Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Chemistry, 2012, 2, 40-44 doi:10.4236/ampc.2012.24B012 Published Online December 2012 (http ://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Photocatalytic of T iO2-SiO2 Thin Films Co-Doped with Fe3+ and Thio-Urea in the Degradation of Formaldehyde by Indoor and Out door Visible Li gh t s Charuwan Kaewtip, Kamolporn Accanit, Nat-a-nong Chaowai, Kanokpun Areerat, Pasuree Reanjaruan, Virote Boonumnauyvitaya Ch emical Engineering Department, King Mongkut’s University of Technology Thonburi, Bangkok, Thailand Email: virote.boo@kmutt.ac.th Received 2012 ABSTRACT In this work the photocatalytic activity of TiO2-SiO2 thin films co-doped with Fe3+ and thio-urea in the degradation of the gaseous formaldehyde was investigaged by indoor and outdoor visible lights. The films were synthesized by Peroxo Titanic Acid (PTA) me- thod. The physicochemical properties of prepared samples were characterized using SEM and UV-vis absorption spectroscopy. It was found that the average film thickness of all coated samples was about 394 ± 5 nm. The band gap energy of un-doped and co-dop ed ph oto catalysts was 3 .0 8 and 2.8 8 eV , resp ecti vel y. The p ho tocat alytic exp eri men tal resu lts showed that the co-doped TiO2- SiO2 thin film yield higher photocatalytic efficiency. Under the outdoor light (sunlight in the shade condition) irradiation, with the initial concentrations of formaldehyde of 1000, 3000 and 5000 ppmV, the efficiencies of formaldehyde degradation were 94.7%, 89.5% and 85.1%, respectively. Under the indoor light (the fluorescent lamp) irradiation, with the same formaldehyde initial concen- trations, the photocatal ytic act iviti es w er e 87.4%, 85.3% and 81.5%, respectivel y. Keywords: Perxo Titanic Acid; Titanium Dioxide; Sunlight in the Shade; Formaldehyde 1. Introduction Formaldehyde is a toxic volatile organic compound (VOCs), which causes cancer and is harmful to health when uptake into human bodies. Therefore, purification of ambient air form this toxic gas is essential for improving indoor air quality and hu- man being’s health [1]. Titanium dioxide (TiO2) is a promising tool for environmental purification due to its specific optical and electronic properties, low cost, chemical stability and non-toxicity [2-4]. However, the need of an ultraviolet (UV) excitation which accounts for only a small fraction of the total solar energy (∼5%) hinders its utility for limited applications [5] . Many attempts have been made to enhance the utilization of solar energy by doping the base photocatalyst with co-dopants elements such as C and N [6], Fe3+ and C [7], and Fe3+ and N [8,9]. Ohno et al. reported that the effect of adsorbing Fe3+ on the N- or S- doped TiO2 and found that the photocatalytic effi- ciency under visible light region was about twice as high as without Fe3+ doping [10]. One element can extend the response of TiO2 to visible light, the other can act as electron and hole traps. Then TiO2 can respond to visible light and present a high catalytic activity [11]. In ou r previ ous work [ 12], TiO2-SiO2 thin films with co- do- pants of Fe3+ and N,S yielded high efficiency in formaldehyde degradation. However, the study was limited in small lab scale using fluorescent lamp as the light source. The objective of this work is to confirm the practical use of these films by studying the efficiency of formaldehyde degradation using co-doped TiO2-SiO2 thin films in a large cubic glass chamber under the outdoor light compared with the indoor fluorescent light. 2. Experimental 2.1. Catalysts Preparation All reagents were of analytical grade and used without further purification. The co-doped TiO2-SiO2 thin films were prepared using the peroxo titanic acid (PTA) approach combined with the sol–gel method. The procedures for co-doped PTA sol (so- lution A) preparing were as follows: First, 4.3 g of Titanyl sul- fate (TiOSO4) was added to 150 cm3 deionized water. While under vigorous stirring, 26 cm3 of NH4OH (3 mol/dm3) was added to the solution. Next, the white precip itates were filtered and sufficiently washed four to six times with distilled water to remove residues of NH4+ and SO42− ions, then dispersed ho- mogeneously in 112.5 ml of distilled water. The resulting sol was peptized in 25 cm3 of hydrogen peroxide (30%), and then stirred for 15 min. The obtained orange transparent sol was kept under reflux at 100°C. Before adding the co-dopants, the PTA sol was kept at room temperature for 24 h. Dopants added into the TiO2 based on the mass of TiO2 1.64 g in PTA sol. Finally, CSN2H4 of 0.125 wt.% and Fe(NO3)3 of 1.0 wt.% were added into the PTA sol. The S iO2 (solution B) sol was prepared via the conden sation reaction of methyltrimethoxysilane (MTMOS). First, 4.3 cm3 of MTMOS were hydrolyzed with the mixture of 8.22 cm3 of methano l, 2 cm3 of H 2O, and 0.5 cm3 of HCl (0.055M) aqueous solution. After the solutions were vigorously stirred at 50°C fo r 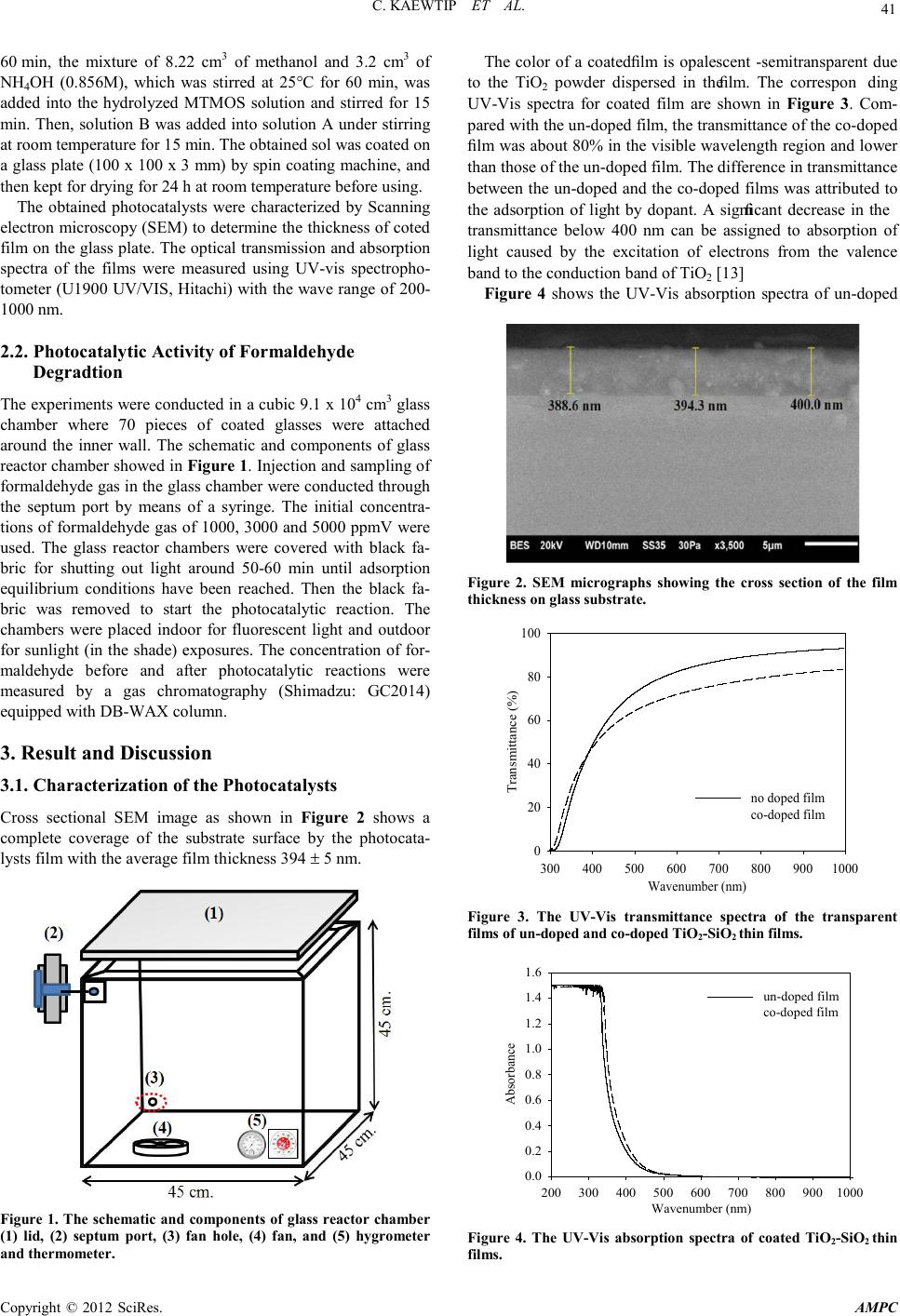 C. KAE WTIP ET AL. Copyright © 2012 SciRes. AMPC 41 60 min, the mixture of 8.22 cm3 of methanol and 3.2 cm3 of NH4OH (0.856M), which was stirred at 25°C for 60 min, was added into the hydrolyzed MTMOS solution and stirred for 15 min. Then, solution B was added into solution A under stirring at room temperature for 15 min. The obtained sol was coated on a glass plat e (100 x 100 x 3 mm) by spin coating machine, and then kept for drying for 24 h at room temperature before using. The obtained photocatalysts were characterized by Scanning electron microscopy (SEM) to determine the thickness of coted film on the glass plate. The optical transmission and absorption spectra of the films were measured using UV-vis spectropho- tometer (U1900 UV/VIS, Hitachi) with the wave range of 200- 1000 nm. 2.2. Photocatalytic Activity of Formaldehyde Degradtion The experiments were conducted in a cubic 9.1 x 104 cm3 glass chamber where 70 pieces of coated glasses were attached around the inner wall. The schematic and components of glass reactor chamber showed in Figure 1. Injection and sampling of formaldehyde gas in the glass chamber were conducted through the septum port by means of a syringe. The initial concentra- tions of formaldehyde gas of 1000, 3000 and 5000 ppmV were used. The glass reactor chambers were covered with black fa- bric for shutting out light around 50-60 min until adsorption equilibrium conditions have been reached. Then the black fa- bric was removed to start the photocatalytic reaction. The chambers were placed indoor for fluorescent light and outdoor for sunlight (in the shade) exposures. The concentration of for- maldehyde before and after photocatalytic reactions were measured by a gas chromatography (Shimadzu: GC2014) equipped with DB-WAX column. 3. Result and Discussion 3.1. Characterization of the Photocatalysts Cross sectional SEM image as shown in Figure 2 shows a complete coverage of the substrate surface by the photocata- lysts film with the average film thickness 3 94 ± 5 nm. Figure 1. The schematic and components of glass reactor chamber (1) lid, (2) septum port, (3) fan hole, (4) fan, and (5) hygrometer and thermometer. The color of a coated film is opalescent-semitransparent due to the TiO2 powder dispersed in the film. The corresponding UV-Vis spectra for coated film are shown in Figure 3. Com- pared with the un-doped film, the transmittan ce o f the co -doped film was about 80% in the visible wavelength region and lower than those of the un-doped film. The difference in transmittance between th e un-doped and the co-doped films was attributed to the adsorption of light by dopant. A significant decrease in the transmittance below 400 nm can be assigned to absorption of light caused by the excitation of electrons from the valence band to the co nductio n ba nd of TiO2 [13] Figure 4 shows the UV-Vis absorption spectra of un-doped Figure 2. SEM micrographs showing the cross section of the film thickness on glass substrate. Wavenumber (n m) 300 400 500 600 700 800 9001000 Transmittance (%) 0 20 40 60 80 100 no doped film co-doped fi lm Figure 3. The UV-Vis transmittance spectra of the transparent films of un-doped and co-doped TiO2-Si O 2 th in fi lms. Wavenumber (nm) 200 300 400 500 600 700 800 9001000 Absorbance 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 un-doped film co-doped film Figure 4. The UV-Vis absorption spectra of coated TiO2-SiO2 thin f i lms. 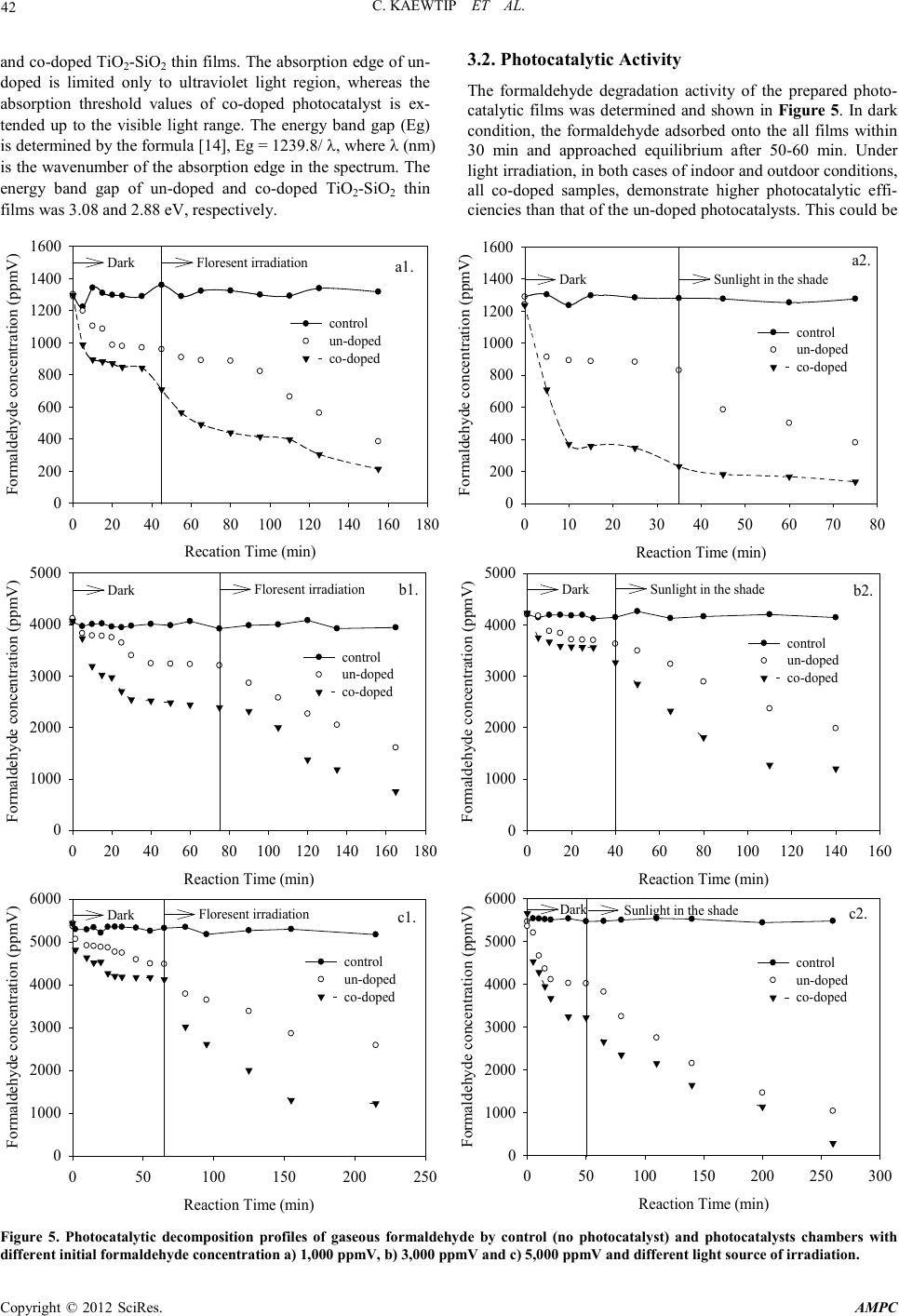 C. KAE WTIP ET AL. Copyright © 2012 SciRes. AMPC 42 and co -doped TiO2-SiO2 thin films. The absorption edge of un- doped is limited only to ultraviolet light region, whereas the absorption threshold values of co-doped photocatalyst is ex- tended up to the visible light range. The energy band gap (Eg) is determined by the formula [14], Eg = 1239.8/ λ, where λ (nm) is the wavenumber of the absorption edge in the spectrum. The energy band gap of un-doped and co-doped TiO2-SiO2 thin films was 3.08 and 2.88 eV, respectivel y. 3.2. Photocatalytic Activity The formaldehyde degradation activity of the prepared photo- catalytic films was deter mined and shown in Figure 5. In dark condition, the formaldehyde adsorbed onto the all films within 30 min and approached equilibrium after 50-60 min. Under ligh t i rr adia ti on, in bo th cas es o f in d oo r and ou t door con d iti ons , all co-doped samples, demonstrate higher photocatalytic effi- ciencies t han that of the un-doped photocatalysts. This could be Reaction Time (min) 050100 150 200 250 300 Formaldehy de concentration (ppmV) 0 1000 2000 3000 4000 5000 6000 control un-doped co-doped Dark Sunlight in the shade c2. Reaction Time (min) 050100 150 200 250 Formaldehy de concentration (ppmV) 0 1000 2000 3000 4000 5000 6000 control un-doped co-doped Dark Floresent irradiation c1. Reaction Time (min) 020406080100 120 140 160 Formaldehy de concentration (ppmV) 0 1000 2000 3000 4000 5000 control un-doped co-doped Dark Sunlight in the shade b2. Reaction Time (min) 020406080100 120 140 160 180 Formaldehy de concentration (ppmV) 0 1000 2000 3000 4000 5000 control un-doped co-doped Dark Floresent irradiation b1. Reaction Time (min) 010 20 30 40 50 60 70 80 Formaldehy de concentration (ppmV) 0 200 400 600 800 1000 1200 1400 1600 control un-doped co-doped Dark Sunlight in the shade a2. Recation Time (min) 020406080100 120 140 160180 Formaldehy de concentration (ppmV) 0 200 400 600 800 1000 1200 1400 1600 control un-doped co-doped Dark Floresent irradiation a1. Figure 5. Photocatalytic decomposition profiles of gaseous formaldehyde by control (no photocatalyst) and photocatalysts chambers with different initial formaldehyde concentration a) 1,000 ppmV, b) 3,000 ppmV and c) 5,000 ppmV and different light source of irradiation. 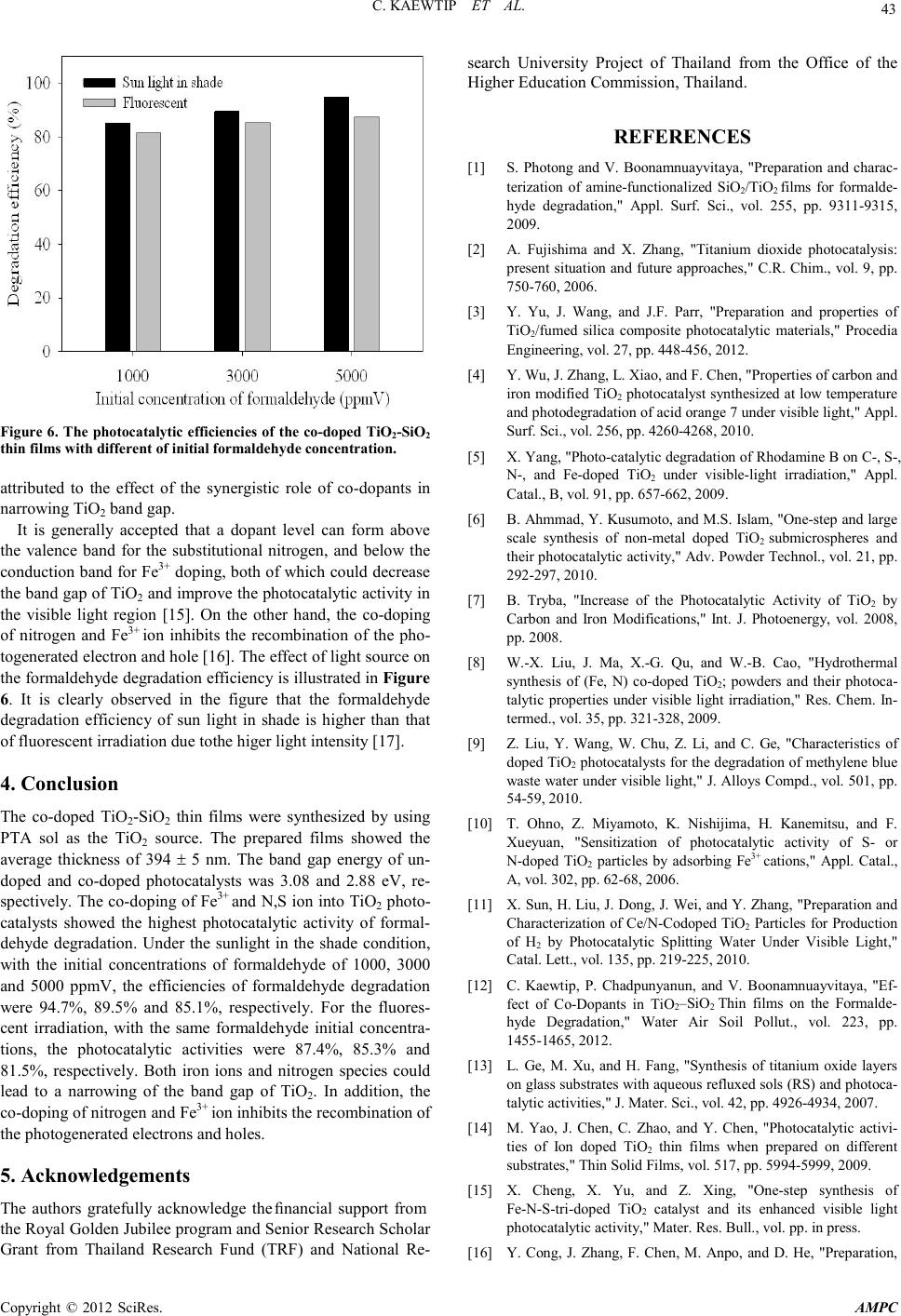 C. KAE WTIP ET AL. Copyright © 2012 SciRes. AMPC 43 Fig ure 6. The photocatalytic efficiencies of the co-doped TiO2-Si O 2 thin films with different of initial formaldehyde concentration. attributed to the effect of the synergistic role of co-dopants in narrowing TiO2 band gap. It is generally accepted that a dopant level can form above the valence band for the substitutional nitrogen, and below the conduction band for Fe3+ doping, both of which cou ld decrease the band gap of TiO2 and improve the photocatalytic activity in the visible light region [15]. On the other hand, the co-doping of nitrogen and Fe3+ ion inhibits the recombination of the pho- togener ated electr on and ho le [16]. The effect o f ligh t s ou rce on the formaldeh yde degrad atio n efficienc y is illustrated i n Figure 6. It is clearly observed in the figure that the formaldehyde degradation efficiency of sun light in shade is higher than that of fluorescent irradiation due tothe higer light intensity [17]. 4. Conclusion The co-doped TiO2-SiO2 thin films were synthesized by using PTA sol as the TiO2 source. The prepared films showed the average thickness of 394 ± 5 nm. The band gap energy of un- doped and co-doped photocatalysts was 3.08 and 2.88 eV, re- spectivel y. The co -doping of Fe3+ and N,S ion into TiO2 photo- catalysts showed the highest photocatalytic activity of formal- dehyde degradation. Under the sunlight in the shade condition, with the initial concentrations of formaldehyde of 1000, 3000 and 5000 ppmV, the efficiencies of formaldehyde degradation were 94.7%, 89.5% and 85.1%, respectively. For the fluores- cent irradiation, with the same formaldehyde initial concentra- tions, the photocatalytic activities were 87.4%, 85.3% and 81.5%, respectively. Both iron ions and nitrogen species could lead to a narrowing of the band gap of TiO2. In addition, the co-doping of nitrogen and Fe3+ ion inhibits the recombination of the photogenerated electrons and holes. 5. Acknowledgements The authors gratefully acknowledge the financial support from the Ro yal Gold en Jubi lee p rogram and S eni or Research Sch olar Grant from Thailand Research Fund (TRF) and National Re- search University Project of Thailand from the Office of the Higher Education Commission, Thailand. REFERENCES [1] S. Photong and V. Boonamnuayvitaya, "Preparation and charac- terization of amine-functionalized SiO2/TiO2 films for formalde- hyde degradation," Appl. Surf. Sci., vol. 255, pp. 9311-9315, 2009. [2] A. Fujishima and X. Zhang, "Titanium dioxide photocatalysis: present situation and future approaches," C.R. Chim., vol. 9, pp. 750-760, 2006. [3] Y. Yu, J. Wang, and J.F. Parr, "Preparation and properties of TiO2/fumed silica composite photocatalytic materials," Procedia Engineering, vol. 27, pp. 448-456, 2012. [4] Y. Wu, J. Zhang, L. Xiao, and F. Chen, "Properties of carbon and iron modified TiO2 photocatalyst synthesized at low temperature and photodegradation of acid orange 7 under visible light," Appl. Surf. Sci., vol. 2 56, pp. 4260-4268, 2010 . [5] X. Yang, "Photo-catalytic degradation of Rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradiation," Appl. Catal., B, vo l . 91, pp . 65 7-6 62, 2009. [6] B. Ahmmad, Y. Kusumoto, and M.S. Islam, "One-st ep and la rge scale synthesis of non-metal doped TiO2 submicrospheres and their photocatalytic activity," Adv. Powder Technol., vol. 21, pp. 292-297, 2010. [7] B. Tryba, "Increase of the Photocatalytic Activity of TiO2 by Carbon and Iron Modifications," Int. J. Photoenergy, vol. 2008, pp. 2008. [8] W.-X. Liu, J. Ma, X.-G. Qu, and W.-B. Cao, "Hydrothermal synthesis of (Fe, N) co-doped TiO2; powders and their photoca- talytic properties under visible light irradiation," Res. Chem. In- termed., vol. 35, pp. 321-328, 2009. [9] Z. Liu, Y. Wang, W. Chu, Z. Li, and C. Ge, "Characteristics of doped Ti O2 photocatalysts for the degradation of methylene blue waste water u nder visible light, " J. Alloys C ompd., vol. 501, pp. 54-59, 2010. [10] T. Ohno, Z. Miyamoto, K. Nishijima, H. Kanemitsu, and F. Xueyuan, "Sensitization of photocatalytic activity of S- or N-doped TiO2 particles by adsorbing Fe3+ cations," Appl. Catal., A, vol. 3 02, pp. 62-68, 2006. [11] X. Sun, H. Liu, J. Dong, J. Wei, and Y. Zhang, "Preparation and Characterization of Ce/N-Codoped TiO2 Particles for Production of H2 by Photocatalytic Splitting Water Under Visible Light," Cata l. Lett., vol. 135, pp. 219 -225, 2010. [12] C. Kaewtip, P. Chadpunyanun, and V. Boonamnuayvitaya, "Ef- fect of Co-Dopants in TiO2–SiO2 Thin films on the Formalde- hyde Degradation," Water Air Soil Pollut., vol. 223, pp. 1455-1465, 2012. [13] L. Ge, M. Xu, and H. Fang, "Synthesis of titanium oxide layers on glass subst rates with aq ueous refluxed sols (RS) and ph otoca- taly tic activities," J. Mate r . Sci ., vol. 42, pp. 492 6-4934, 2007. [14] M. Yao, J. Chen, C. Zhao, and Y. Chen, "Photocatalytic activi- ties of Ion doped TiO2 thin films when prepared on different sub st r ates," Thin Solid Films, vol. 517 , pp. 5994-5999, 2009. [15] X. Cheng, X. Yu, and Z. Xing, "One-step synthesis of Fe-N-S-tri-doped TiO2 catalyst and its enhanced visible light photocatalytic activity," Mater. Res. Bull., vol. pp. in press. [16] Y. Cong, J. Zhang, F. Chen, M. Anpo, and D. He, "Preparation,  C. KAE WTIP ET AL. Copyright © 2012 SciRes. AMPC 44 Photocatalytic Activity, and Mechanism of Nano-TiO 2 Co-Doped with Nitrogen and Iron (III)," J. Phys. Chem. C, vol. 111, pp. 10618-106 23, 2007. [17] T. Oyama, "Solar photocatalysis, photodegradation of a com- merci al detergen t in aqueous TiO2 dispersions under sunlight ir- radiation," Sol. Energy, in press. |

