 Crystal Structure Theory and Applications, 2012, 1, 92-96 http://dx.doi.org/10.4236/csta.2012.13017 Published Online December 2012 (http://www.SciRP.org/journal/csta) Crystal and Molecular Structure of 2-Amino-3-Ethyl Carboxamido-4-Metyl-5-Carboxy Ethyl Thiophene Dhananjay Dey1, Venugopal Prakash2, Vasu3, Janardhanan Saravanan4, Deepak Chopra1* 1Department of Chemistry, Indian Institute of Science Education and Research, Bhopal, India 2Shirdi Sai Engineering College, Bangalore, India 3Vivekananda Degree College, Bangalore, India 4PES College of Pharmacy, Bangalore, India Email: dchopra@iiserb.ac.in Received October 18, 2012; revised November 15, 2012; accepted November 28, 2012 ABSTRACT The crystal and molecular structure of 2-Amino-3-ethyl carboxamido-4-methyl-5-carboxy ethyl thiophene (C11H16N2O3S) has been investigated from single crystal X-ray diffraction data. The primary focus is to investigate the molecular ge- ometry of this compound in the solid state along with the associated inter and intra-molecular hydrogen bonding and related weak interactions present in this molecule. This compound crystallizes in the monoclinic space group P21/c with cell parameters, a = 8.1344(3) Å, b = 13.7392(4) Å, c = 11.4704(4) Å, β = 100.769(2)˚, V = 1259.36 (7) Å3, D = 1.352 g·cm–3, Z = 4. The molecular geometry is stabilized by intra-molecular N-H…O=C and C-H…O interactions along with intramolecular C-H…N and C-H…O interactions which contribute towards the stability of the crystal packing. Keywords: Crystal; Molecular Conformation; Intermolecular Interactions; Spectroscopy; Diffraction 1. Introduction Thiophene derivatives [1] are of importance in medicinal chemistry and have recently been incorporated into new pharmaceutical and chemical compounds tested as anti- inflammatory agents [2]. This class of compounds ex- hibit pharmacological activity [3-5]. These are also use- ful in polymer chemistry because of their mechanical strength, ease of fabrication, flexibility in design, stabil- ity, resistance to corrosion and low cost [6]. In view of the importance of this class of heterocycles from a bio- logical and pharmaceutical perspective, we report in this manuscript the synthesis of 2-Amino-3-ethyl carbox- amido-4-methyl-5-carboxy ethyl thiophene. The com- pound has been purified and characterized spectroscopi- cally using FT-IR, 1H and 13C NMR techniques. The pu- rity of the phase has been established by powder X-Ray diffraction. Structural characterization of this compound has been achieved via single crystal X-ray diffraction study. Finally, an investigation of the CSD for related compounds containing the thiophene core has also been performed to compare the changes in geometry which accompany the introduction of a 3-ethyl carboxamide and 5-carboxy ester moiety on the thiophene ring. 2. Experimental 2.1. Synth esis of 2- Am ino-3-Ethyl Carboxamido- 4-Methyl-5-Carboxy Ethyl Thiophene A mixture of ethyl acetoacetate (5.2 g; 0.04 mol), ethyl cyanocetate (4.52 g; 0.04 mol) and sulphur powder (1.28 g; 0.04 mol) in ethanol (40 ml) were added in a round bottomed flask. To this, morpholine (4.0 ml) was added dropwise with stirring [Scheme 1]. The mixture was stirred further for 1 h at 45˚C - 50˚C, cooled overnight in ice and the solid product obtained was filtered, washed and recrystallised from ethanol. Pink coloured crystals were obtained and these were used for diffraction pur- poses. Melting point: 106˚C. Scheme 1 *Corresponding author. C opyright © 2012 SciRes. CSTA 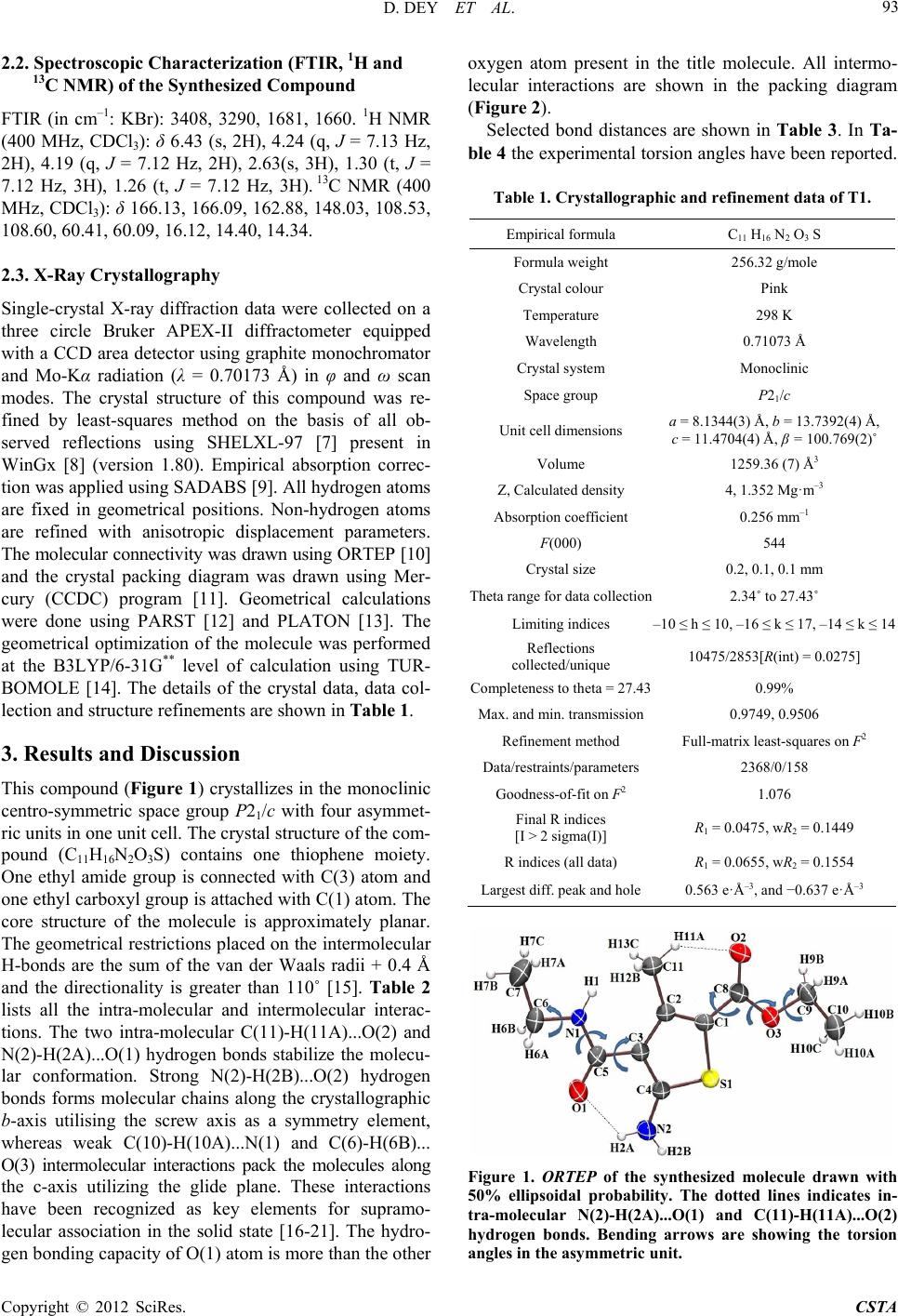 D. DEY ET AL. 93 2.2. Spec tr oscopic Characterization (FTI R , 1H and 13C NMR) of the Synthesized Compound FTIR (in cm–1: KBr): 3408, 3290, 1681, 1660. 1H NMR (400 MHz, CDCl3): δ 6.43 (s, 2H), 4.24 (q, J = 7.13 Hz, 2H), 4.19 (q, J = 7.12 Hz, 2H), 2.63(s, 3H), 1.30 (t, J = 7.12 Hz, 3H), 1.26 (t, J = 7.12 Hz, 3H). 13C NMR (400 MHz, CDCl3): δ 166.13, 166.09, 162.88, 148.03, 108.53, 108.60, 60.41, 60.09, 16.12, 14.40, 14.34. 2.3. X-Ray Crystall ogra ph y Single-crystal X-ray diffraction data were collected on a three circle Bruker APEX-II diffractometer equipped with a CCD area detector using graphite monochromator and Mo-Kα radiation (λ = 0.70173 Å) in φ and ω scan modes. The crystal structure of this compound was re- fined by least-squares method on the basis of all ob- served reflections using SHELXL-97 [7] present in WinGx [8] (version 1.80). Empirical absorption correc- tion was applied using SADABS [9]. All hydrogen atoms are fixed in geometrical positions. Non-hydrogen atoms are refined with anisotropic displacement parameters. The molecular connectivity was drawn using ORTEP [10] and the crystal packing diagram was drawn using Mer- cury (CCDC) program [11]. Geometrical calculations were done using PARST [12] and PLATON [13]. The geometrical optimization of the molecule was performed at the B3LYP/6-31G** level of calculation using TUR- BOMOLE [14]. The details of the crystal data, data col- lection and structure refinements are shown in Table 1. 3. Results and Discussion This compound (Figure 1) crystallizes in the monoclinic centro-symmetric space group P21/c with four asymmet- ric units in one unit cell. The crystal structure of the com- pound (C11H16N2O3S) contains one thiophene moiety. One ethyl amide group is connected with C(3) atom and one ethyl carboxyl group is attached with C(1) atom. The core structure of the molecule is approximately planar. The geometrical restrictions placed on the intermolecular H-bonds are the sum of the van der Waals radii + 0.4 Å and the directionality is greater than 110˚ [15]. Table 2 lists all the intra-molecular and intermolecular interac- tions. The two intra-molecular C(11)-H(11A)...O(2) and N(2)-H(2A)...O(1) hydrogen bonds stabilize the molecu- lar conformation. Strong N(2)-H(2B)...O(2) hydrogen bonds forms molecular chains along the crystallographic b-axis utilising the screw axis as a symmetry element, whereas weak C(10)-H(10A)...N(1) and C(6)-H(6B)... O(3) intermolecular interactions pack the molecules along the c-axis utilizing the glide plane. These interactions have been recognized as key elements for supramo- lecular association in the solid state [16-21]. The hydro- gen bonding capacity of O(1) atom is more than the other oxygen atom present in the title molecule. All intermo- lecular interactions are shown in the packing diagram (Figure 2). Selected bond distances are shown in Table 3. In Ta- ble 4 the experimental torsion angles have been reported. Table 1. Crystallographic and refine ment data of T1. Empirical formula C11 H16 N2 O3 S Formula weight 256.32 g/mole Crystal colour Pink Temperature 298 K Wavelength 0.71073 Å Crystal system Monoclinic Space group P21/c Unit cell dimensions a = 8.1344(3) Å, b = 13.7392(4) Å, c = 11.4704(4) Å, β = 100.769(2)˚ Volume 1259.36 (7) Å3 Z, Calculated density 4, 1.352 Mg·m–3 Absorption coefficient 0.256 mm–1 F(000) 544 Crystal size 0.2, 0.1, 0.1 mm Theta range for data collection2.34˚ to 27.43˚ Limiting indices –10 ≤ h ≤ 10, –16 ≤ k ≤ 17, –14 ≤ k ≤ 14 Reflections collected/unique 10475/2853[R(int) = 0.0275] Completeness to theta = 27.430.99% Max. and min. transmission0.9749, 0.9506 Refinement method Full-matrix least-squares on F2 Data/restraints/parameters2368/0/158 Goodness-of-fit on F2 1.076 Final R indices [I > 2 sigma(I)] R1 = 0.0475, wR2 = 0.1449 R indices (all data) R1 = 0.0655, wR2 = 0.1554 Largest diff. peak and hole0.563 e·Å–3, and −0.637 e·Å–3 Figure 1. ORTEP of the synthesized molecule drawn with 50% ellipsoidal probability. The dotted lines indicates in- tra-molecular N(2)-H(2A)...O(1) and C(11)-H(11A)...O(2) hydrogen bonds. Bending arrows are showing the torsion angles in the asymmetric unit. Copyright © 2012 SciRes. CSTA 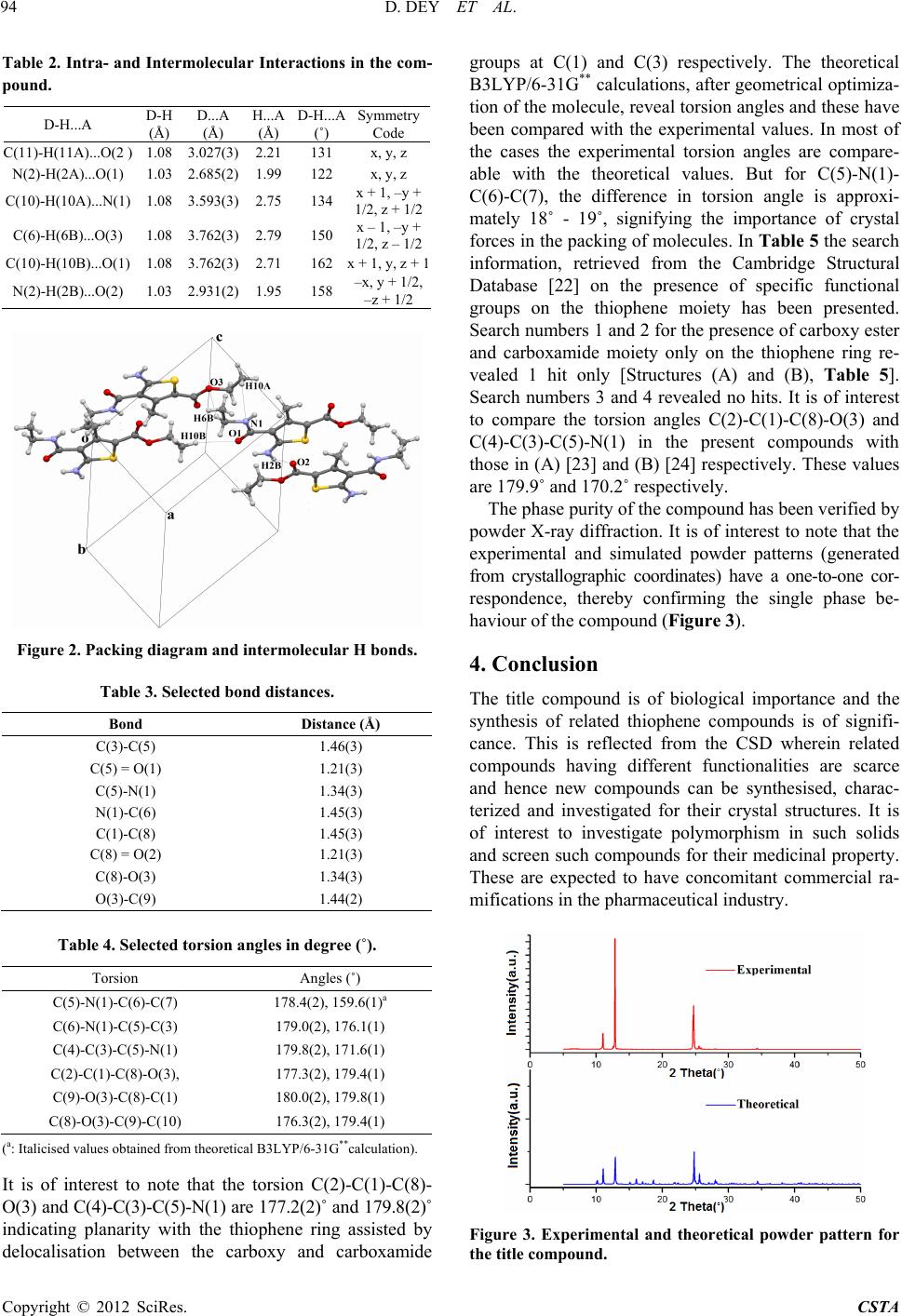 D. DEY ET AL. 94 Table 2. Intra- and Intermolecular Interactions in the com- pound. D-H...A D-H (Å) D...A (Å) H...A (Å) D-H...A (˚) Symmetry Code C(11)-H(11A)...O(2 ) 1.08 3.027(3)2.21 131 x, y, z N(2)-H(2A)...O(1) 1.03 2.685(2)1.99 122 x, y, z C(10)-H(10A)...N(1) 1.08 3.593(3)2.75 134 x + 1, –y + 1/2, z + 1/2 C(6)-H(6B)...O(3) 1.08 3.762(3)2.79 150 x – 1, –y + 1/2, z – 1/2 C(10)-H(10B)...O(1) 1.08 3.762(3)2.71 162 x + 1, y, z + 1 N(2)-H(2B)...O(2) 1.03 2.931(2)1.95 158 –x, y + 1/2, –z + 1/2 Figure 2. Packing diagram and intermolecular H bonds. Table 3. Selected bond distanc e s. Bond Distance (Å) C(3)-C(5) 1.46(3) C(5) = O(1) 1.21(3) C(5)-N(1) 1.34(3) N(1)-C(6) 1.45(3) C(1)-C(8) 1.45(3) C(8) = O(2) 1.21(3) C(8)-O(3) 1.34(3) O(3)-C(9) 1.44(2) Table 4. Selected torsion angles in degree (˚). Torsion Angles (˚) C(5)-N(1)-C(6)-C(7) 178.4(2), 159.6(1)a C(6)-N(1)-C(5)-C(3) 179.0(2), 176.1(1) C(4)-C(3)-C(5)-N(1) 179.8(2), 171.6(1) C(2)-C(1)-C(8)-O(3), 177.3(2), 179.4(1) C(9)-O(3)-C(8)-C(1) 180.0(2), 179.8(1) C(8)-O(3)-C(9)-C(10) 176.3(2), 179.4(1) (a: Italicised values obtained from theoretical B3LYP/6-31G**calculation). It is of interest to note that the torsion C(2)-C(1)-C(8)- O(3) and C(4)-C(3)-C(5)-N(1) are 177.2(2)˚ and 179.8(2)˚ indicating planarity with the thiophene ring assisted by delocalisation between the carboxy and carboxamide groups at C(1) and C(3) respectively. The theoretical B3LYP/6-31G** calculations, after geometrical optimiza- tion of the molecule, reveal torsion angles and these have been compared with the experimental values. In most of the cases the experimental torsion angles are compare- able with the theoretical values. But for C(5)-N(1)- C(6)-C(7), the difference in torsion angle is approxi- mately 18˚ - 19˚, signifying the importance of crystal forces in the packing of molecules. In Table 5 the search information, retrieved from the Cambridge Structural Database [22] on the presence of specific functional groups on the thiophene moiety has been presented. Search numbers 1 and 2 for the presence of carboxy ester and carboxamide moiety only on the thiophene ring re- vealed 1 hit only [Structures (A) and (B), Table 5]. Search numbers 3 and 4 revealed no hits. It is of interest to compare the torsion angles C(2)-C(1)-C(8)-O(3) and C(4)-C(3)-C(5)-N(1) in the present compounds with those in (A) [23] and (B) [24] respectively. These values are 179.9˚ and 170.2˚ respectively. The phase purity of the compound has been verified by powder X-ray diffraction. It is of interest to note that the experimental and simulated powder patterns (generated from crystallographic coordinates) have a one-to-one cor- respondence, thereby confirming the single phase be- haviour of the compound (Figure 3 ). 4. Conclusion The title compound is of biological importance and the synthesis of related thiophene compounds is of signifi- cance. This is reflected from the CSD wherein related compounds having different functionalities are scarce and hence new compounds can be synthesised, charac- terized and investigated for their crystal structures. It is of interest to investigate polymorphism in such solids and screen such compounds for their medicinal property. These are expected to have concomitant commercial ra- mifications in the pharmaceutical industry. Figure 3. Experimental and theoretical powder pattern for the title compound. Copyright © 2012 SciRes. CSTA 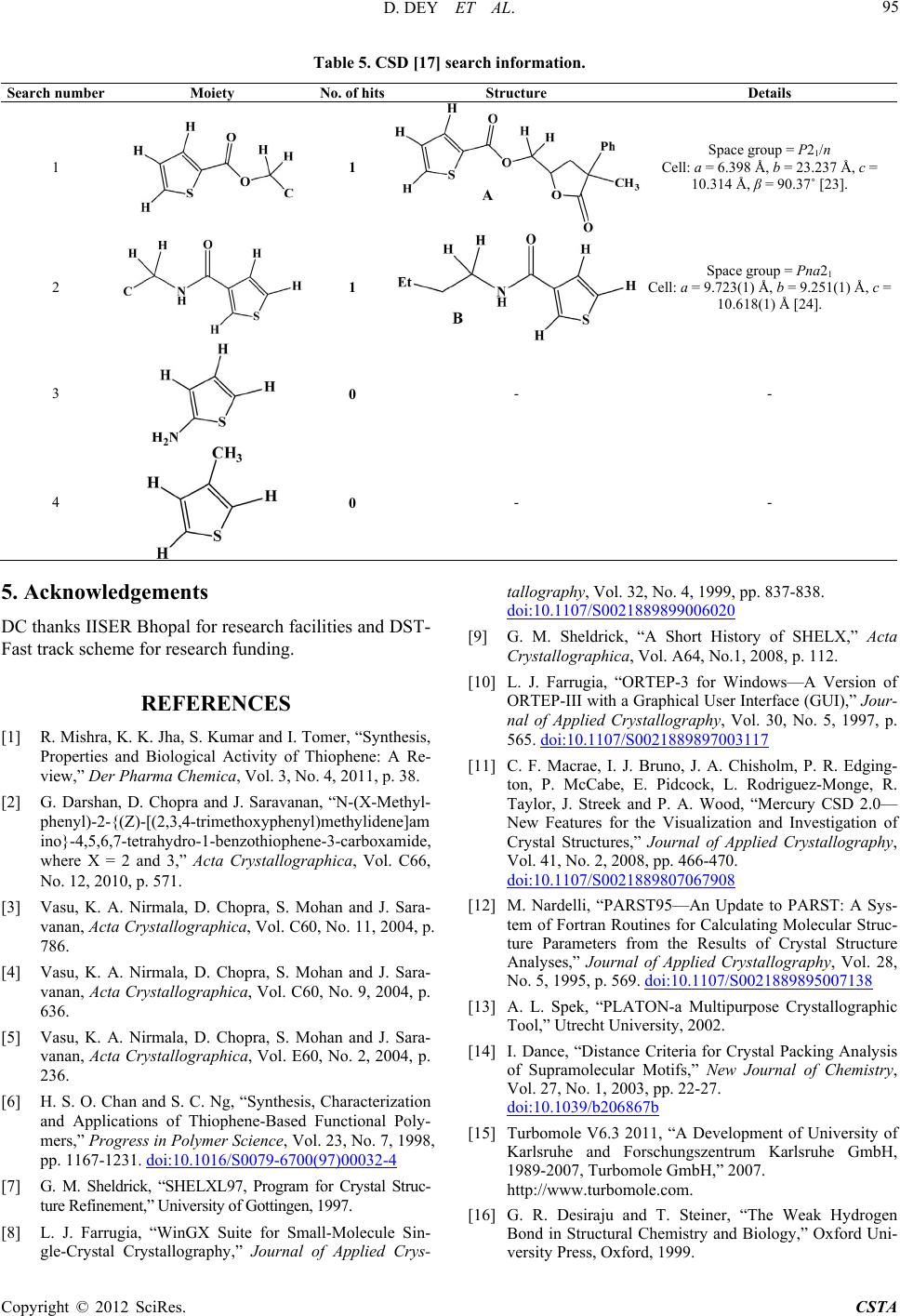 D. DEY ET AL. Copyright © 2012 SciRes. CSTA 95 Table 5. CSD [17] search information. Search number Moiety No. of hitsStructure Details 1 1 Space group = P21/n Cell: a = 6.398 Å, b = 23.237 Å, c = 10.314 Å, β = 90.37˚ [23]. 2 1 Space group = Pna21 Cell: a = 9.723(1) Å, b = 9.251(1) Å, c = 10.618(1) Å [24]. 3 0 - - 4 0 - - 5. Acknowledgements DC thanks IISER Bhopal for research facilities and DST- Fast track scheme for research funding. REFERENCES [1] R. Mishra, K. K. Jha, S. Kumar and I. Tomer, “Synthesis, Properties and Biological Activity of Thiophene: A Re- view,” Der Pharma Chemica, Vol. 3, No. 4, 2011, p. 38. [2] G. Darshan, D. Chopra and J. Saravanan, “N-(X-Methyl- phenyl)-2-{(Z)-[(2,3,4-trimethoxyphenyl)methylidene]am ino}-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxamide, where X = 2 and 3,” Acta Crystallographica, Vol. C66, No. 12, 2010, p. 571. [3] Vasu, K. A. Nirmala, D. Chopra, S. Mohan and J. Sara- vanan, Acta Crystallographica, Vol. C60, No. 11, 2004, p. 786. [4] Vasu, K. A. Nirmala, D. Chopra, S. Mohan and J. Sara- vanan, Acta Crystallographica, Vol. C60, No. 9, 2004, p. 636. [5] Vasu, K. A. Nirmala, D. Chopra, S. Mohan and J. Sara- vanan, Acta Crystallographica, Vol. E60, No. 2, 2004, p. 236. [6] H. S. O. Chan and S. C. Ng, “Synthesis, Characterization and Applications of Thiophene-Based Functional Poly- mers,” Progress in Polymer Science, Vol. 23, No. 7, 1998, pp. 1167-1231. doi:10.1016/S0079-6700(97)00032-4 [7] G. M. Sheldrick, “SHELXL97, Program for Crystal Struc- ture Refinement,” University of Gottingen, 1997. [8] L. J. Farrugia, “WinGX Suite for Small-Molecule Sin- gle-Crystal Crystallography,” Journal of Applied Crys- tallography, Vol. 32, No. 4, 1999, pp. 837-838. doi:10.1107/S0021889899006020 [9] G. M. Sheldrick, “A Short History of SHELX,” Acta Crystallographica, Vol. A64, No.1, 2008, p. 112. [10] L. J. Farrugia, “ORTEP-3 for Windows—A Version of ORTEP-III with a Graphical User Interface (GUI),” Jour- nal of Applied Crystallography, Vol. 30, No. 5, 1997, p. 565. doi:10.1107/S0021889897003117 [11] C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edging- ton, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. Streek and P. A. Wood, “Mercury CSD 2.0— New Features for the Visualization and Investigation of Crystal Structures,” Journal of Applied Crystallography, Vol. 41, No. 2, 2008, pp. 466-470. doi:10.1107/S0021889807067908 [12] M. Nardelli, “PARST95—An Update to PARST: A Sys- tem of Fortran Routines for Calculating Molecular Struc- ture Parameters from the Results of Crystal Structure Analyses,” Journal of Applied Crystallography, Vol. 28, No. 5, 1995, p. 569. doi:10.1107/S0021889895007138 [13] A. L. Spek, “PLATON-a Multipurpose Crystallographic Tool,” Utrecht University, 2002. [14] I. Dance, “Distance Criteria for Crystal Packing Analysis of Supramolecular Motifs,” New Journal of Chemistry, Vol. 27, No. 1, 2003, pp. 22-27. doi:10.1039/b206867b [15] Turbomole V6.3 2011, “A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, Turbomole GmbH,” 2007. http://www.turbomole.com. [16] G. R. Desiraju and T. Steiner, “The Weak Hydrogen Bond in Structural Chemistry and Biology,” Oxford Uni- versity Press, Oxford, 1999.  D. DEY ET AL. 96 [17] G. R. Desiraju, “C–H...O and Other Weak Hydrogen Bonds. From Crystal Engineering to Virtual Screening,” Chemical Communication, 2005, pp. 2995-3001. doi:10.1039/b504372g [18] G. R. Desiraju, “Hydrogen Bridges in Crystal Engineer- ing: Interactions without Borders,” Accounts of Chemical Research, Vol. 35, No. 7, 2002, pp. 565-573. doi:10.1021/ar010054t [19] S. S. Kuduva, D. C. Craig, A. Nangia and G. R. Desiraju, “Cubanecarboxylic Acids. Crystal Engineering Conside- rations and the Role of C−H...O Hydrogen Bonds in De- termining O−H...O Networks,” Journal of the American Chemial Society, Vol. 121, No. 9, 1999, p. 1936. doi:10.1021/ja981967u [20] T. Steiner, Chemical Communication, No. 8, 1997, pp. 727-734. doi:10.1039/a603049a [21] G. R. Desiraju, “The C−H···O Hydrogen Bond: Struc- tural Implications and Supramolecular Design,” Accounts of Chemical Research, Vol. 29, No. 9, 1996, pp. 441-449. doi:10.1021/ar950135n [22] CSD Version 5.33, “The Following Constraints Were Applied: No Refcode Restrictions Applied, 3D Coordi- nates Determined, R Factor ≤ 0.1, No Errors, Not Poly- meric, No Ions, Only Organics,” 2011. [23] I. F. Cottrell, A. R. Cowley, L. J. Croft, L. Hymns, M. G. Moloney, E. J. Nettleton, H. K. Smithies and A. L. Thompson, “Acyloxylactonisations Mediated by Lead Tetracarboxylates,” Tetrahedron, Vol. 65, No. 12, 2009, pp. 2537-2550. doi:10.1016/j.tet.2009.01.042 [24] R. W. Gable, M. J. Laws, C. H. Schiesser, Acta Crystal- lographica, Vol. C53, No. 5, 1997, p. 641. Copyright © 2012 SciRes. CSTA
|