Paper Menu >>
Journal Menu >>
 Advances in Materials Physics and Chemistry, 2012, 2, 9-12 doi:10.4236/ampc.2012.24B003 Published Online December 2012 (http://www.SciRP.org/journal/ampc) Antibacterial Activity of TiO2/Ti Composite Photocatalyst Films Treated by Ultrasonic Cleaning Yun Lu1, Lian Hao2, Yutaka Hiraka wa2, Hiromasa Sato2 1Graduate School & Faculty of Engineering, Chiba University, Yayoi-cho, Inage-ku, Chiba, Japan 2Graduate School, Chiba University, Yayoi-cho, Inage-ku, Chiba, Japan Email: luyun@faculty.chiba-u.jp, haoliang2005abcd@126.com Received 2012 ABSTRACT In this work, TiO2/Ti composite films were fabricated by 2-setp MCT and the following high temperature oxidation. Antibacterial activity of the composite films treated by ultrasonic cleaning to increase the performance reliability was examined. The prepared TiO2/Ti composite films showed high photocatalytic activity in the degradation of methylene blue solution. It is obvious that TiO2/Ti composite films have antibacterial activity under UV irradiation. Keywords: Mechanical Coating Technique; TiO2/Ti Composite Film; Photocatatlyst; Antibacterial Activity 1. Introduction In recent years, TiO2 photocatalysts have showed a high poten- tial in the environmental and energy fields, including self- cleaning Surfaces, air and water purification systems, steriliza- tion, hydrogen evolution and photoelectrochemical conversion, among others [1-3]. To lower the recycling cost and increase the degradation efficiency of pollutants, investigations of TiO2 photocatalysts are oriented toward the immobilization in the form of films [4,5]. Numerous techniques including physical vapor deposition (PVD), chemical vapor deposition (CVD), and sol-gel method, among others have been used to fabricate TiO2 photocatalyst films to increase their photocatalytic activity [6-8]. However, some disadvantages limit the applications of these techniques. For example, complicated and large scale equip- ments are required and their processes can be operated only in vacuum for PVD and CVD. In addition, the production cost is relatively high. In this condition, we developed ball milling and proposed a novel coating technique called mechanical coating technique (MCT) to fabricate TiO2 photocatalyst films on alumina (Al2O3) balls [9-12]. In MCT, collision, friction and abrasion among Ti powder, alumina balls and the inner wall of the bowl are util- ized effectively to form Ti films on alumina balls. After that, TiO2 films or TiO2/Ti composite films were fabricated by the following high-temperature oxidation. Although the TiO2 re- sultants had rutile crystal type, they showed relatively high photocatalytic activity [11]. Further, we developed 2-step MCT based on the concept of MCT to prepare TiO2/Ti composite films by coating nano-TiO2 powder particles on the Ti films without oxidation process [9,12]. The TiO2/Ti composite films showed high photocatalytic activity in the degradation of me- thylene blue solution. It is expected that the TiO2/Ti composite films on alumina balls are applied in the environmental and energy fields. In this work, TiO2/Ti composite films on alumna balls were fabricated by 2-step MCT and the following high temperature oxidation. Ultrasonic cleaning was performed for the alumina balls with the TiO2/Ti composite films to increase the perform- ance reliability. Photocatalytic activity and antibacterial activity of the TiO2/Ti composite films were evaluated and discussed. 2. Experimental 2.1. F abric ation of TiO2/Ti Composite Films First, Ti powder with an average diameter of 30 μm and a pu- rity of 99.1% was used as the coating material. Alumina (Al2O3) balls with an average diameter of 1 mm were used as the sub- strates. Ti powder and Al2O3 balls were charged into a bowl made of alumina with a dimension of Φ75 × 70 mm (250 ml in volume). Then the mechanical coating was carried out by a planetary ball mill (Pulverisette 6, Fritsch). The rotation speed of the ball mill was set at 300 rpm and the milling time was 10 h. During the fabrication, milling operation was performed 10 min followed by 2 min intermittence to avoid the overheating of the bowl and the contents. The schematic diagram of MCT can be found in our published work [9]. Secondly, TiO2/Ti composite films were fabricated. The Al2O3 balls coated with Ti films and anatase TiO2 nanopowder of 7 nm in average diameter (ST-01, purity: 99.99 %, Ishihara Sangyo) were used as the substrate and the coating material, respectively. The rotation speed of planetary ball mill was 300 rpm. MCT was carried out for 3 h. Planetary ball mill (Pulver- isette 6, Fritsch) was also employed for MCT in the second step. Further, to enhance the photocatalytic activity and, the alu- mina balls with TiO2/Ti composite films were heat-treated at 773 K in air for 10 h. Subsequently, all the samples were cleaned in acetone by ultrasonic (frequency: 28 kHz) for 1.5 h to remove the unstrong adhesions on the surface of the samples to increase the performance reliability. 2.2. Characteri zatio n of TiO 2/Ti Composite Films and Evaluation of Phot o catalytic Activity Copyright © 2012 SciRes. AMPC 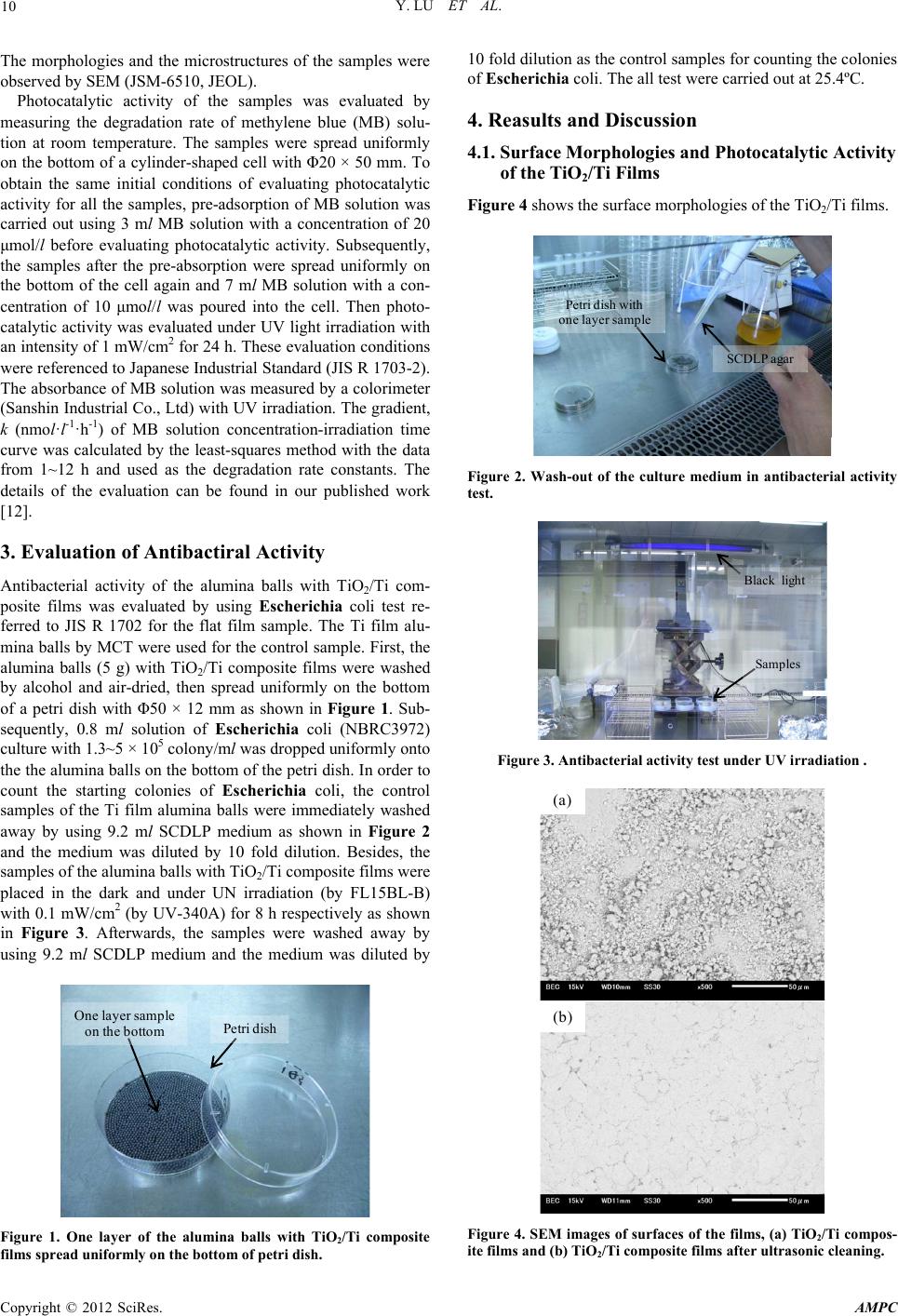 Y. LU ET AL. 10 The morphologies and the microstructures of the samples were observed by SEM (JSM-6510, JEOL). Photocatalytic activity of the samples was evaluated by measuring the degradation rate of methylene blue (MB) solu- tion at room temperature. The samples were spread uniformly on the bottom of a cylinder-shaped cell with Φ20 × 50 mm. To obtain the same initial conditions of evaluating photocatalytic activity for all the samples, pre-adsorption of MB solution was carried out using 3 ml MB solution with a concentration of 20 μmol/l before evaluating photocatalytic activity. Subsequently, the samples after the pre-absorption were spread uniformly on the bottom of the cell again and 7 ml MB solution with a con- centration of 10 μmol/l was poured into the cell. Then photo- catalytic activity was evaluated under UV light irradiation with an intensity of 1 mW/cm2 for 24 h. These evaluation conditions were referenced to Japanese Industrial Standard (JIS R 1703-2). The absorbance of MB solution was measured by a colorimeter (Sanshin Industrial Co., Ltd) with UV irradiation. The gradient, k (nmol·l-1·h-1) of MB solution concentration-irradiation time curve was calculated by the least-squares method with the data from 1~12 h and used as the degradation rate constants. The details of the evaluation can be found in our published work [12]. 3. Evaluation of Antibactiral Activity Antibacterial activity of the alumina balls with TiO2/Ti com- posite films was evaluated by using Escherichia coli test re- ferred to JIS R 1702 for the flat film sample. The Ti film alu- mina balls by MCT were used for the control sample. First, the alumina balls (5 g) with TiO2/Ti composite films were washed by alcohol and air-dried, then spread uniformly on the bottom of a petri dish with Φ50 × 12 mm as shown in Figure 1. Sub- sequently, 0.8 ml solution of Escherichia coli (NBRC3972) culture with 1.3~5 × 105 colony/ml was dropped uniformly onto the the alumina balls on the bottom of the petri dish. In order to count the starting colonies of Escherichia coli, the control samples of the Ti film alumina balls were immediately washed away by using 9.2 ml SCDLP medium as shown in Figure 2 and the medium was diluted by 10 fold dilution. Besides, the samples of the alumina balls with TiO2/Ti composite films were placed in the dark and under UN irradiation (by FL15BL-B) with 0.1 mW/cm2 (by UV-340A) for 8 h respectively as shown in Figure 3. Afterwards, the samples were washed away by using 9.2 ml SCDLP medium and the medium was diluted by Petri dish One layer sample on the bottom Figure 1. One layer of the alumina balls with TiO2/Ti composite films spread uniformly on the bottom of petri dish. 10 fold dilution as the control samples for counting the colonies of Escherichia coli. The all test were carried out at 25.4ºC. 4. Reasults and Discussion 4.1. Surface Mor phologies a nd Photoca taly tic Act ivit y of the TiO2/Ti Films Figure 4 shows the surface morphologies of the TiO2/Ti films. SCDLP agar Petri dish with one layer sample Figure 2. Wash-out of the culture medium in antibacterial activity test. Black light Samples Figure 3. Antibacterial activity test under UV irradiation . (a) (b) Figure 4. SEM images of surfaces of the films, (a) TiO2/Ti compos- ite films and (b) TiO2/Ti composite films after ultrasonic cleaning. Copyright © 2012 SciRes. AMPC 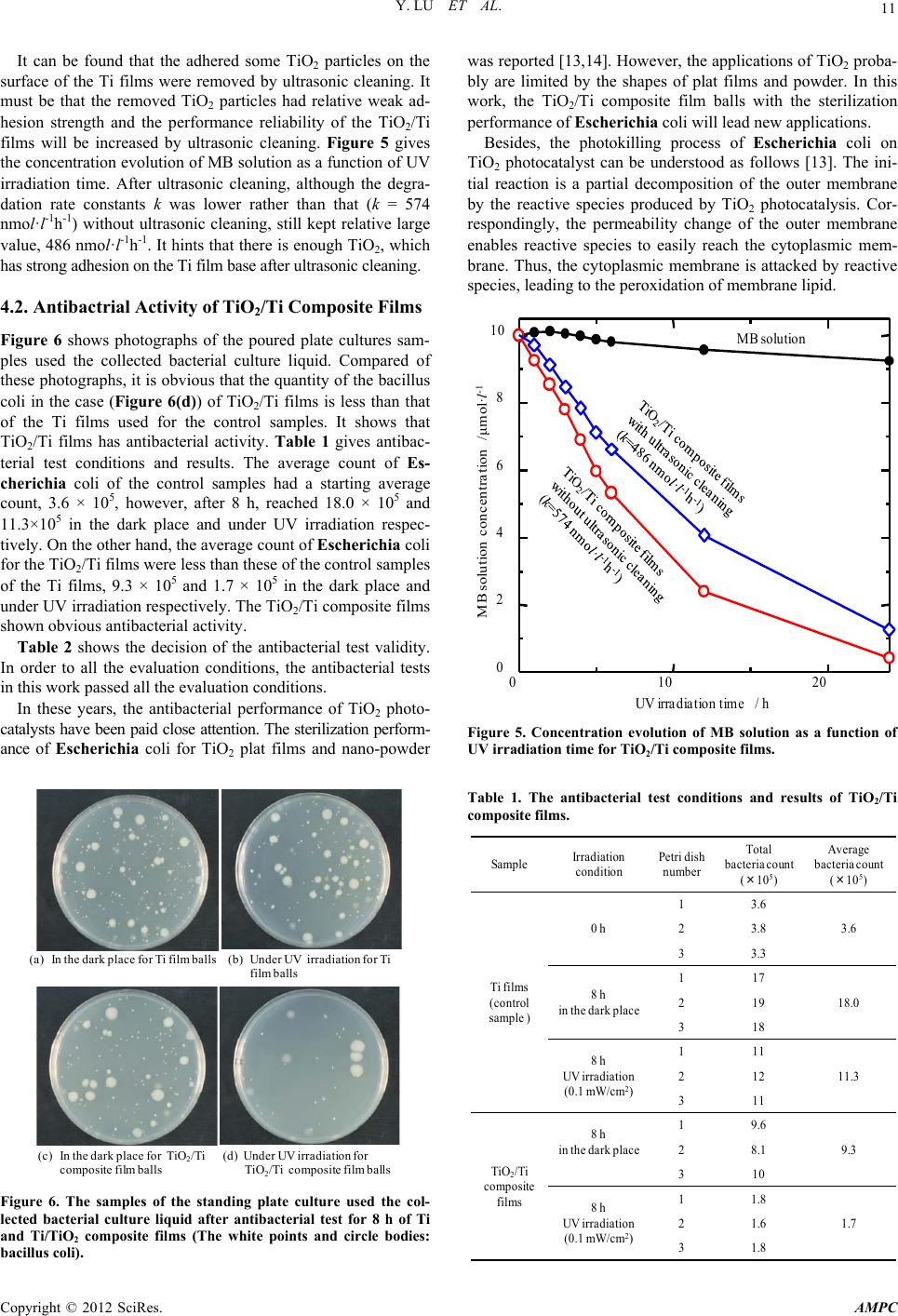 Y. LU ET AL. Copyright © 2012 SciRes. AMPC 11 was reported [13,14]. However, the applications of TiO2 proba- bly are limited by the shapes of plat films and powder. In this work, the TiO2/Ti composite film balls with the sterilization performance of Escherichia coli will lead new applications. It can be found that the adhered some TiO2 particles on the surface of the Ti films were removed by ultrasonic cleaning. It must be that the removed TiO2 particles had relative weak ad- hesion strength and the performance reliability of the TiO2/Ti films will be increased by ultrasonic cleaning. Figure 5 gives the concentration evolution of MB solution as a function of UV irradiation time. After ultrasonic cleaning, although the degra- dation rate constants k was lower rather than that (k = 574 nmol·l-1h-1) without ultrasonic cleaning, still kept relative large value, 486 nmol·l-1h-1. It hints that there is enough TiO2, which has strong adhesion on the Ti film base after ultrasonic cleaning. Besides, the photokilling process of Escherichia coli on TiO2 photocatalyst can be understood as follows [13]. The ini- tial reaction is a partial decomposition of the outer membrane by the reactive species produced by TiO2 photocatalysis. Cor- respondingly, the permeability change of the outer membrane enables reactive species to easily reach the cytoplasmic mem- brane. Thus, the cytoplasmic membrane is attacked by reactive species, leading to the peroxidation of membrane lipid. 4.2. Antiba ctrial Activi t y of T iO2/Ti Composite Films 01020 0 2 4 6 8 10 UV irradiation time / h MB solution concentration /μmol·l -1 MB solution Figure 6 shows photographs of the poured plate cultures sam- ples used the collected bacterial culture liquid. Compared of these photographs, it is obvious that the quantity of the bacillus coli in the case (Figure 6(d)) of TiO2/Ti films is less than that of the Ti films used for the control samples. It shows that TiO2/Ti films has antibacterial activity. Table 1 gives antibac- terial test conditions and results. The average count of Es- cherichia coli of the control samples had a starting average count, 3.6 × 105, however, after 8 h, reached 18.0 × 105 and 11.3×105 in the dark place and under UV irradiation respec- tively. On the other hand, the average count of Escherichia coli for the TiO2/Ti films were less than these of the control samples of the Ti films, 9.3 × 105 and 1.7 × 105 in the dark place and under UV irradiation respectively. The TiO2/Ti composite films shown obvious antibacterial activity. Table 2 shows the decision of the antibacterial test validity. In order to all the evaluation conditions, the antibacterial tests in this work passed all the evaluation conditions. In these years, the antibacterial performance of TiO2 photo- catalysts have been paid close attention. The sterilization perform- ance of Escherichia coli for TiO2 plat films and nano-powder Figure 5. Concentration evolution of MB solution as a function of UV irradiation time for TiO2/Ti composite films. Table 1. The antibacterial test conditions and results of TiO2/Ti composite films. (a)In the dark place for Ti film balls(b)Under UV irradiation for Ti film balls (d) Under UV irradiation for Ti O 2 /Ti composite film balls (c)In the dark place for TiO 2 /Ti composite film balls Sample Irradiation condition Petri dish number Total bacteriacount (×10 5 ) Average bacteria count (×10 5 ) Ti films (control sample ) 0 h 13.6 3.623.8 33.3 8 h in the dark place 117 18.0219 318 8 h UV irradiation (0.1 mW/cm 2 ) 111 11.3212 311 TiO 2 /Ti composite films 8 h in the dark place 19.6 9.328.1 310 8 h UV irradiation (0.1 mW/cm 2 ) 11.8 1.721.6 31.8 Figure 6. The samples of the standing plate culture used the col- lected bacterial culture liquid after antibacterial test for 8 h of Ti and Ti/TiO2 composite films (The white points and circle bodies: bacillus coli).  Y. LU ET AL. 12 5. Conclusions TiO2/Ti composite films on alumna balls were fabricated by 2-step MCT and the following high temperature oxidation. Ul- trasonic cleaning was performed for the alumina balls with the TiO2/Ti composite films to increase the performance reliability. Photocatalytic activity and antibacterial activity of the TiO2/Ti composite films were evaluated respectively. After ultrasonic cleaning, although the degradation rate constants k still kept high photocatalytic activity in the degradation of methylene blue solution. TiO2/Ti composite films has obvious antibacte- rial activity. 6. Acknowledgements The authors gratefully acknowledge JFE Techno-Research cor- poration of Japan for providing the antibacterial test. REFERENCES [1] T. Ochiai and A. Fujishima, “Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purifi- cation," J. Photochem. Photobiol. C: Photochem. Rev., 2012, in press. [2] K. Nakata, T. Ochiai, T. Murakami and A. Fujishima, "Pho- toenergy conversion with TiO2 photocatalysis: New materials and recent applications," Electrochim. Acta, 2012, in press. [3] M.N. Chong, B. Jin, C.W.K. Chow and C. Saint, "Recent devel- opments in photocatalytic water treatment technology: A re- view," Water Research, vol.44, pp.2997-3027, 2010. [4] I.M. Arabatzis, T. Stergiopoulos, D. Andreeva, S. Kitova, S.G. Neophytides, P. Falaras, "Characterization and photocatalytic ac- tivity of Au/TiO2 thin films for azo-dye degradation," J. Catal., vol.220, pp. 127-135, 2003. [5] M. Miyauchi, A. Nakajima, T. Watanabe and K. Hashimoto, " Photocatalysis and photoinduced hydrophilicity of various metal oxide thin films," Chemistry of Materials, vol.14, pp. 2812-2816, 2002. [6] C.C. Chen, W.J. Yang and C.Y. Hsu, "Fabrication of electron passes in nano-TiO2 layer by high-velocity oxy-fuel method for dye-sensitized solar Cells," Superlattices and Microstructure, vol.46, pp. 461-468 (2009). [7] A. Mills, N. Elliott, I.P. Parkin, S.A. O’Neill and R.J. Clark, "Novel TiO2 CVD films for semiconductor photocatalysis," J. Photochem. Photobi. A: Chemistry, vol.151, pp. 171-179, 2002. [8] C. Trapalis, N. Todorova, M. Anastasescu, C. Anastasescu, M. Stoica, M. Gartner, M. Zaharescu and T. Stoica, "Atomic force microscopy study of TiO2 sol–gel films thermally treated under NH3 atmosphere," Thin Solid Films, vol.517, pp. 6243-6247, 2009. [9] Y. Lu, H. Yoshida, H. Nakayama, L. Hao, M. Hirohashi, "For- mation of TiO2/Ti composite photocatalyst film by 2-step me- chanical coating technique," Materials Science Forum, vol. 675-677, pp. 1229-1232, 2011. [10] Y. Lu, L. Hao, K. Toh, H. Yoshida, "Fabrication of TiO2/Cu composite photocatalyst thin film by 2-step Mechanical Coat- ing Technique and its photocatalytic activity," Advanced Materi- als Research, vol.415-417, pp. 1942-1948, 2012. [11] H. Yoshida, Y. Lu, H. Nakayama, M. Hirohashi, "Fabrication of TiO2 film mechanical coating technique and its photocatalytic activity," Journal of Alloys and Compounds, vol.475, pp. 383-386, 2009. [12] L. Hao, Y. Lu, H. Asanuma, J. Guo, "The influence of the proc- essing parameters on the formation of iron thin films on alu- mina balls by mechanical coating technique," Journal of Materi- als Processing Technology, vol.212, pp. 1169-1176, 2012. [13] K. Sunada, T. Watanabe and K. Hashimoto, "Studies on photo- killing of bacteria on TiO2 thin film," J. Photochem. Photobio. A: Chemistry, vol.156, pp.227-233, 2003. [14] K.J. Shieh, M. Li, Y.H. Lee, S. D. Sheu, Y.T. Liu and Y.C. Wang, "Antibacterial performance of photocatalyst thin film fab- ricated by defsction effect in visible light," Nanomedicine: Nonatechnology, Biology and Medicne, vol.2, pp.121-126, 2006. Copyright © 2012 SciRes. AMPC |

