. Piqué, R.A. McGill and D.B.Chrisey, “Growth of organic thin films by the matrix assisted pulsed laser evaporation (MAPLE) technique,” Thin Solid Films, vols. 355-3 56, pp. 536-541 , 1999. [2] D.B. Chrisey, A. Piqué and R.A. McGill, “Laser deposition of polymer and biomaterial films,” Chem. Rev., vol. 103, pp. 553-576, 2003. [3] W. Göppel and K. D. Schierbaum, “SnO2 sensors: current status and future prospects,” Sens. Actuators B, vol. 26/27, pp. 1–12, 1995. [4] S. Carquigny, “Ammonia gas sensor based on electrosynthesized polypyrrole films,” Tala nta, vol. 78, pp. 199-206, 200 9. [5] D. Delmare and C. Bied-Charreton, “Grafting of cobalt porphy- rins in sol-gel matrices:application to the detection of amines,” Sens.Actuator s B, vol. 62, pp.136-142, 2000. [6] S. Nespu rek , “Solub le p htha locyan in es: Pers pect iv e mater ial s for electr o nics ,” Mo l . Liq. Cryst., vol . 46 8, p p. 3 55-373, 2007. [7] Y.Q. Chen, W.M. Zhang and G.A. Li, “Saw gas sensor with copper tetrasulfonated phthalocyanine film,” Sens. Actuators B, vol. 20, pp. 247-249, 1994. [8] R. Fryček, F.Vysloužil, V. Myslík, M.Vrňata, D. Kopecký, O. Ekrt, P. Fitl, M.Jelínek, T. Kocourek and R. Šipula, “Deposition of organic metalocomplexes for sensor applications by MAPLE,” Sens. Actuat ors B, vol. 12 5, pp. 189-1949 , 2007. [9] V. Myslík, F.Vysloužil, M. Vrňata, Z. Rozehnal, M. Jelínek, R. Fryček and M. Kovanda, “Phase ac-sensitivity of oxidic and acetylacetonic gas sensors,” Sens. Actuators B, vol. 89, pp. 205-211, 2003. [10] P. Fitl, V. Myslík, M. Vrňata, J. Náhlík, D. Kopecký, J. Vlček, J. Hofmann, J. Lančok, “Sensing properties of tin acetylaceto- nate-based thin films doped with platinum,” Sens. Mater., vol.24, pp. 75-86, 2012. [11] M. Fukui, “Structural characterization of phthalocyanine Lang- muir-Blodgett multilayer assemblies by ft-ir spectroscopy,” Chem. Phys. Let t., vol. 177, pp. 247-251, 1991. |

