Paper Menu >>
Journal Menu >>
 Advances in Materials Physics and Chemistry,2012, 2,25-27 doi:10.4236/ampc.2012.24B007 PublishedOnline December 2012 (http://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes.AMPC Thermoelectrical Investigation of RareEarth Sulfide Materials V. V. Sokolov1,V.V.Bakovetz 1,S.M.Luguev 2,N.V.Lugueva 2 1NikolaevInstitute of Inorganic Chemistry SB RAS, NIIC SBRAS, Novosibirsk, Russia 2AmirkhanovInstituteof Physic DSC RAS,IPh DSC RAS, Makhachkala, Dagestan. Russia Email:sokolov@niic.nsc.ru, luguev.if@mail.ru Received 2012 ABSTRACT Results are presented on synthesis and crystal growthof Gd2S3-Dy 2S3solid solution sulfides and study of their thermoelectric properties in the range of temperatures 80-400 K. Gd0.2Dy0.8S1.48 composition has the best values ofthermoelectric efficiency 0.39 x 10-3/K at 400 K. Keywords:Gd2S3 -Dy2S3 SolidSolutionSulfides;Synthesis; Growth of Crystals; Thermoelectric Properties 1. Introduction Interest in investigation of rare-earth Ln2S3 sulfides with the structure ofThorium phosphide isbound with possible applica- tion of compositions on their base as working elements of thermoelectric energy convertersfor high temperatures. The researches of GdSy (1.45≤y≤1.50) [1,2]showed that some compositions at 1000 K havemore high thermoelectric efficiency ofZ then Ge-Sisolid solution compositions. The structure of Ln2S3 sulfides allows to fill vacancy with rare- earth andother metals and to change their thermoelectric prop- erties.Toincreasethermoelectric efficiency ofmaterial itis usual todecrease its thermalconductivity creatingthere addi- tional centers of phonon scattering. Such centers for the GdSy system may bepresented bythe dopingparamagnetic ionsof rare-earth elements scattering phonons and decreasing thermal conductivity but not modifying electrical characteristicsof the compound. The research ofGd1-xDyxS1.48 compositions con- tainingparamagnetic Dy ions showed thatin thissystem near Gd0.2Dy0.8S1.48 havethe minimumthermal conductivity[3]. Therefore is of interest to study dynamics of thermoelectric properties ofthese compositions independence from tempera- ture. In this work the synthesis, preparation ofcrystals in Gd2S3 - Dy2S3 system and their thermoelectricproperties in the range of temperatures 80-400 K are presented. 2. Experimental 2.1. Preparation o f Gd2S3 and D y 2S3 Sulfides SulfidesGd2S3and Dy2S3werepreparedfromhigh-purity rare earthoxides (99.95%)by H2Ssulfidizingat 950– 10000C [4]. The mixtures of sulfides forcrystallization were prepared with 0.1 mol. step. 2.2. GrowthofGd2S3 - Dy2S3 SolidSolution Crystals Directedcrystallization fromsulfide melts at 1700-20000Cin carbon and glass-carbon containers with HF heating was used. Crystallization of prepared compositions was carried out under inert gas atmosphere for preparation of nonstoichiometric with electronconductivity crystals [5]. Crystallization velocity was 5 - 30 mm/h.The diameter of the obtainedcylindrical samples of crystals was 10 mm and height - 1030 mm. 2.3. Methods ofCharacterization of Crystals -XRD- parametersofcubicTh3P4type - Gravimetric measurements of density - Measurements of Seebeck coefficientat 200C - Chemical and gas-chromatografic analysis of composition [6] 2.4. Study of Thermoelectrical Properties To study thermoelectricalproperties the samples werecut from the central part of ingots that was the most uniform. At temperature measurements the composition changeon sulfur therefore uncertainty in an index at sulfur+-0.01 is possible. Measurements of thermal conductivity coefficient were performed with absolute stationary method. The equipment simultaneously with measurementof in the same samplesallowedto measureelectricalconductivity and Seebeck coefficient at 80-400 K. 3. Results and Discussion 3.1. Characterization o f Prepared Cr y stals Results of characterization of prepared crystals of Gd2S3- Dy2S3solid solution is presented inTable 1. All crystalshavecubicstructure ofTh3P4typewithlinear dependence of cell parameters from composition. The same dependence is in density of crystals from composition. Deviationof compositionfrom stoichiometry from Gd2S3to Dy2S3agree with their phase diagrams. Increasing ofSeebeck coefficient fromGd2S3to Dy2S3to 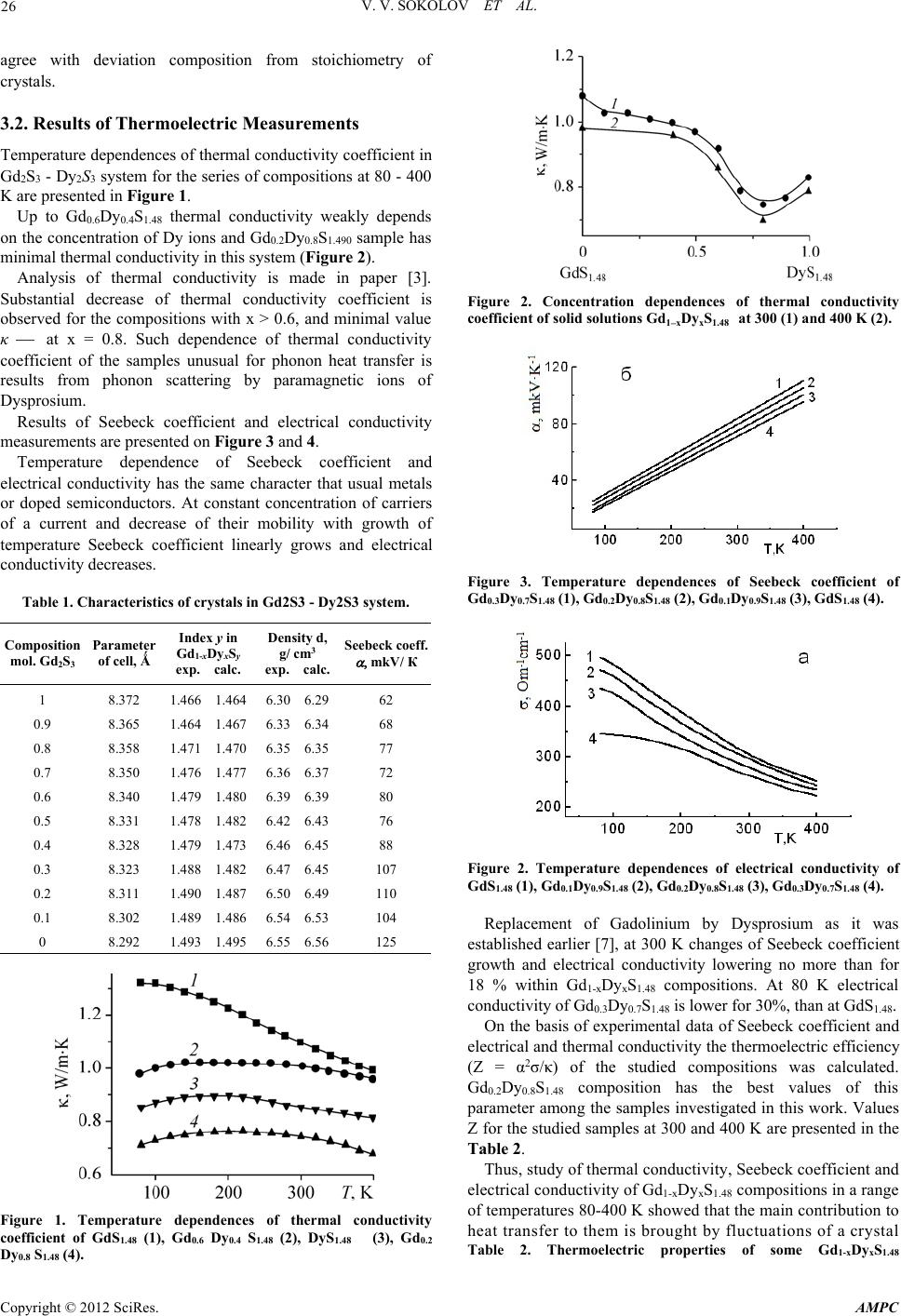 V. V. SOKOLOVET AL. Copyright © 2012 SciRes.AMPC 26 agree withdeviation composition from stoichiometry of crystals. 3.2. Results of Thermoelectric Measurements Temperature dependencesof thermal conductivity coefficient in Gd2S3-Dy 2S3system for the seriesof compositions at80 -400 K are presented in Figure 1. Up to Gd0.6Dy0.4S1.48thermal conductivity weakly depends on the concentration of Dy ions and Gd0.2Dy0.8S1.490sample has minimal thermalconductivity in this system (Figure 2). Analysis of thermal conductivity is made in paper [3]. Substantial decrease of thermal conductivity coefficient is observed for the compositions with x >0.6, and minimal value κ at x = 0.8.Suchdependence ofthermal conductivity coefficientof thesamplesunusual forphononheattransfer is results from phonon scattering by paramagneticions of Dysprosium. Results of Seebeck coefficient and electrical conductivity measurements are presented on Figure 3 and 4. Temperature dependence of Seebeck coefficient and electrical conductivity has the same character that usual metals or dopedsemiconductors.At constantconcentration ofcarriers of acurrentanddecreaseoftheirmobilitywith growthof temperature Seebeck coefficient linearly growsand electrical conductivity decreases. Table 1.Characteristics of crystals in Gd2S3- Dy2S3system. Composition mol. Gd2S3 Parameter of cell, Ǻ Indexyin Gd1-xDyxSy exp. calc. Density d, g/ cm3 exp. calc. Seebeck coeff. mkV/ К 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 8.372 8.365 8.358 8.350 8.340 8.331 8.328 8.323 8.311 8.302 8.292 1.466 1.464 1.464 1.467 1.471 1.470 1.476 1.477 1.479 1.480 1.478 1.482 1.479 1.473 1.488 1.482 1.490 1.487 1.489 1.486 1.493 1.495 6.30 6.29 6.33 6.34 6.35 6.35 6.36 6.37 6.39 6.39 6.42 6.43 6.46 6.45 6.47 6.45 6.50 6.49 6.54 6.53 6.55 6.56 62 68 77 72 80 76 88 107 110 104 125 Figure 1. Temperature dependences of thermal conductivity coefficient of GdS1.48 (1), Gd0.6 Dy0.4 S1.48 (2), DyS1.48 (3), Gd0.2 Dy0.8 S1.48 (4). Figure 2. Concentration dependencesof thermalconductivity coefficient of solid solutions Gd1xDyxS1.48 at 300(1) and400 K (2). Figure 3.Temperature dependences ofSeebeck coefficient of Gd0.3Dy0.7S1.48 (1), Gd0.2Dy0.8S1.48 (2), Gd0.1Dy0.9S1.48 (3), GdS1.48 (4). Figure 2. Temperature dependences ofelectrical conductivity of GdS1.48 (1), Gd0.1Dy0.9S1.48 (2), Gd0.2Dy0.8S1.48 (3), Gd0.3Dy0.7S1.48 (4). Replacement of Gadolinium by Dysprosium asit was established earlier [7], at 300 K changes of Seebeck coefficient growth and electrical conductivity lowering no more than for 18 % within Gd1-xDyxS1.48compositions. At 80 K electrical conductivity of Gd0.3Dy0.7S1.48is lower for 30%, than at GdS1.48. On the basisof experimental dataof Seebeck coefficientand electrical and thermalconductivity thethermoelectric efficiency (Z = α2σ/κ) ofthe studied compositionswas calculated. Gd0.2Dy0.8S1.48 composition has the best values of this parameteramong thesamples investigatedin thiswork. Values Z forthe studiedsamplesat 300and 400K arepresentedin the Table 2. Thus, studyofthermalconductivity,Seebeck coefficient and electricalconductivity of Gd1-xDyxS1.48compositions inarange of temperatures80-400Kshowed thatthemain contributionto heat transfer tothem isbrought byfluctuations of acrystal Table 2. Thermoelectric properties of some Gd1-xDyxS1.48  V. V. SOKOLOVET AL. Copyright © 2012 SciRes.AMPC 27 composition. Composition -, mkV·K-1 300 К , Om-1cm-1 300 K , Wm -1K-1 300 K Z·103,K -1 300КZ·103,K -1 400K GdS1.48 723041.040.16 0.27 Gd0.3Dy0.7S1.48 832620.800.23 0.34 Gd0.2Dy0.8S1.48 802780.740.24 0.39 Gd0.1Dy0.9S1.48 752980.840.20 0.32 lattice.It is established that replacement of atoms of a Gadolinium byDysprosium reducesthermal conductivity with growth of deficiency of a crystals and additional scattering of phonon on paramagnetic Dy ions. On the basis of experimental datainallstudied temperatureintervalthe Gd0.2Dy0.8S1.48 sample has maximal value of thermoelectric efficiency. REFERENCES [1]G.G. Gadzhiev, S.M.Luguev,V.V. Sokolov, B.N. Magdiev., N.V. Lugueva.Inbook.:Transfer ofcarriers ofa chargeand heat in semiconductors. Makhachkala, 1986,page 87-93.(on Russian) [2]S.M. Luguev, V.V. Sokolov, N.V. Lugueva. Advanced Materials and Proceessing.Proceedingsof Russia-JapanSeminar, September 15-20, 2007. Novosibirsk, 2007, р.71-75. [3]S.M. Luguev, N.V. Lugueva, V.V. Sokolov. Temperature and concentration dependences of thermal conductivity of solid solutions of gadolinium anddysprosium//Thermophysics and Aeromechanics, vol. 19,No. 3,pp. 375-380, 2012. [4]V.V. Sokolov, A.A. Kamarzin, L.N.Trushnikova,M.V. Savelyeva Optical materials containing rare earth Ln2S3sulfides // J. Alloysand Comp.vol.225, No. 2,.pp. 567570, 1995. [5]A.A.Kamarzin,K.E.Mironov,V.V. SokolovGrowthand Properties of Lanthanum and Rare Earth Metal Sesquisulfide Crystals// J.CrystalGrowth. vol.52, pp.619 –622, 1981. [6]L.S. Chuchalina, I.G. Vasilyeva, A.A. Kamarzin Non-direc t method ofgas-chromatografic analysisof determination of lanthanum sulfide composition//Journal of Analytical Chemistry vol. 33,n. 1, pp.190-192,1978. (on Russian) [7]S.M. Luguev,N.V. Lugueva, V.V. Sokolov. Thermoelectricsand their applications. Reports of theX-th interstateseminar. , Ioffe PTI, pp.179-183,2007. (onRussian) |

