Paper Menu >>
Journal Menu >>
 Creative Education 2012. Vol.3, Supplement, 17-20 Published Online December 2012 in SciRes (http://www.SciRP.org/journal/ce) DOI:10.4236/ce.2012.37B004 Copyright © 2012 SciRes. 17 Effect of External Carbon Source and Bed Turnover Rate on Denitrificaion Jihee So ng , Younghee Kim, Namjong Yoo R&D Department, Ilshin Environmental Engineering Co., Ltd., Seoul, Korea Email: njyoo@okilshin.co.kr Received 2012 This study investigated the effect of external carbon source and bed turnover rate on biological denitrifi- cation in the pilot-scale IPNR (Ilshin Phosphorus and Nitrate Removal) process. IPNR process is an up-flow reactor filled with Si-media which removes phosphorus physically and nitrate nitrogen biologi- cally using secondary effluent from sewage treatment plant. Phosphorus (PO4-P) was removed using HFO (Hydrous ferric oxide) by adsorption as well as nitrate nitrogen (NO3-N) was transformed to nitrogen gas (N2) by denitrifying bacteria using external carbon source in the same reactor. Methanol was used as an external carbon source for denitrification with various dosing concentration and Si-media bed turnover rate was varied from 6.4 ~ 7.75 day-1. The concentrations of PO4-P and NO3-N in the secondary effluent were from 0.1 to 1.75 mg/L and from 1.1 to 31.9 mg/L, respectively. The results demonstrated that high amount of NO3-N removed from 31.9 to 1.2 mg/L, at the same time PO4-P removed from 1.75 to 0.41mg/L using secondary effluent. Denitrification was found more stable with 6.4 bed turnover/day and 130% over dosing of theoretical methanol dose for denitrification. Keywords: Denitrification; Carbon Source; Bed Turnover Rate; Phosphorus Removal Introduction One of the major conce rns for wastewater trea t me nt is removal of phosphorus and nitrogen which could cause eutrophication in river and lakes. According to EPA and Forsberg & Ryding, the standard for eutrophication is 0.02 ~ 0.025 mg/L as T-P and 0.6 ~ 1.5 mg/L as T-N [1,2], on the other hand, the water quality regulation comprehenses 20 mg/L as T-N without winter ease and 0.2 ~ 0.5 mg/L as T-P had been enforced recently in Korea which means phosphorus and nitrogen needs to be removed below water quality regulation to control eutrophication. There are various processes operating in the world in order to remove phosphorus and nitrogen commonly divided chemical and biological treatment. Most of phosphorus removal process uses flocculation with aluminum sulfate (Al2(SO4)3, alum), PAC (Poly aluminium chloride), Fe2(SO4)3, FeCl3 and polymer followed by sedimentation. Flocculation and sedimentation process is simple and effective process for phosphorus removal however high cost due to uses of plenty of flocculant [3]. Equa- tion (1) and (2) show the formation of M-PO4-P precipitate using aluminum or iron salts for flocculant [4]. Al2(SO3)3∙14H2O+2H3(PO4) → 2Al(PO4)+3H2SO4+18H2O (1) FeCl3∙(6H2O)+H2PO4+2HCO3 → FePO4+3Cl+2CO2+H2O (2) Biological nitrogen removal process includes A/O (Anaero- bic-Oxic), A 2/O, modified bardenpho, UCT (University of cape town), phostrip, VIP (Virginia initiative plat), SBR (Seqencing batch reacor), Bio-denipho and so on. These processes have developed with long period throughout the world. Nevertheless, operating these processes has some problem such as large site needs for installation, a lot of uses of external carbon source for denitrificaiton and unstable treatment results [5]. The principal of nitrification and denitrification which uses various carbon sources is well known in the world and it is shown below (3~6) [6]. Ammonia oxidation to nitrate requires 4.57 g O2/g NH4-N oxidized to NO3-N and consumes 7.14 g alkalinity (as CaCO3) per g NH4-N oxidized. For denitrification 1 mole of hydroxide alkalinity is produced per mole of NO3 reduced or one equiva- lent OH per equivalent N. 50 mg alkalinity as CaCO3 per 14 mg N reduced or 3.57 mg alkalinity as CaCO3 produced per mg NO3-N reduced. NH4++2O2+2HCO3- → NO3- + 2CO2 + 3H2O (3) 5CH3OH+6NO3- → 3N2+5CO2+7H2O+6OH- (4) 5CH3CHOH+12NO3- → 6N2+10CO2+9H2O+12OH- (5) 5CH3COOH+8NO3- → 4N2+10CO2+6H2O+8OH- (6) To minimize the amount of external carbon source and chemical flocculant uses and footprint for installation, an effi- cient process for removal of phosphorus and nitrogen has to be developed. IPNR process is the one solution for nitrate and phosphorus treatment at the same time with high efficiency, less sludge waste and less flocculant and carbon source uses using less process site. Materials and Methods Experimental Setup IPNR process is an up-flow single reactor and able to remove phosphorus and nitrate nitrogen simultaneously. The reactor was conducted by using FRP (Glass fiber reinforced plastic) tank of 2.7 m internal diameter and 5.5 m height. Figure 1 is the schemetic diagram of the IPNR process. IPNR reactor was filled with media which can occur adsorption between media and HFO, followed by PO4-P. By adding methanol as a carbon source, denitrifying bacteria can grow in the pore of and 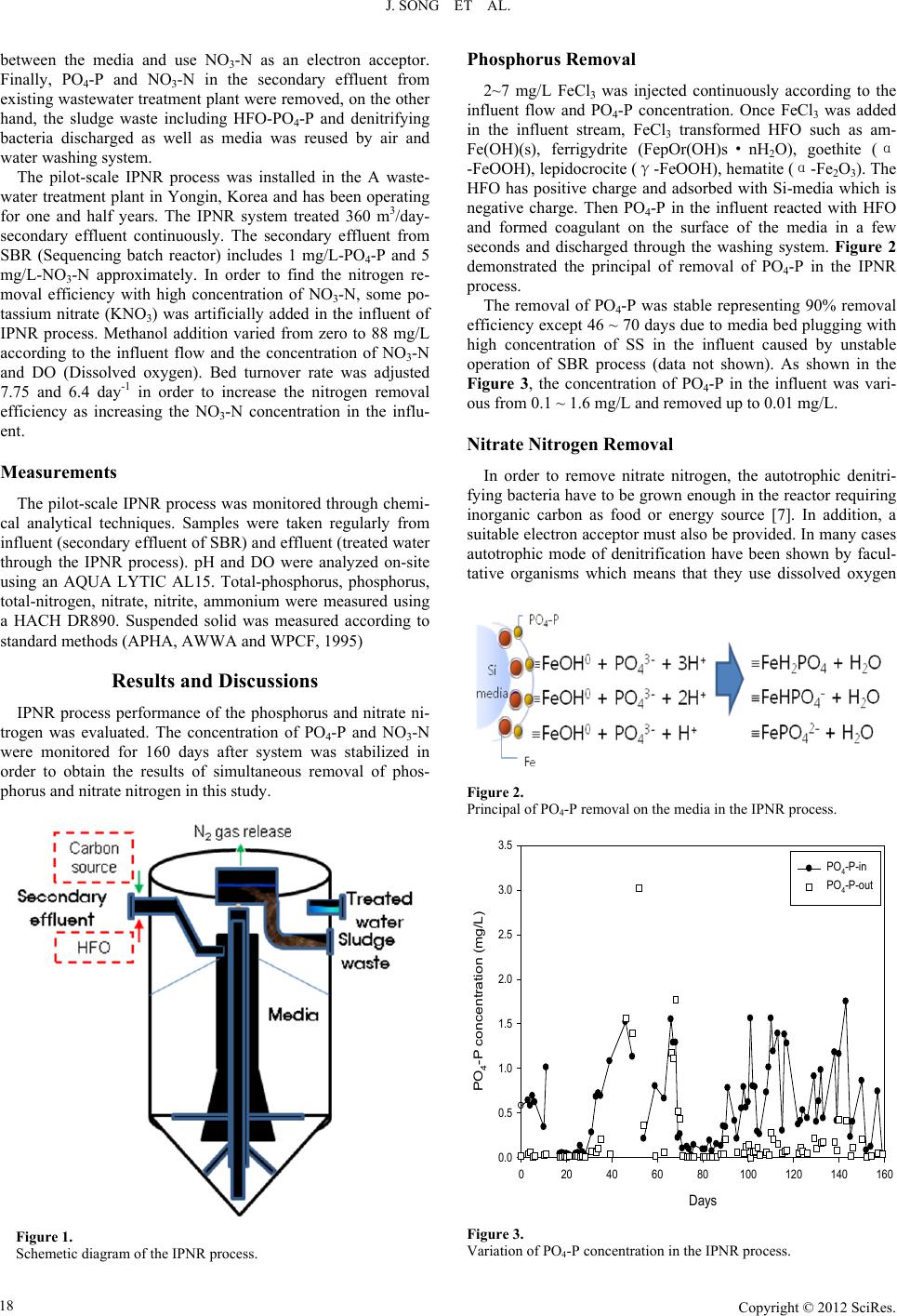 J. SONG ET AL. Copyright © 2012 SciRes. 18 between the media and use NO3-N as an electron acceptor. Finally, PO4-P and NO3-N in the secondary effluent from existing wastewater treatment plant were removed, on the other hand, the sludge waste including HFO-PO4-P and denitrifying bacteria discharged as well as media was reused by air and water washing system. The pilot-scale IPNR process was installed in the A waste- water treatment plant in Yongin, Korea and has been operating for one and half years. The IPNR system treated 360 m3/day- secondary effluent continuously. The secondary effluent from SBR (Sequencing batch reactor) includes 1 mg/L-PO4-P and 5 mg/L-NO3-N approximately. In order to find the nitrogen re- moval efficiency with high concentration of NO3-N, some po- tassium nitrate (KNO3) was artificially added in the influent of IPNR process. Methanol addition varied from zero to 88 mg/L according to the influent flow and the concentration of NO3-N and DO (Dissolved oxygen). Bed turnover rate was adjusted 7.75 and 6.4 day-1 in order to increase the nitrogen removal efficiency as increasing the NO3-N concentration in the influ- ent. Measurements The pilot-scale IPNR process was monitored through chemi- cal analytical techniques. Samples were taken regularly from influent (secondary effluent of SBR) and effluent (treated water through the IPNR process). pH and DO were analyzed on-site using an AQUA LYTIC AL15. Total-phosphorus, phosphorus, total-nitrogen, nitrate, nitrite, ammonium were measured using a HACH DR890. Suspended solid was measured according to standard methods (APHA, AWWA and WPCF, 1995) Results and Discu ssions IPNR process performance of the phosphorus and nitrate ni- trogen was evaluated. The concentration of PO4-P and NO3-N were monitored for 160 days after system was stabilized in order to obtain the results of simultaneous removal of phos- phorus and nitrate nitrogen in this study. Figure 1. Schemetic diagram of the IPNR process. Phosphorus Removal 2~7 mg/L FeCl3 was injected continuously according to the influent flow and PO4-P concentration. Once FeCl3 was added in the influent stream, FeCl3 transformed HFO such as am- Fe(OH)(s), ferrigydrite (FepOr(OH)s·nH2O), goethite (α -FeOOH), lepidocrocite (γ-FeOOH), he ma tit e (α-Fe2O3). The HFO has positive charge and adsorbed with Si-media which is negative charge. Then PO4-P in the influent reacted with HFO and formed coagulant on the surface of the media in a few seconds and discharged through the washing system. Figure 2 demonstrated the principal of removal of PO4-P in the IPNR process. The removal of PO4-P was stable representing 90% removal efficiency except 46 ~ 70 days due to media bed plugging with high concentration of SS in the influent caused by unstable operation of SBR process (data not shown). As shown in the Figure 3, the concentration of PO4-P in the influent was vari- ous from 0.1 ~ 1.6 mg/L and removed up to 0.01 mg/L. Nitrate Nitrogen Remo val In order to remove nitrate nitrogen, the autotrophic denitri- fying bacteria have to be grown enough in the reactor requiring inorganic carbon as food or energy source [7]. In addition, a suitable electron acceptor must also be provided. In many cases autotrophic mode of denitrification have been shown by facul- tative organisms which means that they use dissolved oxygen Figure 2. Principal of PO4-P removal on the media in the IPNR process. Days 020 40 60 80100120140160 PO 4 -P concentration (mg/L) 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 PO 4 -P-in PO 4 -P-out Figure 3. Variation of PO4-P concentration in the IPNR process.  J. SONG ET AL. Copyright © 2012 SciRes. 19 when it is aerobic condition or other electron acceptor like NO3-N or NO2-N in anaerobic condition [8]. In this study, me- thanol was used as a carbon source in the anaerobic condi- tion. The theoretical concentration of methanol for denitrifica- tion representated on (7) [9]. 2.9x[NO3-N (mg/L)] + 0.9x[DO (mg/L)] (mg/L) (7) The effect of methanol on denitrification was investigated. The methanol injection was gradually increased from zero to 88 mg/L in order to find the optimized condition for denitrification. At the beginning of the period, the methanol was injected automatically according to the influent flow and the concentration of NO3-N and DO to grow denitrifying bacteria. DO was controlled under 1 ~ 2 mg/L for efficient denitrification. When denitrifying bacteria was stabilized methanol was increased through the stages (Ⅰ~Ⅵ). The amount of methanol injection is shown in Table 1. As the concentration of NO3-N increased in the influent, the amount of methanol injection was increased from 23.9 to 88.1 mg/L. Basically 100% injection of theoretical methanol concentration was enough to remove nitrate nitrogen in the low concentration of NO3-N. When the concentration of NO3-N in the influent was 15~20 mg/L, however, the methanol injection was increased to 130% for stable removal of NO3-N (stage Ⅳ~Ⅴ). When the concentration of NO3-N up to 30 mg/L, the efficiency was decreased dramatically due to lack of carbon source (stage Ⅴ~Ⅵ). We found that 130% methanol injection of theoretical concentration was required for stable denitrification in the IPNR process. This value could be cost effective compared with conventional biological nitrogen removal process because of less external carbon source uses. One of the important factors is bed turnover rate which is re- lated with HRT (Hydraulic retention time). At the beginning of period, the bed turnover was 7.75 day-1 which made the deni- trifying bacteria condition unstable. The denitrifying bacteria require at least 30 min to reduce NO3-N or NO2-N to N2 gas. For more stable denitrification reaction, the bed turnover de- creased down to 6.4 day-1. Finally, we found that stable denitri- fication in the IPNR reactor. Phosphorus and nitrate nitrogen removal test of the pilot- scale IPNR process suggested that approximately 90% of si- multaneous removal for both PO4-P and NO3-N has been suc- cessful using secondary effluent from sewage wastewater treatment plant with 130% overdose of theoretical methanol concentration and 6.4 bed turnover/day. In the optimal condi- tion, 1.75 mg/L PO4-P removed to 0.41 mg/L and mostly 0.01 mg/L was measured in the effluent of IPNR process as maxi- mum efficiency. Although 31.9 mg/L NO3-N was decreased to Table 1. The amount of m ethanol in the pilot -scale IPNR process. The amount of methnoal Stage number Ⅰ Ⅱ Ⅲ Ⅳ Ⅴ Ⅵ Methanol injection percentage based on the theoretical concentration (%) 100 100 100 130 150 150 Theoretical concentration of mothanol (mg/L)a 23.9 29.9 44.4 59.0 66.6 88.1 Concentration of NO3-N (mg/L) 7 10 15 15 15 20 Concentration of DO (mg/L) 4 1 1 1 1 1 Days 020 40 60 80100120140160 Concentration of injected carbon source (mg/L) 0 20 40 60 80 100 MeOH-in Days 020 40 60 80100120140160 NO 3 -N concentration (mg/L) 0 5 10 15 20 25 30 35 NO 3 -N-in NO 3 -N-out 7.75 bed turnover/day6.4 bed turnover/day Ⅰ Ⅱ Ⅲ Ⅳ Ⅴ Ⅵ Figure 4. Variation of NO3-N concentration in the IPNR process. Conclusion 1.2 mg/L, most of the data showed that 20~25 mg/L of NO3-N removed to below than 1 mg/L which has 95~96 % removal efficie ncy. It seems tha t more than 30 mg/L a s NO3-N could be removed by adding more carbon source in this condition. It is possible that phosphorus and nitrate nitrogen can be treated at the same time and in the same reactor. Also, we found that there is no competition between HFO and denitrifying bacteria on the surface of media showing high efficiency of removal of phosphorus and nitrate nitrogen simultaneously. It could make cost effective sewage wastewater treatment plant for both PO4-P and NO3-N by decreasing uses of coagulant and carbon source as well as footprint for installation. Acknowledgment This project was named as “Field application of reactive fil- teration system for total phosphorus/nitrogen removal from effluents of sewage and/or wastewater treatment plants and reservoirs” and funded by Korea Environmental Industry and Technology Institute of Korean government as “Eco-Innovation project, 2011”. REFERENCES U.S. E.P.A, “Water quality criteria research of the U.S. Environmental Protection Agency”, Proceings of an EPA sponsored symposium, EPA-600/3-76-079, 1976. Forsberg, C. & S. O. Ryding, “Eutrophication paramters and trophic state indices in 30 Swedish waste-rec eiving lakes”, Arch . Hydrobiol., vol. 89, pp. 189~207, 1980. Water Environment Federation, Nutrient Removal, Chapter 7. Chemi- cal phosphorus removal, 2010. WEF and ASCE, “Design of municipal wastewater treatment plants-WEF manual of p ractice 8 and ASCE manuals an d reports on  J. SONG ET AL. Copyright © 2012 SciRes. 20 engineeing practice No. 76, 5th Ed. Water Environment Feferation and Americal Society of Civil Engineers Environment & Water Re- sources Institute, 2009 Francisco J. Cervantes, Spysros G. Pavlostathis and Adrianus C. van Haandel, Advanced biological treatment processes for industrial wastewaters, 2006. Tchobanoglous, G., F.L. Burton, and H.D. Stensel, wastewater engi- neeing: Treatme nt and reuse, 2003. Gilda Carvalho, Paulo c. Lemos, Adrian Oehmen, Maria A.M. Reis, “Denitrifying phosphorus removal: Linking the process performance with the microbial community structure”, Water Research, vol. 41, pp. 4383-4396, 2007. H.S. Shin and J.W. Kwak, Nitrogen and phosphorus treatment technol- ogy, Environmental Management Research Center, Korea, 2002. George T. Moore, “Nutrient control design manual”, U.S. E.P.A, Aug. 2010. |

