Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 45-48 doi:10.4236/ampc.2012.24B013 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Apatite Deposition on ZrO2 Thin Fi lms by DC Unbal an ced Magnetron Sputtering Arisara Thaveedeetrakul1, Virote Boonamnuayvitaya2, Nirun Witit-anun2,3 1Department of Chemical Engi neering, Faculty of Engineering, KMUTT, Bangkok, Thailand 2Department of physics, Faculty of Science, Burapha University, Chon Buri, Thailand 3Thailand Center of E xcellenc e in Physics /CHE, Ministry of Ed ucation, Bangkok, Thailand Email: virote.boo@kmutt.ac.th Received 2012 ABSTRACT Zirconia thin films deposited on 316L stainless-steel substrate were prepared by DC unbalanced magnetron sputtering from a metal- lic zirconium target at low temperature with the target-to-substrate distance (dt-s) of 100 mm and sputtering power of 180 W. High purity gas of Ar as the working gas and O2 as the reactive gas were used. The depositions were performed for 120 min at a total pressure of 0.5 Pa. The effect of thermal treatment on the HA formation was investigated. The bioactivity was assessed by investi- gating the formation of hydroxyapatite (HA) on the surface soaked in simulated body fluids (SBF). Films structure, surface mor- phology and chemical composition of the ZrO2 films and HA formation were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and FT-IR spectroscopy. The XRD results demonstrate the ZrO2 films are monoclinic phase. The an- nealed films sho w the higher fil m crystalline due to the rearran gement of fil m stru cture. After b eing immersed th e samples in SBF, the bone-like apatite was observed on all ZrO2 films, but a denser and more continuous HA layer were observed on annealed films due to the crystallinity of ZrO2 films. Keywords: Component; Zirconium Dioxide; Magnetron Sputtering; Hydroxyapatite; Simulated Body Fluid 1. Introduction Thin films of zirconium dioxide (ZrO2) or zirconia are widely used in protecti ve and thermal b arrier co atings [1], optical filter [2] , oxygen sensor, microelectronic devices and biocompatibility of bone implants [3-5]. It was considered an attractive ceramic for biomedical application due to its inertness, high strength, corrosion resistance, and fracture toughness. The ZrO2 have three crystalline polymorphs, namely: monoclinic, tetragonal and cubic [6]. Both the monoclinic and tetragonal phases exhibit excellent biocompatibility properties on th eir surfaces [7]. Bioactivity is widely accepted as the essential requirement for an artificial to exhibit chemical bonding to living tissues upon the formation of a bone-like apatite layer on its surface in any simulated body environment [8]. The formation of a bone-like apatite layer on biomaterials is assumed to be the precondition for their osteoinductivity to induce bone formation on the biomaterials in non-osseous site. The research method of bone-like apatite formation in vitro commonly is to immerse specimen in simulated body fluid (SBF) and hydroxyapatite (HA) layer can be formed on all kinds of bio active materials [9] . Th e in v itro tests of zirconium hydrogel coating have found new bone-like apatite layer formation on the surface [7]. Clinical studies of ZrO2 thin films are now expected to be the useful as bone substitutes even under highly loaded conditions such as is found in femoral and knee joint since they exhibit high fracture toughness as well as high bond-bonding ability [10]. There are many methods to prepare the ZrO2 thin films., however, the sputtering technique is a very attractive process for producing metallic oxide with good uniformity at low temperature [11]. This technique has not yet been well studied for the deposition of ZrO2 films with the purpose of the applications mentioned. In this study, the ZrO2 thin films were deposited by DC unba- lanced magnetron sputtering technique followed by the thermal treatments. All samples were immersed in a SBF solution for demonstration the bone-like apatite on the ZrO2 films. The effect of the pretreatment on the formation of the HA was in- vestigated. 2. Experimental Reactive ma gn etro n s putt erin g, with zir coniu m target o f 54 m m diameter, was used to produce the ZrO2 thin films with the sputtering power of 180 W. The coatings were deposited on 316L-stainless steel type sub strate, 10 mm × 1 0 mm × 1 mm in size. The chemical pretreatment of the substrates was carried out by cleansing with propanol and acetone in ultrasonic bath for 15 min. The sputtering gases argon with a purity of 99.999% and reactive oxygen with a purity 99.999% of which flow rates of 1 and 4 sccm, respectively, were introduced to the chamber separately and controlled by the mass flow controllers (MKS type 247D). The target was sputt er cleaned for 15 min at the argon pressure lower than 0.005 Pa to remove impurities from target surface. The dt-s was adjusted to 100 mm and the base pressure of the system was 0.005 Pa. The depositions were performed for 120 min at a total pressure of 0.5 Pa. Some as-deposited ZrO2 films were annealed in air at the temperature of 800˚C for 1 h. 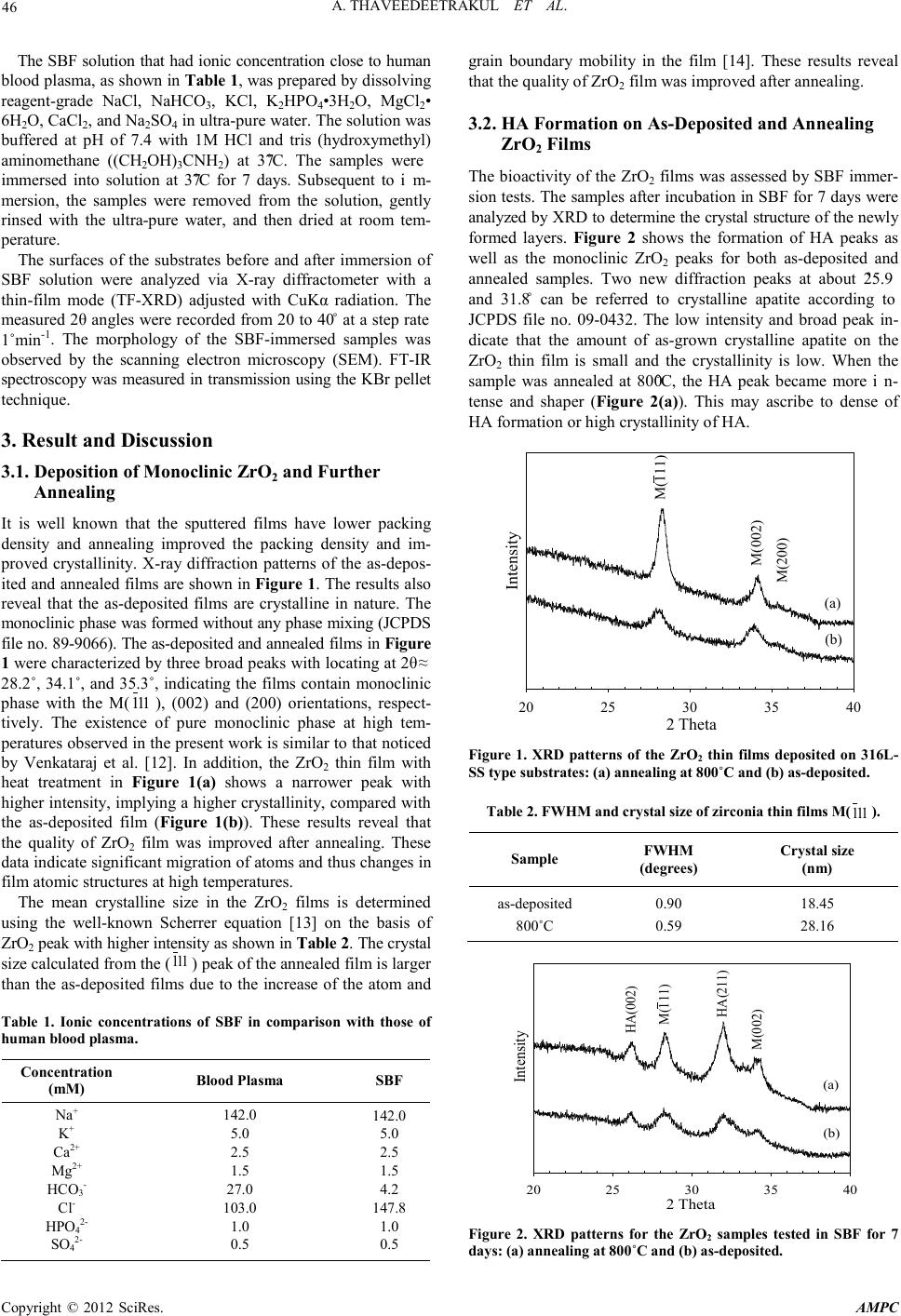 A. THAVEEDEETRAKUL ET AL. Copyright © 2012 SciRes. AMPC 46 The SBF solution that had ionic concentration close to human blood plasma, as shown in Table 1, was prepared by dissolving reagent-grade NaCl, NaHCO3, KCl, K2HP O 4•3H 2O, MgCl2• 6H2O, CaCl2, and Na2SO4 in ultra-pu re water. The so lution was buffered at pH of 7.4 with 1M HCl and tris (hydroxymethyl) aminomethane ((CH2OH)3CNH2) at 37˚C. The samples were immersed into solution at 37˚C for 7 days. Subsequent to im- mersion, the samples were removed from the solution, gently rinsed with the ultra-pure water, and then dried at room tem- perature. The surfaces of the substrates before and after i mmersion of SBF solution were analyzed via X-ray diffractometer with a thin-film mode (TF-XRD) adjusted with CuKα radiation. The measured 2θ angles were recorded from 20 to 40˚ at a step rate 1˚min-1. The morphology of the SBF-immersed samples was observed by the scanning electron microscopy (SEM). FT-IR spectroscopy was measured in transmission using the KBr pellet techn ique. 3. Result and Discussion 3.1. Deposit ion of Monoclinic ZrO2 and Further Annealing It is well known that the sputtered films have lower packing density and annealing improved the packing density and im- proved crystallinity. X-ray diffraction patterns of the as-depos- ited and annealed films are shown in Figure 1. The resu lts also reveal that the as-deposited films are crystalline in nature. The monoclinic phase was formed without any phase mixing (JCPDS file no. 89-9066). The as-deposited and annealed films in Figure 1 were char acteri zed by th ree broad peaks with locating at 2θ ≈ 28.2˚, 34.1˚, and 35.3˚, indicating the films contain monoclinic phase with the M( 111 ), (002) and (200) orientations, respect- tively. The existence of pure monoclinic phase at high tem- peratures observed in the present work is similar to that noticed by Venkataraj et al. [12]. In addition, the ZrO2 thin film with heat treatment in Figure 1(a) shows a narrower peak with higher intensity, implying a higher crystallinity, compared with the as-deposited film (Figure 1(b)). These results reveal that the quality of ZrO2 film was improved after annealing. These data indicate significant migration of atoms and thus changes in film atomic structures at h igh temperatures. The mean crystalline size in the ZrO2 films is determined using the well-known Scherrer equation [13] on the basis of ZrO2 peak with higher intensity as shown in Table 2. Th e c rystal size calculated from the ( 111 ) peak of the anneal ed film is lar ger than the as-deposited films due to the increase of the atom and Table 1. Ionic concentrations of SBF in comparison with those of human blood plasma. Concentration ( mM) Blood Plasma SBF Na+ K+ Ca2+ Mg2+ HCO3- Cl- HPO42- SO42- 142.0 5.0 2.5 1.5 27.0 103.0 1.0 0.5 142.0 5.0 2.5 1.5 4.2 147.8 1.0 0.5 grain boundary mobility in the film [14]. These results reveal that the quality of ZrO2 film was improved after anneali ng. 3.2. HA Formation on As-Deposited a nd A nnealing ZrO 2 Films The bioactivity of the ZrO2 films was assessed by SBF immer- sion test s. The samples after incu bation in SBF for 7 d ays were anal yzed by XRD to determi ne the cr ystal stru cture o f the newl y formed layers. Figure 2 shows the formation of HA peaks as well as the monoclinic ZrO2 peaks for both as-deposited and annealed samples. Two new diffraction peaks at about 25.9˚ and 31.8˚ can be referred to crystalline apatite according to JCPDS file no. 09-0432. The low intensity and broad peak in- dicate that the amount of as-grown crystalline apatite on the ZrO2 thin film is small and the crystallinity is low. When the sample was annealed at 800˚C, the HA peak became more in- tense and shaper (F igure 2(a)). This may ascribe to dense of HA formation or high crystallinity of HA. 2 Theta 20 25 30 35 40 Intensity M(111) M(002) M(200) (a) (b) Figure 1. XRD patterns of the ZrO2 thin films deposited on 316L- SS type substrate s : (a) annealing a t 800˚C and (b) as-deposited. Tabl e 2. FWHM and cryst al size of zirc onia thin fi lms M( 111 ). Sample FWHM (degrees) Crystal size (nm) as-deposited 800˚C 0.90 0.59 18.45 28.16 2 Theta 20 25 30 35 40 Intensity HA(002) M(111) HA(211) M(002) (a) (b) Figure 2. XRD patterns for the ZrO2 samples tested in SBF for 7 days: ( a) annealing at 800˚C and (b) as-deposited.  A. THAVEEDEETRAKUL ET AL. Copyright © 2012 SciRes. AMPC 47 The calcium and phosphate ions required for hydroxyapatite generatio n on the film surface were derived from the S BF. The result obtained from the present study indicates that Zr-OH groups, abundant on the surface of the thin film, are able to induce ap atite nucleatio n in a similar manner as Si -OH, Ti-OH, and Ta-OH gro up do. Neverth eless, Uchida et al . [7] found the apatite-forming ability of the gels with tetragonal or monoclinic structure was apparently much higher than that of the gel with amorphous structure. This implies that not all types of Zr-OH groups , but only Zr-OH group with specific arrangements based on tetragonal or monoclinic structure, are effective in inducing apatit e nucleation. The microstructures of the as-deposited and annealed ZrO2 films after soaked in SBF for 7 days were observed by SEM as shown in Figure 3. Both the samples can induce hydroxyapa- tite form on the films surface. The HA particl es was island -like and spherically shaped with diameter size about 2 to 3 µm. For the as-deposited sample (Figure 3(a)), only a small amount of HA particles formed sparsely scattered on the surface of the sample. The morphology is very similar to that of the deposited apatite on the surface of zirconia gel through biomimetic processing utilizing SBF [7]. However, a den ser and more con- tinuous HA layer was observed on annealed films (Figure 3(b)). The small HA particle size under the aggregation is about 600 nm in diameter. These results indicate that the annealed ZrO2 films with a high crystallinity are more bioactive compared with low crystallized structure. (a) (b) Figure 3. SEM micrographs of HA formation after soaking in S BF solution for 7 days: (a) as-deposited and (b) annealing at 800˚C. Wavenumber (cm -1 )60012001800240030003600 Transmission (%) H2OCO32- PO43- PO43- OH- CO32- Figure 4. FT-IR spectra of HA formed on the sample s in SBF for 7 days. In order to confirm the structure of the Ca-P layer, we per- formed FTIR analysis, as shown in Figure 4. The phosphate group itself has a tetrahedral symmetry; resulting in four vibra- tional modes (symmetric stretch (ν1) at 958 cm-1; asymmetric stretch (ν2) at 430 - 460 cm-1; (ν3) at 1041-1090 cm-1; (ν4) at 575 - 610 cm-1) [15]. An OH- absorption peak at 3440 cm-1 can be seen in the spectra. The absorption peak at 1635 cm-1 can be assigned to absorbed H2O groups, which is a common characte- ristic of precipitates in aqueous solutions [16]. Furthermore, peaks between 1400 - 1450 cm-1 are due to the C−O stretching of C O32- groups, which indicate that the apatites formed on the ZrO2 thin films are bone-like carbonate-containi ng apatite [ 16]. 4. Conclusion Zirconium dioxide thin films of monoclinic phase have been deposited by DC unbalanced magnetron sputtering and the film was subjected to thermal treatment. The annealed films show the higher film crystalline due to the rearrangement of film structure. After being immersed the samples in SBF, the bone-like apatite was observed on all ZrO2 films, but a denser and more continuous HA layer was ob served on annealed films due to the crystallinity of ZrO2 films. These results show that the sp ut tered Zr O 2 thin films exhibit good bioactivity. 5. Acknowledgements This work was supported by the Royal Golden Jubilee of Thailand Research Fund and the Department of Chemical En- gineering at King Mongkut’s University of Technology Thon- buri. REFERENCES [1] B. Leclercq, R. Mévrel, V. Liedtke, W. Hohenauer, Thermal conductivity of zirconia-based ceramics for therma l barri er coat- ing, Materialwissenschaft und Werkstofftechnik, vol. 34, pp. 406-409, 2003. [2] Q. Zhang, X. Li, J. Shen, G. Wu, J. Wang, L. Chen, ZrO2 thin films and ZrO2/SiO2 optical reflection filters deposited by sol–gel method, Mater. Lett., vol. 45, pp. 311-314, 20 0 0. [3] M. Uchida, H.-M. Kim, F. Miyaji, T. Kokubo, T. Nakamura, Apatite formation on zirconium metal treated with aqueous  A. THAVEEDEETRAKUL ET AL. Copyright © 2012 SciRes. AMPC 48 NaOH, Biomaterials, vol. 23, pp. 313-317, 2002. [4] J. Chevalier, What future for zirconia as a biomaterial?, Bioma- terials, vol. 27, pp. 535-543, 2006. [5] X. Liu, A. Huang, C. Ding, P.K. Chu, Bioactivity and cytocom- patibility of zirconia (ZrO2) films fabricated by cathodic arc de- posi tion, Biomaterials , vol. 27, pp. 3904-3911, 2006. [6] A.M. Alper, High Temperature Oxide Part II: Oxides of Rare Earths, Titanium, Zirconium, Hafnium, Niobium and Tantalum, Academic Press, New York, 1970. [7] M. Uchida, H.-M. Kim, T. Kokubo, K. Tanaka, T. Nakamura, Structural dependence of apatite formation on zirconia gels in a sim ulated body flui d, J. Cera m. Soc. Jp n., vol. 110, pp. 710 -715, 2002. [8] T. Kokubo, Bioceramics and their Clinical Applications, Wood- head, Engl and, 200 8. [9] T. Kokubo, H. Takadama, How useful is SBF in predicting in vivo bone bioactivity?, Biomaterials, vol. 27, pp. 2907-2915, 2006. [10] C. Piconi, G. Maccauro, Zirconia as a ceramic biomaterial, Bio- materials, vol. 20, pp. 1-25, 1999. [11] Anderson, Sputtering by particle bombardment. I. Physical sput- tering of single element solids, Springer-Verlag Berlin and Hei- delberg GmbH & Co. K 1982. [12] S. Venkataraj, O. Kappertz, C . Liesch, R. Detemple, R. Jayavel, M. Wuttig, Thermal stability of sputtered zirconium oxide films , Vacuum, vol. 75, pp. 7-16, 2004. [13] B. D. Cullity, S.R. Stock, Elements of X-Ray Diffraction, third ed., Prentice Hall, New Jersey, 2001. [14] M.M. Larijani, D. Najafi, M. Eshghabadi, Th e effect of oxi dation temperature on the nano crystalline structure of ZrO2 films depo- sited on silicon and glass substrates, Crystal Research and Tech- nology, vol. 46, pp. 956-960, 2011. [15] G.S. Kumar, E.K. Girija, A. Thamizhavel, Y. Yokogawa, S.N. Kalkura, Synthesis and characterization of bioactive hydroxya- patite–calcite nanocomposite for biomedical applications, J. Colloid Interfac e Sci. , vol. 349, pp . 56-62, 2010. [16] D. Chen, E.H. Jordan, M. Gell, M. Wei, Apatite formation on alkaline-t reat ed d ens e TiO2 coatings d eposi ted usin g th e solution precursor plasma spray process, Acta Biomater., vol. 4, pp. 553-559, 2008. |

