Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 38-39 doi:10.4236/ampc.2012.24B011 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Synthesis and Characterization of Poly(1-methoxy-4-octyloxy)-Para-Phenylene Vinylen for Light-Emitting Diodes Application Piched Anuragudom Department of Chemistry, Faculty of Liberal Arts and Science, Kasetsart University, Kamphaeng, Nakhon Pathom, Thailand Email: faaspca@ku.ac.th Received 2012 ABSTRACT In this study, the conjugated polymer, poly(1-methoxy-4-octyloxy)-para-phenylene vinylene (MO-p-PPV) was synthes ized and ch a- racterized . M O-p-PPV was synth esi zed acco rd in g to Gilch po lymeri zatio n mechanis m by using 4-methoxyphenol as starting material in the p resence of potassi um tert-butoxide (1M in THF). The product was further purified by multiple precipitations in different sol- vents such as methanol, tetrahydrofuran, isopropyl alcohol and hexane. The final product was dried to afford MO-p-PPV as a red solid. The resulting polymer was completely soluble in common organic solvents. The structure of monomer and optical properties of polymer were chara cterized by p roton nuclear magneti c resonance (1H-NMR) spectroscopy, UV-vis spectroscopy, and fluorescence spectroscopy. The UV-vis spectrum showed absorption maxima for MO-p-PPV at 491 nm. Si milarly, flu orescen ce spectru m sho wed λma x e mission at 540 nm. Keywords: Component; Po ly( 1-Methoxy-4-Oc tylox y)-Para-Phenyl ene V in ylen; Gilch Polymerization; Light-Emitting Diodes 1. Introduction During the past decade, an explosive growth of activity in the area of organic electroluminescence has occurred in both acade- mia and industry [1]. As the potential base material in organic light-emitting diodes (OLEDs), conjugated polymers have been widely explored. For example, since the discovery of electro- luminescence in poly(p-phenylene vinylene) (PPV) [2,3], a wide variety of conjugated and semi-conjugated polymers have been used as the active emissive layer in OLED devices [4-7]. Polymer light-emitting diodes (PLEDs) are promising devices, especially for next generation active matrix displays. Solution deposition techniques, homogeneous large area thin films, re- duced manufacturing process complexity, low-cost, high lumi- nescence efficiency, large spectral range, and relatively simple device stru ctures are so me of the main r easons for an increased interest in polymer materials for LED’s [ ]. M. T. Bernius, M. Inbasekaran, J. O'Brien, and W. Wu, Adv. Mater., 12, 1737 (2000). We report here the preparation of poly(1-methoxy-4-octy- lox y) -para-phenylene vinylene (MO-p-PPV) for light emitting diodes application by using Gilch polymerization route. A typ- ical procedure for the synthesis was described in the Experi- mental sect ion. 2. Experiment 2.1. Materials 4-methoxyphenol, 1-bromooctane, Sodium bromide, parafor- maldehyde, potassium tert-butoxide (1 M solution in THF), KOH, glacial acetic acid, conc. H2SO4 were purchased from Aldrich Chemical Co. and used without further purification unless otherwise noted. THF was dried and purified by frac- tional distillation over sodium/benzophenone and handled in a moisture-fr ee atmosphere. 2.2. Measurements 1H and 13C NMR spectra were recorded using a Bruker avance 400 MHz, and chemical shifts were recorded in ppm. The data were processed using NUTS NMR Utility Transform Software (Acron NMR). The UV-vis spectra were recorded on a Perkin Elmer Lambda 19 UV-VIS -NIR spectrophotometer with base- line corrections and normalizations carried out using WinLab software. Fluorescence spectra were collected on a Perkin El- mer Luminescence S pectro meter LB 50. Methoxy-4-octyloxy benzene (1) 4-methoxyphenol (10.0 g, 0.083 mol) was dissolved by 100 ml ethanol, 6.0 g (0.12 mol) of KOH and octyl bromide (22.4 g, 0.12 mol) were added and strired at 70 oC for 24 h. After the reaction , precip itat e was collect ed b y filtrati on and wash ed with ethanol. White crystalline 1 (67% yield) was obtained. 1,4-Bis(bromomethyl)-methoxy-4-octyloxy benzene (2) Methoxy-4-octyloxy benzene (0.169 mol), Sodium bromide (0.097 mol), paraformaldehyde (0.166 mol) were dissolved in 24 ml of glacial acetic acid. 50% conc. H2SO4 in glacial acetic acid was added and the reaction was heated at 70oC for 24 h. Then saturated NaHCO3 was added until the red color disap- peared. The mixture was extracted three times with dichloro- methane. The organic extracts were combined, washed with brine, and dried with magnesium sulfate. Upon filtering the solution and evaporating the solvent, a white solid was obtained, which was recrystallized in hexane and washed with cool me- 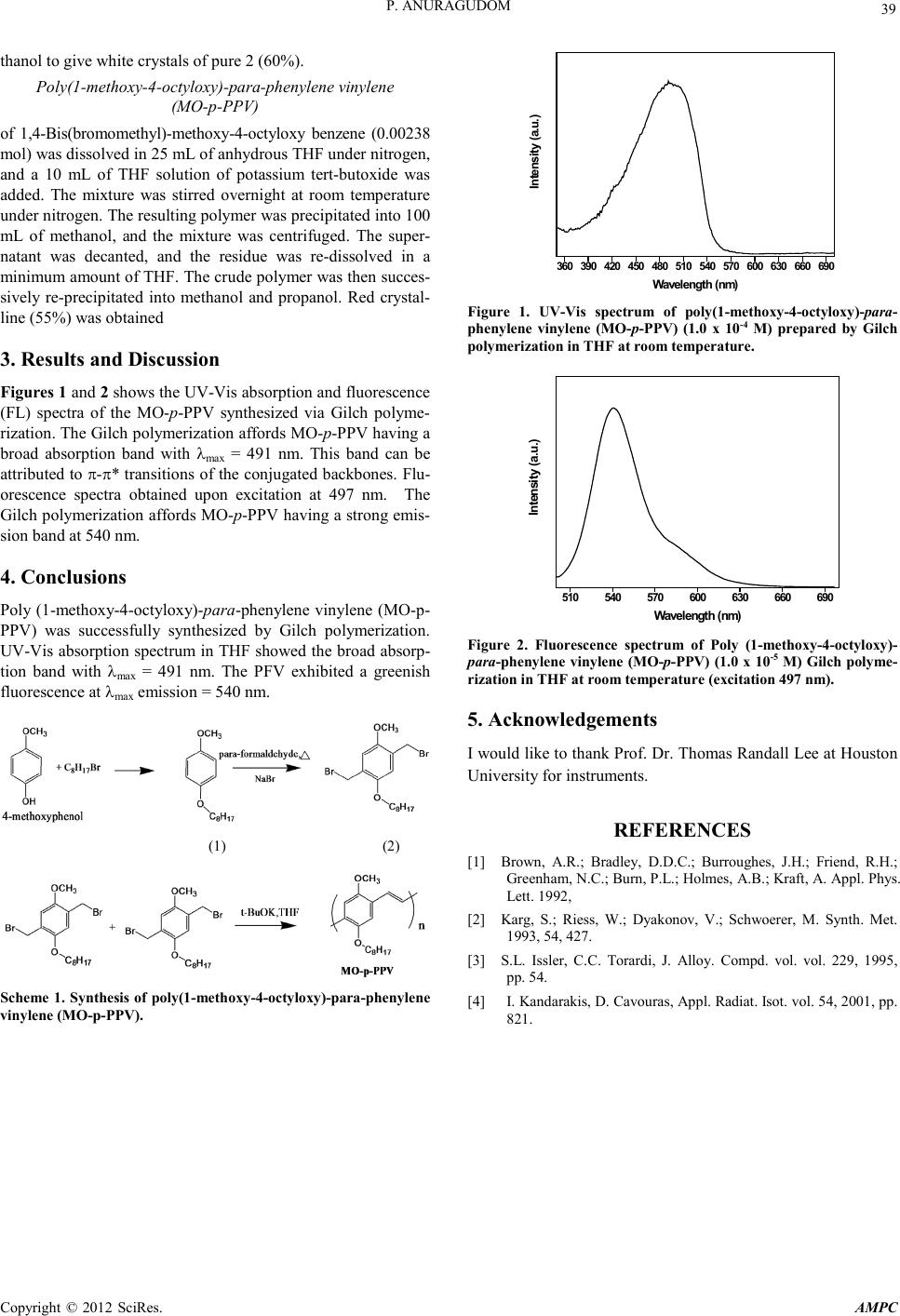 P. ANU RAGUDOM Copyright © 2012 SciRes. AMPC 39 thanol to give white crystals of pure 2 (60%). Poly(1-met hoxy-4-octyloxy)-par a-phen ylene vinylene (MO-p-PPV) of 1,4-Bis(bromomethyl)-methoxy-4-octyloxy benzene (0.00238 mol) was dissolved in 25 mL of anhydrous THF under nitrogen, and a 10 mL of THF solution of potassium tert-butoxide was added. The mixture was stirred overnight at room temperature under nitrogen. The resulting polymer was precipitated into 100 mL of methanol, and the mixture was centrifuged. The super- natant was decanted, and the residue was re-dissolved in a minimum amount of THF. The crude polymer was then succes- sively re-precipitated into methanol and propanol. Red crystal- line (55%) was obtained 3. Results and Discussion Figures 1 and 2 shows the UV-Vis absorption and fluorescen ce (FL) spectra of the MO-p-PPV synthesized via Gilch polyme- rization. The Gilch polymerization affords MO-p-PPV having a broad absorption band with λmax = 491 nm. This band can be attributed to π-π* transitions of the conjugated backbones. Flu- orescence spectra obtained upon excitation at 497 nm. The Gilch polymerization affords MO-p-PPV having a strong emis- sion band at 540 nm. 4. Conclusions Po ly (1 -methoxy-4-octyloxy)-para-phenylene vinylene (MO-p- PPV) was successfully synthesized by Gilch polymerization. UV-Vis absorption spectrum in THF showed the broad absorp- tion band with λmax = 491 nm. The PFV exhibited a greenish fluorescence at λmax emission = 540 nm. (1) (2) Scheme 1. Synthesis of poly(1-met hoxy-4-octyloxy)-para-phenylene vinylene (MO-p-PPV). 360 390 420 450 480 510 540 570600 630 660 690 Intensity (a.u.) W avelength (nm) Figure 1. UV-Vis spectrum of poly(1-met hox y-4-octyloxy)-para- phenylene vinylene (MO-p-PPV) (1.0 x 10-4 M) prepared by Gilch polymerization in THF at room temperature. 510 540 570 600 630 660 690 Intensity (a.u.) W avelength (nm) Figure 2. Fluorescence spectrum of Poly (1-me th oxy-4-octyloxy)- para-phenylene vinylene (MO-p-PPV) (1.0 x 10-5 M) Gilch polyme- rization in THF at room temperature (excitation 497 nm). 5. Acknowledgements I would like to thank Prof. Dr. Thomas Randall Lee at Houston University for instruments. REFERENCES [1] Brown, A.R.; Bradley, D.D.C.; Burroughes, J.H.; Friend, R.H.; Greenham, N.C.; Burn, P.L.; Holmes, A.B.; Kraft, A. Appl. Phys. Lett. 1992, [2] Karg, S.; Riess, W.; Dyakonov, V.; Schwoerer, M. Synth. Met. 1993, 54, 427. [3] S.L. Issler, C.C. Torardi, J. Alloy. Compd. vol. vol. 229, 1995, pp. 54. [4] I. Kandarakis, D. Cavouras, Appl. Radiat. Isot. vol. 54, 2001, pp. 821. |

