Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mist ry, 2012, 2, 21-24 doi:10.4236/ampc.2012.24B006 Published Online December 2012 (htt p://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Fluoride Process ing of Titaniu m-Containing Minerals N. M. Laptash , I. G. Maslennikova Institute of Chemistry, Far Eastern Branch of RAS, Vladivostok, Russi a Email: laptash@ich.dvo.ru Received 2012 ABSTRACT Fluoride processing of natural ilmenite with the use of ammonium hydrogen difluoride (NH4HF2) as an effecti ve flu or in atin g agent is suggested. Chemistry, composition, structure, thermal and hydrolytic properties of fluorination products were investigated. Ammo- nium fluoro- and oxofluorotitanates are suitable for preparing of titanium dioxide as pigmentary product or as doped by nitrogen and fluorine. Keywords: Ilmenite; Fluorination Reactions; Ammonium Hydrogen Difluoride; Ammonium Fluoro- and Oxofluorometallates; Thermal Behavior; Hydrolysis; N-F-TiO2. 1. Introduction Titanium dioxide (TiO2) has long been at the center of photo- catalyst research due to its catalytic efficiency coupled with wide availability, biocompatibility, chemical stability, low cost, and safety toward both humans and the environment. It is much more effective as photocatalyst in the form of nanoparticles modified by doping with cations and anions [1,2]. The nitrogen and fluorine-doped titanium dioxide (N–F–TiO2) nan omaterials exhibit high photocatalytic activity for water-splitting and pho- todegradation of organic pollutants [3–8]. It was shown that co-doping with nitrogen and fluorine is advantageous for the reduction of defect formation and lowers the energy cost for the incorporation of nitrogen owing to the charge compensation effect between the donor (F) and acceptor (N) [9]. Multifunctional properties and vast applications of nano– TiO2 require its production in a mass scale. The large-quantity production of rutile nanorods from ilmenite sands was recently suggested [10,11]. Ilmenite (FeTiO3) is abundant feedstock for industrial production of TiO2. At pr esent, ilmenite is commonly used in industry for making white pigment via a sulfate or chlo- rine route having serious disadvantages, such as the treatments of byproducts in the former and the lack of raw rutile minerals in the latter. Fluoride processing of titanium-bearing minerals can serve as an alternative. Ammonium hydrogen difluoride (NH4HF2, solid, melting point is 126oC, boiling point is 240oC) was recogni zed as versati le fluorinating agent for recovering of titanium-containing raw materials [12,13]. It should be noted that foundation of ilmenite processing with ammonium hydro- gen difluoride was created by Svendsen as early as the thirties [14,15]. The suggested methods comprised fluorination with molten NH4HF2 followed by sublimation of fluoride titanium compound but the detailed chemistry was not completely un- derstood. Since the fluorination products are ammonium fluoro- or oxoflluorotitanates, it is reasonable to consider them as pre- cursors for the N–F–TiO2 obtaining. Indeed, the N–F–TiO 2 nanoparticles of anatase crystalline structure were recently prepared by a facile method of (NH4)2TiF6 pyrolysis [16]. The synthesis of N–F-codoped TiO2 powders with a homogenous anatase structure via a thermal decomposition of different ammonium oxofluorotitanate pre- cursors at 550oC was reported [17]. Uniform ammonium oxof- luorotitanate (NH4TiOF3) mesocrystals and their conversion to mesocr ystals of TiO2 were descr ibed [1 8-20]. Titanium oxyflu- oride TiOF2 was synthesized for obtaining of thermally stable TiO2 of high photocatalytic activity [21] and for its use as anode material for lithium-ion battery [22]. The synthesis of the above precursors from natural ilmenite and investigation of their physicochemical properties is the aim of presen t paper . 2. Fluorination of Ilmenite with NH4HF2 Interaction of ilmenite with NH4HF2 proceeds exothermally at room temperature under grinding the initial components [23]. Similar reactions when two solids interact under mechanical grinding with the formation of a new compound were being studied by Indian authors since 1982 [24]. One should concen- trate attention on nonstoichiometric composition of fluorinating products due to some OH– groups substituting for fluorine since water mol ecules are formed during fluor ination: FeTiO3 + (5 –0.5y)NH4HF2 = NH4H2xFeOxF3 + (NH4)3Ti(OH)yF7-y + (1–0.5y)NH3 + (3–x–y)H2O (x ≤ 0.3, y ≤ 0.4). (1) The main titanium fluoride product is a double salt isostruc- tural with (NH4)3TiF7 = (NH4)2TiF6∙NH4F which was isolated in a single crystal form from fluoride aqueous solution. Its crystal structure was determined. The parameters of tetragonal unit cell were changed under X-rays, the stable phase is cha- racterized by the following parameters: sp. gr.P4nc; a = 11.97, b = 11.68 Å; z = 8. One of the three independent Ti octahedra is diso rd ered so a ph ase tr ansi tion (P T) at abou t 280 K takes place. Fe(II) forms a cubic fluoroperovskite type structure and easily oxidized in air and in aqueous solution, and Fe(III) fluoride compound crystallizes in cubic fluoroelpasolite structure. Its octahedral single crystals of the (NH4)xFe(OН)3-xF2x (х = 2.70–2.85) composition were grown. Usually, natural ilmenite 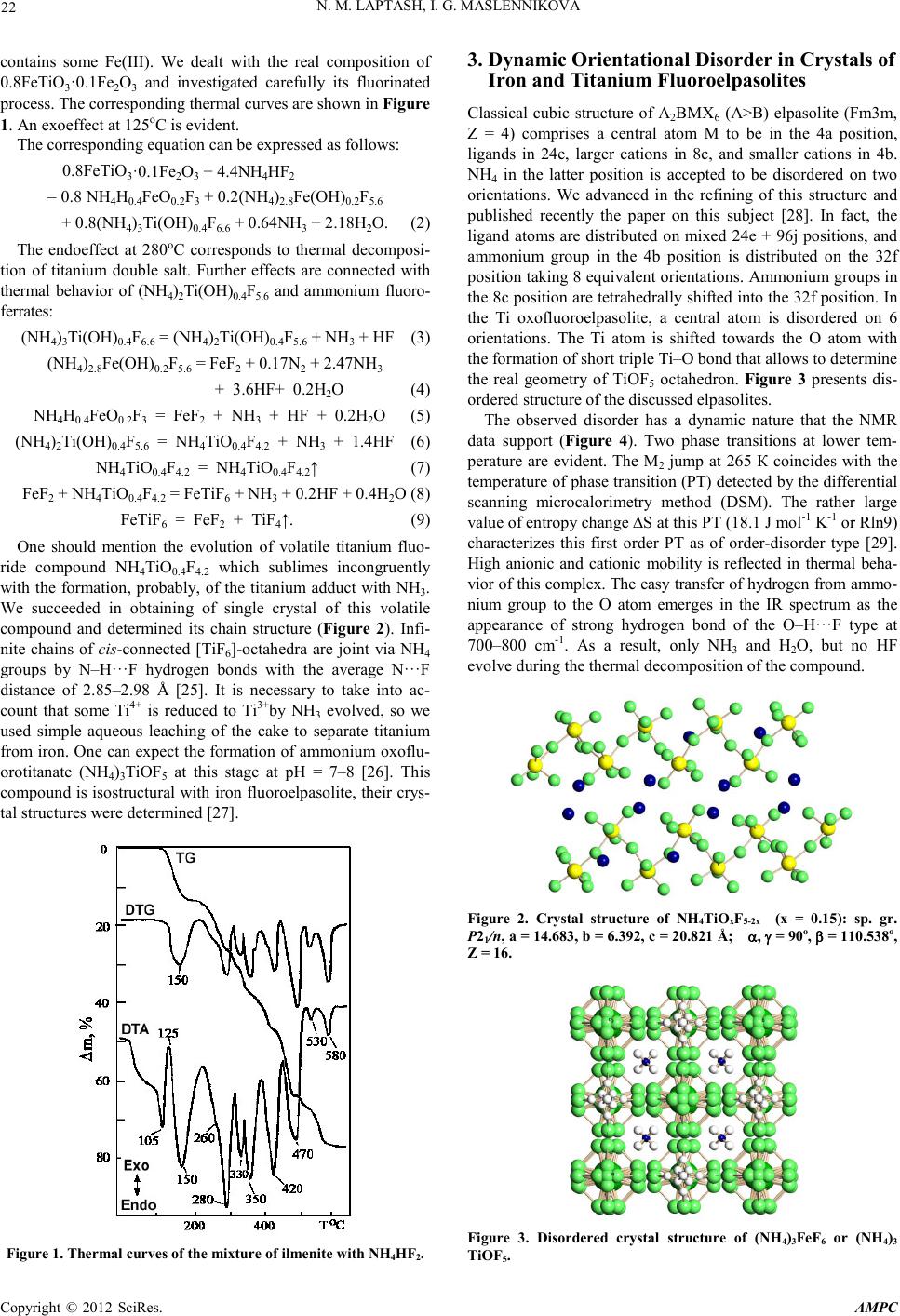 N. M. LAPTASH, I. G. M ASLENNIKOVA Copyright © 2012 SciRes. AMPC 22 contains some Fe(III). We dealt with the real composition of 0.8Fe TiO3·0.1Fe2O3 and investigated carefully its fluorinated process. The corresponding thermal curves are shown in Figure 1. An exoeffect at 125oC is evident. The corresponding equation can be expressed as follows: 0.8Fe TiO3·0.1Fe2O3 + 4.4NH4HF2 = 0.8 NH4H0.4FeO0.2F3 + 0.2(NH4)2.8Fe(OН)0.2F5.6 + 0.8(NH4)3Ti(OН)0.4F6.6 + 0.64NH3 + 2.18H2O. (2) The endoeffect at 280oC corresponds to thermal decomposi- tion of titanium double salt. Further effects are connected with thermal behavior of (NH4)2Ti(OН)0.4F5.6 and ammonium fluoro- ferrat es : (NH4)3Ti(O Н)0.4F6.6 = (NH4)2Ti(OН)0.4F5.6 + NH3 + HF (3) (NH4)2.8Fe(OН)0.2F5.6 = FeF2 + 0.17N2 + 2. 4 7N H 3 + 3.6HF+ 0.2H2O (4) NH4Н0.4FeO0.2F3 = FeF2 + NH3 + HF + 0.2H2O (5) (NH4)2Ti(O Н)0.4F5.6 = NH4TiO 0.4F4.2 + NH3 + 1.4HF (6 ) NH4TiO0.4F4.2 = NH4TiO 0.4F4.2↑ (7) FeF2 + NH4TiO0.4F4.2 = FeTiF6 + NH3 + 0.2HF + 0.4H2O (8) FeTiF6 = FeF2 + TiF4↑. (9) One should mention the evolution of volatile titanium fluo- ride compound NH4TiO0.4F4.2 which sublimes incongruently with the formation, probably, of the titanium adduct with NH3. We succeeded in obtaining of single crystal of this volatile compound and determined its chain structure (Figure 2). Infi- nite chains of cis-con nected [TiF6]-octahedra are joint via NH4 groups by N–H···F hydrogen bonds with the average N···F distance of 2.85–2.98 Å [25]. It is necessary to take into ac- count that some Ti4+ is reduced to Ti3+by NH3 evolved, so we used simple aqueous leaching of the cake to separate titanium from iron. One can expect the formation of ammonium oxoflu- orotitanate (NH4)3TiOF5 at this stage at pH = 7–8 [26]. This compound is isostructural with iron fluoroelpasolite, their crys- tal structures were d etermined [27]. Figure 1. Thermal curves of the mixture of ilmenite with NH4HF2. 3. Dynamic Orientational Disorder in Crystals of Iron and Titanium Fluoroelpasolites Classical cubic structure of A2BMX6 (A>B) elpasolite (Fm3m, Z = 4) comprises a central atom M to be in the 4a position, ligands in 24e, larger cations in 8c, and smaller cations in 4b. NH4 in the latter position is accepted to be disordered on two orientations. We advanced in the refining of this structure and published recently the paper on this subject [28]. In fact, the ligand atoms are distributed on mixed 24e + 96j positions, and ammonium group in the 4b position is distributed on the 32f position taking 8 equivalent orientations. Ammonium groups in the 8c position are tetrahedrally shifted into the 32f position. In the Ti oxofluoroelpasolite, a central atom is disordered on 6 orientations. The Ti atom is shifted towards the O atom with the formation of short triple Ti–O bond that allows to determine the real geometry of TiOF5 octahedron. Figure 3 presents dis- ordered structure of the discussed elpasolites. The observed disorder has a dynamic nature that the NMR data support (Figure 4). Two phase transitions at lower tem- perature are evident. The M2 jump at 265 К coincides with the temperature o f phase tran sition (PT) detect ed by the di fferential scanning microcalorimetry method (DSM). The rather large value of entropy change ∆S at this PT (18.1 J mol-1 K-1 or Rln9) characterizes this first order PT as of order-disorder type [29]. High anionic and cationic mobility is reflected in thermal beha- vior of this complex. The easy transfer of hydrogen from ammo- nium group to the O atom emerges in the IR spectrum as the appearance of strong hydrogen bond of the O–H···F type at 700–800 cm-1. As a result, only NH3 and H2O, but no HF evolve during the thermal decomposition of the compound. Figure 2. Crystal structure of NH4TiOxF5-2x (x = 0.15): sp. gr. P21/n, a = 14.683, b = 6.392, c = 20.821 Å; α, γ = 90 o, β = 110.538o, Z = 16. Figure 3. Disordered crystal structure of (NH4)3Fe F6 or (NH4)3 TiOF5. 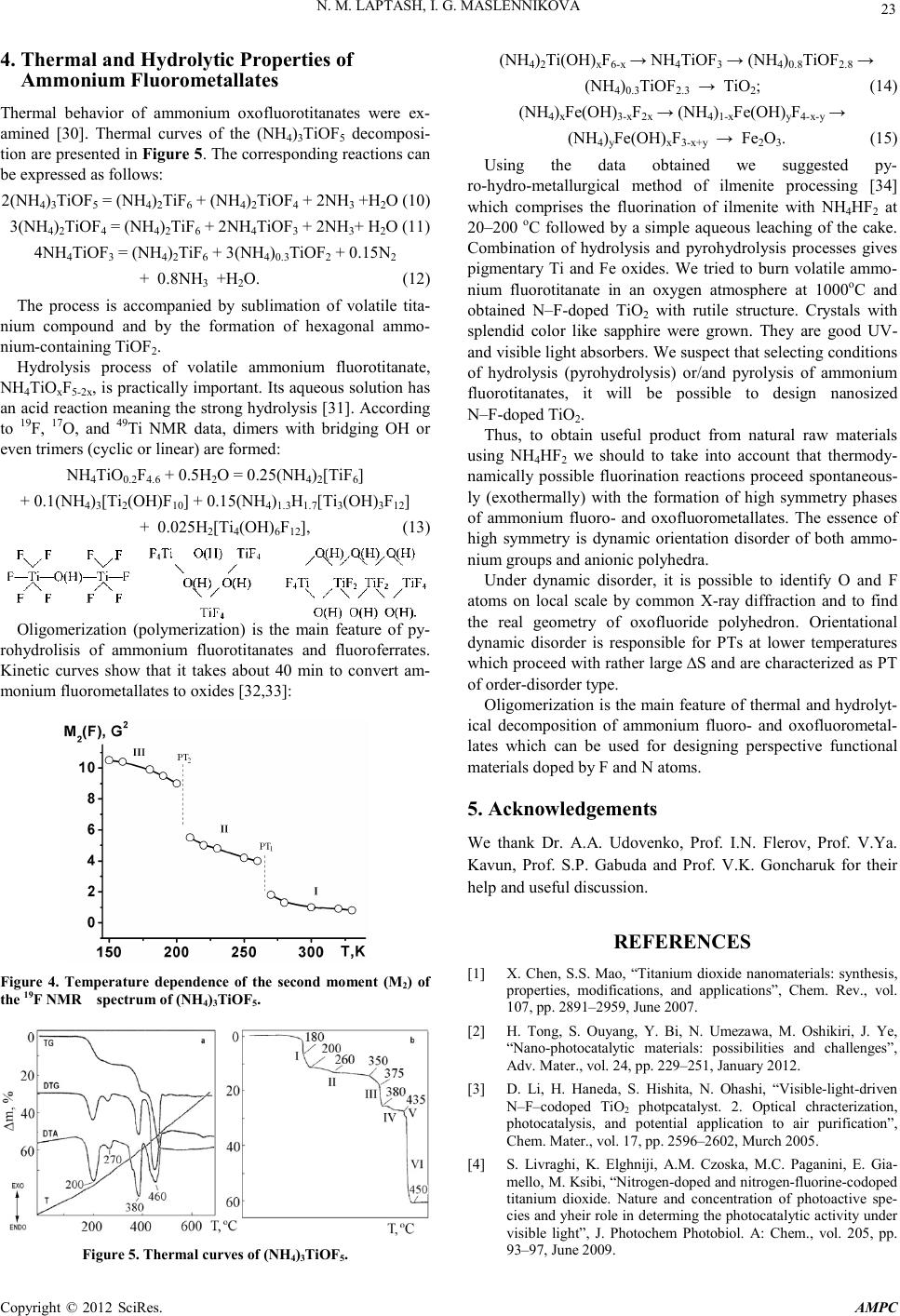 N. M. LAPTASH, I. G. M ASLENNIKOVA Copyright © 2012 SciRes. AMPC 23 4. Thermal and Hydrolytic Properties of Ammon iu m Fluo rometallates Thermal behavior of ammonium oxofluorotitanates were ex- amined [30]. Thermal curves of the (NH4)3TiOF5 decomposi- tion are presented in Figure 5. The corresponding reactions can be expressed as follows: 2(NH4)3TiO F5 = (NH4)2Ti F6 + (N H 4)2TiOF 4 + 2NH3 +H2O (10) 3(NH4)2TiO F4 = (NH4)2Ti F6 + 2NH4TiOF3 + 2NH3+ H2O (11) 4NH4TiO F3 = (NH4)2Ti F6 + 3( NH 4)0.3TiOF2 + 0.15N2 + 0.8NH3 +H2O. (12) The process is accompanied by sublimation of volatile tita- nium compound and by the formation of hexagonal ammo- nium-containing TiOF2. Hydrolysis process of volatile ammonium fluorotitanate, NH4TiOxF5-2x, is p ractically important. Its aqueous solution has an acid reaction meaning the strong hydrolysis [31]. According to 19F, 17O, and 49Ti NMR data, dimers with bridging OH or even trimers ( cyclic or lin ear) are formed: NH4TiO0.2F4.6 + 0.5H2O = 0.25(NH4)2[TiF6] + 0.1(NH4)3[Ti2(OH)F10] + 0.15(NH4)1.3H1.7[Ti3(OH)3F12] + 0.025H2[Ti4( OH) 6F12], (13) Oligomerization (polymerization) is the main feature of py- rohydrolisis of ammonium fluorotitanates and fluoroferrates. Kinetic curves show that it takes about 40 min to convert am- monium fluorometallates to oxides [32,33]: Figure 4. Temperature dependence of the second moment (M2) of the 19F NMR spectrum of (NH4)3TiOF 5. Figure 5. Thermal curves of (NH4)3TiOF5. (NH4)2Ti(OH)xF6-x → NH4TiOF3 → (NH4)0.8TiOF2.8 → (NH4)0.3TiOF2.3 → TiO2; (14) (NH4)xFe(OH)3-xF2x → (NH4)1-хF e(OH)yF4-х-y → (NH4)yFe(OH)xF3-x+y → Fe2О3. (15) Using the data obtained we suggested py- ro-hydro-metallurgical method of ilmenite processing [34] which comprises the fluorination of ilmenite with NH4HF2 at 20–200 oC followed by a simple aqueous leaching of the cake. Combination of hydrolysis and pyrohydrolysis processes gives pigmentary Ti and Fe oxides. We tried to burn volatile ammo- nium fluorotitanate in an oxygen atmosphere at 1000oC and obtained N–F-doped TiO2 with rutile structure. Crystals with splendid color like sapphire were grown. They are good UV- and visible light absorbers. We suspect th at sel ectin g condit ion s of hydrolysis (pyrohydrolysis) or/and pyrolysis of ammonium fluorotitanates, it will be possible to design nanosized N–F-doped TiO2. Thus, to obtain useful product from natural raw materials using NH4HF2 we should to take into account that thermody- namically possible fluorination reactions proceed spontaneous- ly (exothermally) with the formation of high symmetry phases of ammonium fluoro- and oxofluorometallates. The essence of high symmetry is dynamic orientation disorder of both ammo- nium groups and anionic polyhedra. Under dynamic disorder, it is possible to identify O and F atoms on local scale by common X-ray diffraction and to find the real geometry of oxofluoride polyhedron. Orientational dynamic disorder is responsible for PTs at lower temperatures which p roceed with rather large ∆S and are characterized as PT of order-disorder type. Oligomerization is the main feature of thermal and hydrolyt- ical decomposition of ammonium fluoro- and oxofluorometal- lates which can be used for designing perspective functional materials doped by F and N atoms. 5. Acknowledgements We thank Dr. A.A. Udovenko, Prof. I.N. Flerov, Prof. V.Ya. Kavun, Prof. S.P. Gabuda and Prof. V.K. Goncharuk for their help and useful discussion. REFERENCES [1] X. Chen, S.S. Mao, “Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications”, Chem. Rev., vol. 107, pp. 2891–2959 , June 2007. [2] H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri, J. Ye, “Nano-photocatalytic materials: possibilities and challenges”, Adv. Ma ter., vol. 24, pp. 229–251, January 2012. [3] D. Li, H. Haneda, S. Hishita, N. Ohashi, “Visible-light-driven N–F–codoped TiO2 photpcatalyst. 2. Optical chracterization, photocatalysis, and potential application to air purification”, Chem. Mater., vol. 17, pp. 2596–2602, Murch 2005. [4] S. Livraghi, K. Elghniji, A.M. Czoska, M.C. Paganini, E. Gia- mello, M. Ksibi, “Nitrogen-doped and n itrogen -fluori ne-codoped titanium dioxide. Nature and concentration of photoactive spe- cies and yheir role in determing the photocatalytic activity under visible light”, J. Photochem Photobiol. A: Chem., vol. 205, pp. 93–97 , Ju ne 2009.  N. M. LAPTASH, I. G. M ASLENNIKOVA Copyright © 2012 SciRes. AMPC 24 [5] X. Du, J. He, Y. Zhao, “Facile preparation of F and N codoped pinecone-like titania hollow microparticals with visible light photocatalytic activity”, J. Phys. Chem., vol. 113, pp. 14151–14158, August 2009. [6] G.S. Wu, J.L. Wen, S. Nigro, A.C. Chen, “One-step synthesis of N- and F-codop ed mesoporou s TiO 2 photocatalyst with high vis- ible light activity”, Nanotechnology, vol. 21, No. 085701, Feb- ruary 2010. [7] Y. Lv, Z. Fu, B . Yang, J. Xu , M. Wu, C. Zhu, Y. Zh ao, “ Prep a- ration N–F-codoped TiO2 nanorod array by liquid phase deposi- tion as visible light photocatalyst”, Mater. Res. Bull., vol. 46, pp. 361–365, March 2011. [8] W. Wang, C. Lu, Y. Ni, M. Su, W. Huang, Z. Xu, “Preparation and characterization of visible-light-driven N–F–Ta tri-doped TiO2 photocatalyst”, Appl. Surf. Sci., vol. 258, pp. 8696–8703, September 2012. [9] C. Di Valentin, E. Finazzi, G. Pacchioni, A. Selloni, S. Livraghi, A.M. Czoska, M.C. Paganini, E. Giamello, “Density functional theory and electron paramagnetic resonance study on the effect of N–F codoping of TiO2”, Chem. Mater., vol. 20, pp. 3706–37 14, June 2008. [10] J. Yu, Y. Chen, A.M. Glushenkov, “Titanium oxide nanorods extra cted from i lmenit e sands ”, Cryst . Growth @ Desi gn, vo l. 9, pp. 1240–1244, February 2009. [11] T. Tao, A.M. Glushenkov, Q. Chen , H. Hu, D. Zhou, H. Zhang, M. Boese, S. Liu, R. Amal,Y. Chen, “Porous TiO2 with a con- trollable bimodal pore size distribution from natural ilmenite”, Cryst. Eng. Com., vol. 13, pp. 1322–1327, March 2011. [12] G. L. He r wig, “Up g ra d in g tit an i fero u s ores” , Pat . AU No. 428 758, June 1972. [13] A.R. McGregor, A. Rodionov, “F–treatment of titanium mate- rials”, Pat. AU No. 2005100939 , Februa r y 2006. [14] S.S. Svendsen, “Manufacture of titanium compounds”, Pat. US No. 2042434, June1931. [15] S.S. Svendsen, “Treatment of titanium-bearing materials”, Pat. US No. 2042435, Sep tember 1934. [16] D.M. Chen, Z.Y. Jiang, J.Q. Geng, J.H. Zhu, D. Yang, “A facile method to synthesize nitrogen and fluorine co-doped TiO2 nanoparticles by pyrolysis of (NH4)2TiF6”, J . Nan opa rt . R es . , vol. 11, pp. 303–313, February 2009. [17] M. Hojamberdiev, G.Q. Zhu, P. Sujaridworakun, S. Jinawath, P. Liu, J.P. Zhou, “Visible-light-driven N-F-codoped TiO2 powders from different ammonium oxofluoroti tanate precursors ”, Powder Te c hno logy, vol. 218, p p. 1 40 –148, Ma r c h 2 012. [18] L. Zhou, D. Smyth-Boyle, P. O’Brien, “A facile synthesis of uniform NH4TiO F3 mesocrystals and their conversion to TiO2 mesocrystals”, J. Am. Chem. Soc., vol. 130, pp. 1309–1320, January 2008. [19] L. Zhou, P. O’Brien, “Ammonium oxofluorotitanate: morpholo- gy control and conversion to anatase TiOF2 mesocrystals and their conversion to TiO2”, Phys. Status Solidy A – Appl. Mater Sci., vol. 205, pp. 2317–2323, October 2008. [20] M-K. Lee, T-H. Shih, “Growth evolution of ammonium oxotrif- luorotitanate discoid crystal on glass prepared by ammonium hexafluorotitanate and boric acid”, J. Phys. D: Appl. Phys., vol. 43, No. 025402, January 2010. [21] K.L. Lv, J.G. Yu, L.Z. Cui, S.L. Chen, M. Li, “Preparation of thermally sdtable antase TiO2 photocatalyst from TiOF2 precur- sor and its photocatalytic activity”, J. Alloys Compounds, vol. 509, pp. 4557–4562, March 2011. [22] L. Chen, L.F. Shen, P. Nie, X.G. Zhang, H.S. Li, “Facile hydro- thermal synthesis of single crystalline TiOF2 nanocubes and their phase transitions to TiO2 hollow nanocages as anode materials for lithium-ion battwery”, Electrochim. Acta, vol. 62, pp. 408–415, February 2012. [23] N.M. Laptash, I.G. Maslennikova, L.N. Kurilenko, N.M. Mish- chenko, “Ilmenite fluorination by ammonium hydrodifluoride. New ammonium oxofluorotitanate”, Russ. J. Inorg. Chem., vol. 46, pp. 28–34, January 2001 (Translated from Zh. Neorgan. Khim., vol. 46, pp. 33–39). [24] B.R. Wani, U.R.K. Rao, K.S. Venkateswarlu, A.S. Gokhale, “Thermal behaviour of (NH4)3VO2F4 and Na(NH4)2VO2F4”, Thermochim. Acta, vol. 58 , pp. 87–95, October 1982. [25] I.G. Maslennikova, N.M. Laptash, T.A. Kaidalova, V.Ya. Ka vun, “Volatile ammonium fluorotitanate“, Spectroscopy Letters, vol. 34, pp. 775–781, 2001. [26] N.M Laptash., I.G. Maslennikova, Т.А. Кaidalova, “Ammonium Oxofluorotitanates”, J. Fluorine Chem., vol. 99, pp. 133–137, November 1999. [27] A.A. Udovenko, N.M. Laptash, I.G. Maslennikova, “Orientation disorder in ammonium elpasolites. Crystal structures of (NH4)3AlF6, (NH4)3TiOF5 and (NH4)3FeF6“, J. Fluorine Chem., vol. 124, pp. 5–15, November 2003. [28] A.A. Udovenko, N.M. Laptash, “Dynamic orientational disorder in crystals of fluroelpasolites, structural refinement of (NH4)3AlF6, (NH4)3TiOF5, and Rb2KTiOF 5“, Acta Crystallogr., vol. B67, pp. 447–454, Dec ember 2011. [29] I.N. Flerov, V.D. Fokina, A.F. Bovina, N.M. Laptash, “Phase transitions in perovskite-like oxyfluorides (NH4)3WO3F3 and (NH4)3TiOF5“, Solid State S ci., vo l . 6, p p. 367–370, April 2004. [30] N.M. Laptash, E.B. Merkulov, I.G. Maslennikova, “Thermal behaviour of ammonium oxofluorotitanates (IV)“, J. Therm. Anal. Calorym., vol. 63, pp. 197–204, 2001. [31] N.M. Laptash, M.A. Fedotov, I.G. Maslennikova, “Hydrolysis of volatile ammonium oxofluorotitanate (IV) according to 19F, 17O, 49Ti. NMR data“, Russ. J. Struct. Chem., vol. 45, pp. 74–82, January-February 2004 (Translated from Zh. Strukt. Khim., vol. 45, pp . 77–85). [32] I.G. Maslennikova, N.M. Laptash, A.P. Golikov, “Kinetics of pyrohydrolysis of (NH4)2TiF6 и (NH4)2Ti OF4“, Russ. J. Inorg. Chem. , vol. 46 , pp. 186–191, February 2001 (Translated from Zh. Neorgan. Khim., vol. 46, pp. 233–238). [33] I.G. Maslennikova, N.M. Laptash, A.P. Golikov, “Pyrohydroly- sis of amm onium fluoroferrates“, R uss. J. Inorg. Chem., vol. 47, pp. 705–711, May 2002 (Translated from Zh. Neorgan. Khim., vol. 47, pp. 796-802). [34] P.S. Gordienko, I.G. Maslennikova, N.M. Laptash, V.K. Gon- chruk, A.A. Smol’kov, “Method of titanium-containing raw ma- teria l processing”, Pat. RU No. 2139249, October 1999. |

