Paper Menu >>
Journal Menu >>
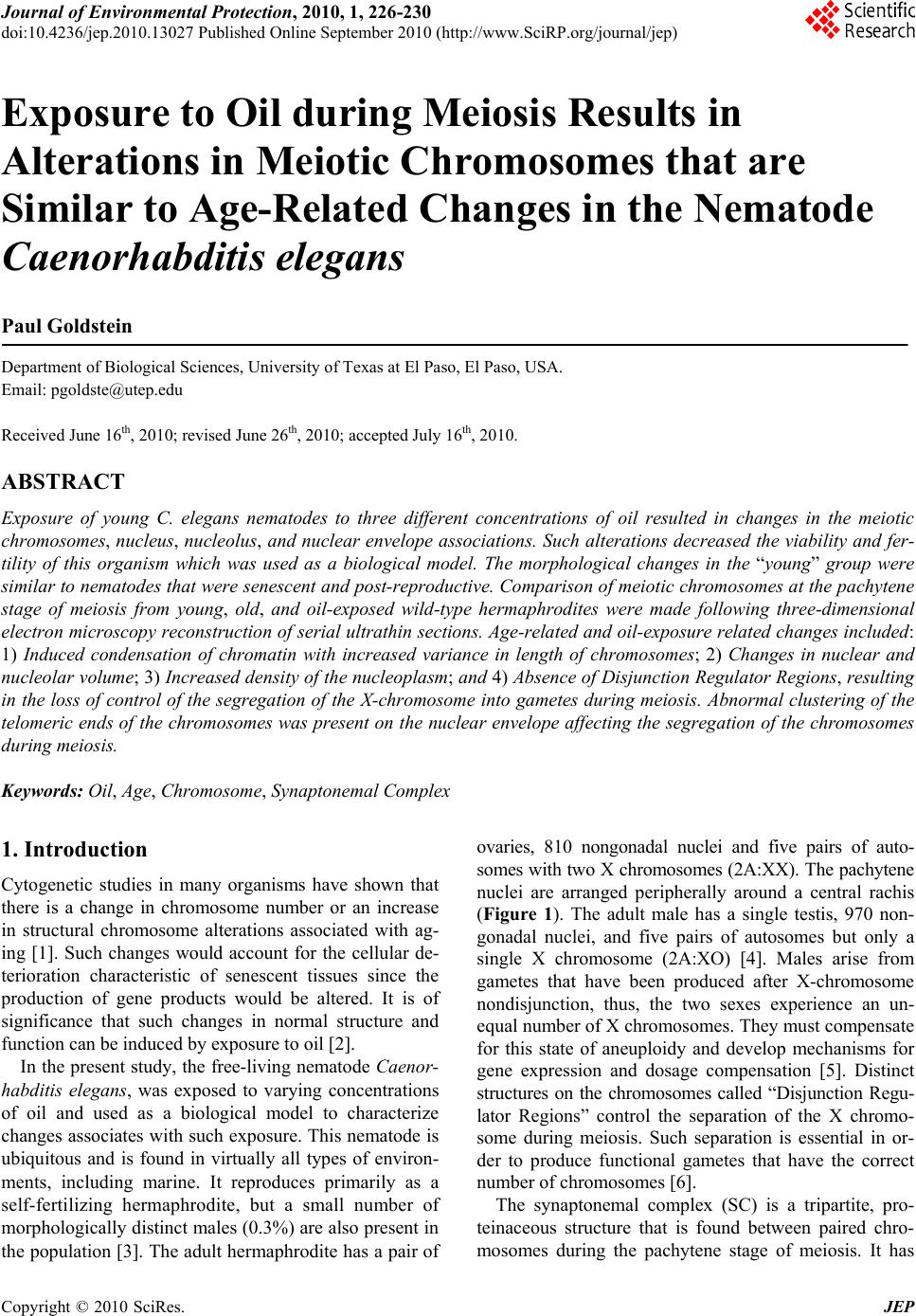 Journal of Environmental Protection, 2010, 1, 226-230 doi:10.4236/jep.2010.13027 Published Online September 2010 (http://www.SciRP.org/journal/jep) Copyright © 2010 SciRes. JEP Exposure to Oil during Meiosis Results in Alterations in Meiotic Chromosomes that are Similar to Age-Related Changes in the Nematode Caenorhabditis elegans Paul Goldstein Department of Biological Sciences, University of Texas at El Paso, El Paso, USA. Email: pgoldste@utep.edu Received June 16th, 2010; revised June 26th, 2010; accepted July 16th, 2010. ABSTRACT Exposure of young C. elegans nematodes to three different concentrations of oil resulted in changes in the meiotic chromosomes, nucleus, nucleolus, and nuclear envelope associations. Such alterations decreased the viability and fer- tility of this organism which was used as a biological model. The morphological changes in the “young ” group were similar to nematodes that were senescent and post-reproductive. Comparison of meiotic chromosomes at the pachytene stage of meiosis from young, old, and oil-exposed wild-type hermaphrodites were made following three-dimensional electron microscopy reconstruction of serial ultrathin sections. Age-related and oil-exposure related changes included: 1) Induced condensation of chromatin with increased variance in length of chromosomes; 2) Changes in nuclear and nucleolar volume; 3) Increased density of the nucleoplasm; and 4) Absence of Disjunction Regulator Regions, resulting in the loss of control of the segregation of the X-chromosome into gametes during meiosis. Abnormal clustering of the telomeric ends of the chromosomes was present on the nuclear envelope affecting the segregation of the chromosomes during meiosis. Keywords: Oil, Age, Chromosome, Synaptonemal Complex 1. Introduction Cytogenetic studies in many organisms have shown that there is a change in chromosome number or an increase in structural chromosome alterations associated with ag- ing [1]. Such changes would account for the cellular de- terioration characteristic of senescent tissues since the production of gene products would be altered. It is of significance that such changes in normal structure and function can be induced by exposure to oil [2]. In the present study, the free-living nematode Caenor- habditis elegans, was exposed to varying concentrations of oil and used as a biological model to characterize changes associates with such exposure. This nematode is ubiquitous and is found in virtually all types of environ- ments, including marine. It reproduces primarily as a self-fertilizing hermaphrodite, but a small number of morphologically distinct males (0.3%) are also present in the population [3]. The adult hermaphrodite has a pair of ovaries, 810 nongonadal nuclei and five pairs of auto- somes with two X chromosomes (2A:XX). The pachytene nuclei are arranged peripherally around a central rachis (Figure 1). The adult male has a single testis, 970 non- gonadal nuclei, and five pairs of autosomes but only a single X chromosome (2A:XO) [4]. Males arise from gametes that have been produced after X-chromosome nondisjunction, thus, the two sexes experience an un- equal number of X chromosomes. They must compensate for this state of aneuploidy and develop mechanisms for gene expression and dosage compensation [5]. Distinct structures on the chromosomes called “Disjunction Regu- lator Regions” control the separation of the X chromo- some during meiosis. Such separation is essential in or- der to produce functional gametes that have the correct number of chromosomes [6]. The synaptonemal complex (SC) is a tripartite, pro- teinaceous structure that is found between paired chro- mosomes during the pachytene stage of meiosis. It has 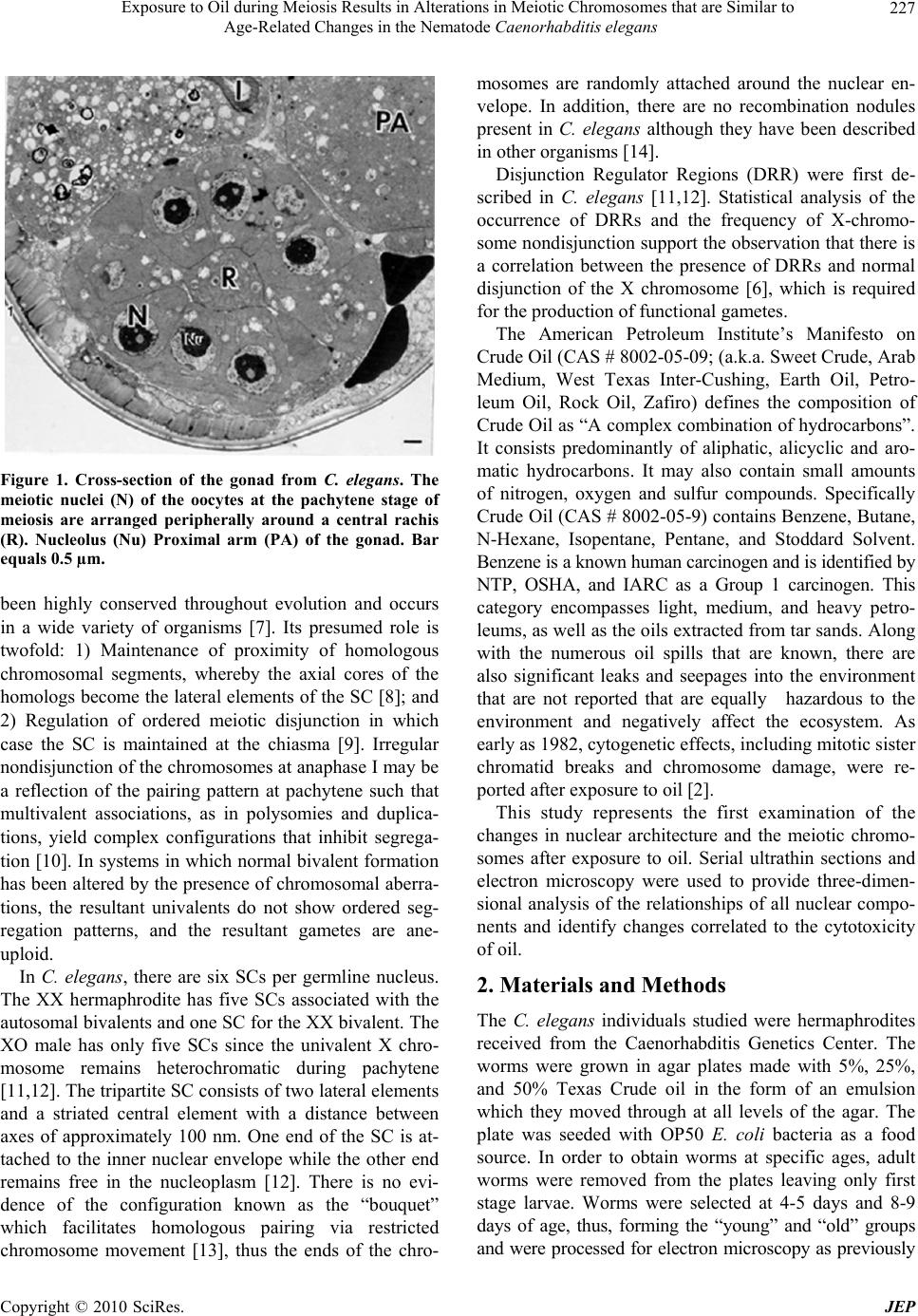 Exposure to Oil during Meiosis Results in Alterations in Meiotic Chromosomes that are Similar to Age-Related Changes in the Nematode Caenorhabditis elegans Copyright © 2010 SciRes. JEP 227 Figure 1. Cross-section of the gonad from C. elegans. The meiotic nuclei (N) of the oocytes at the pachytene stage of meiosis are arranged peripherally around a central rachis (R). Nucleolus (Nu) Proximal arm (PA) of the gonad. Bar equals 0.5 µm. been highly conserved throughout evolution and occurs in a wide variety of organisms [7]. Its presumed role is twofold: 1) Maintenance of proximity of homologous chromosomal segments, whereby the axial cores of the homologs become the lateral elements of the SC [8]; and 2) Regulation of ordered meiotic disjunction in which case the SC is maintained at the chiasma [9]. Irregular nondisjunction of the chromosomes at anaphase I may be a reflection of the pairing pattern at pachytene such that multivalent associations, as in polysomies and duplica- tions, yield complex configurations that inhibit segrega- tion [10]. In systems in which normal bivalent formation has been altered by the presence of chromosomal aberra- tions, the resultant univalents do not show ordered seg- regation patterns, and the resultant gametes are ane- uploid. In C. elegans, there are six SCs per germline nucleus. The XX hermaphrodite has five SCs associated with the autosomal bivalents and one SC for the XX bivalent. The XO male has only five SCs since the univalent X chro- mosome remains heterochromatic during pachytene [11,12]. The tripartite SC consists of two lateral elements and a striated central element with a distance between axes of approximately 100 nm. One end of the SC is at- tached to the inner nuclear envelope while the other end remains free in the nucleoplasm [12]. There is no evi- dence of the configuration known as the “bouquet” which facilitates homologous pairing via restricted chromosome movement [13], thus the ends of the chro- mosomes are randomly attached around the nuclear en- velope. In addition, there are no recombination nodules present in C. elegans although they have been described in other organisms [14]. Disjunction Regulator Regions (DRR) were first de- scribed in C. elegans [11,12]. Statistical analysis of the occurrence of DRRs and the frequency of X-chromo- some nondisjunction support the observation that there is a correlation between the presence of DRRs and normal disjunction of the X chromosome [6], which is required for the production of functional gametes. The American Petroleum Institute’s Manifesto on Crude Oil (CAS # 8002-05-09; (a.k.a. Sweet Crude, Arab Medium, West Texas Inter-Cushing, Earth Oil, Petro- leum Oil, Rock Oil, Zafiro) defines the composition of Crude Oil as “A complex combination of hydrocarbons”. It consists predominantly of aliphatic, alicyclic and aro- matic hydrocarbons. It may also contain small amounts of nitrogen, oxygen and sulfur compounds. Specifically Crude Oil (CAS # 8002-05-9) contains Benzene, Butane, N-Hexane, Isopentane, Pentane, and Stoddard Solvent. Benzene is a known human carcinogen and is identified by NTP, OSHA, and IARC as a Group 1 carcinogen. This category encompasses light, medium, and heavy petro- leums, as well as the oils extracted from tar sands. Along with the numerous oil spills that are known, there are also significant leaks and seepages into the environment that are not reported that are equally hazardous to the environment and negatively affect the ecosystem. As early as 1982, cytogenetic effects, including mitotic sister chromatid breaks and chromosome damage, were re- ported after exposure to oil [2]. This study represents the first examination of the changes in nuclear architecture and the meiotic chromo- somes after exposure to oil. Serial ultrathin sections and electron microscopy were used to provide three-dimen- sional analysis of the relationships of all nuclear compo- nents and identify changes correlated to the cytotoxicity of oil. 2. Materials and Methods The C. elegans individuals studied were hermaphrodites received from the Caenorhabditis Genetics Center. The worms were grown in agar plates made with 5%, 25%, and 50% Texas Crude oil in the form of an emulsion which they moved through at all levels of the agar. The plate was seeded with OP50 E. coli bacteria as a food source. In order to obtain worms at specific ages, adult worms were removed from the plates leaving only first stage larvae. Worms were selected at 4-5 days and 8-9 days of age, thus, forming the “young” and “old” groups and were processed for electron microscopy as previously 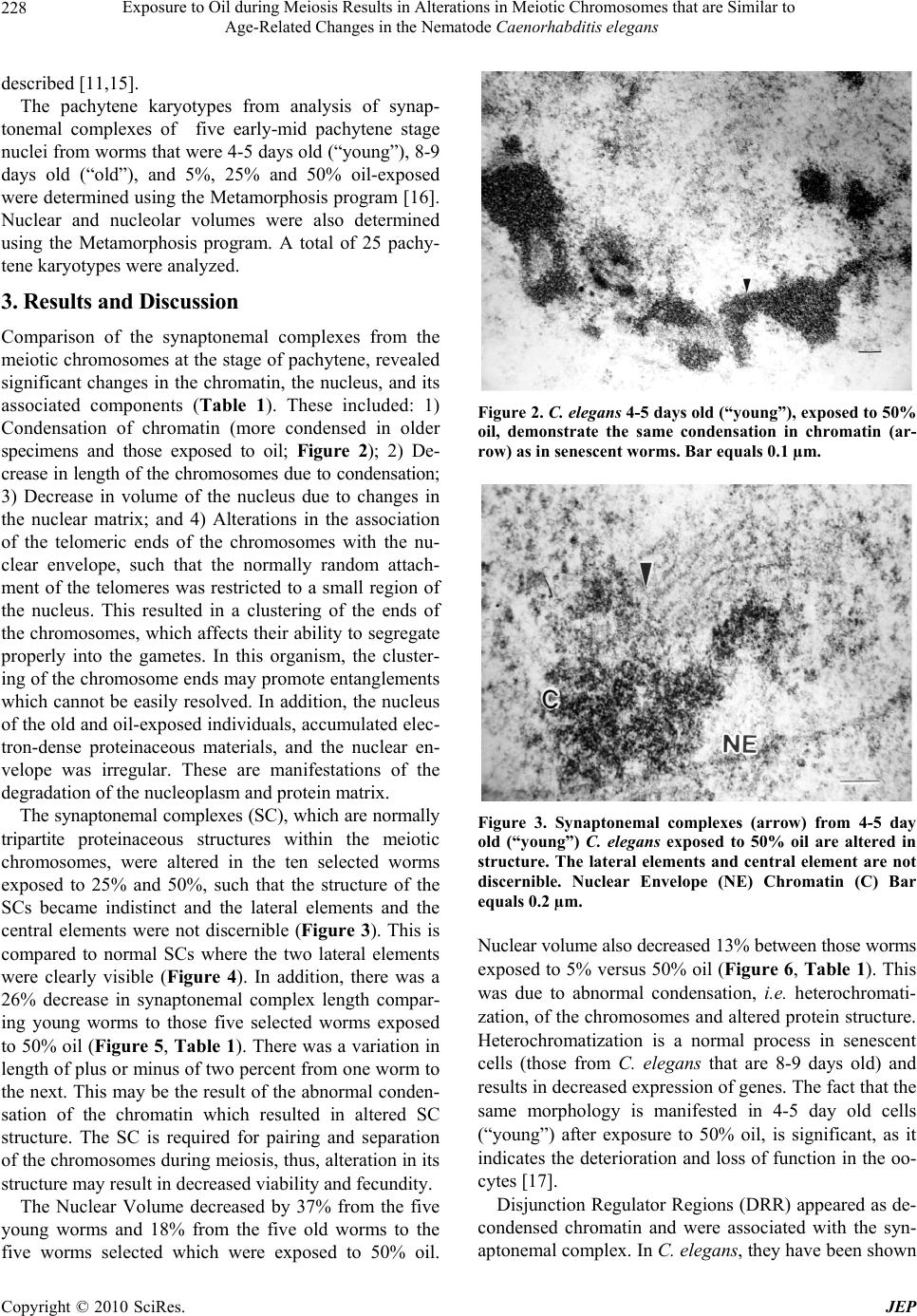 Exposure to Oil during Meiosis Results in Alterations in Meiotic Chromosomes that are Similar to Age-Related Changes in the Nematode Caenorhabditis elegans Copyright © 2010 SciRes. JEP 228 described [11,15]. The pachytene karyotypes from analysis of synap- tonemal complexes of five early-mid pachytene stage nuclei from worms that were 4-5 days old (“young”), 8-9 days old (“old”), and 5%, 25% and 50% oil-exposed were determined using the Metamorphosis program [16]. Nuclear and nucleolar volumes were also determined using the Metamorphosis program. A total of 25 pachy- tene karyotypes were analyzed. 3. Results and Discussion Comparison of the synaptonemal complexes from the meiotic chromosomes at the stage of pachytene, revealed significant changes in the chromatin, the nucleus, and its associated components (Table 1). These included: 1) Condensation of chromatin (more condensed in older specimens and those exposed to oil; Figure 2); 2) De- crease in length of the chromosomes due to condensation; 3) Decrease in volume of the nucleus due to changes in the nuclear matrix; and 4) Alterations in the association of the telomeric ends of the chromosomes with the nu- clear envelope, such that the normally random attach- ment of the telomeres was restricted to a small region of the nucleus. This resulted in a clustering of the ends of the chromosomes, which affects their ability to segregate properly into the gametes. In this organism, the cluster- ing of the chromosome ends may promote entanglements which cannot be easily resolved. In addition, the nucleus of the old and oil-exposed individuals, accumulated elec- tron-dense proteinaceous materials, and the nuclear en- velope was irregular. These are manifestations of the degradation of the nucleoplasm and protein matrix. The synaptonemal complexes (SC), which are normally tripartite proteinaceous structures within the meiotic chromosomes, were altered in the ten selected worms exposed to 25% and 50%, such that the structure of the SCs became indistinct and the lateral elements and the central elements were not discernible (Figure 3). This is compared to normal SCs where the two lateral elements were clearly visible (Figure 4). In addition, there was a 26% decrease in synaptonemal complex length compar- ing young worms to those five selected worms exposed to 50% oil (Figure 5, Table 1). There was a variation in length of plus or minus of two percent from one worm to the next. This may be the result of the abnormal conden- sation of the chromatin which resulted in altered SC structure. The SC is required for pairing and separation of the chromosomes during meiosis, thus, alteration in its structure may result in decreased viability and fecundity. The Nuclear Volume decreased by 37% from the five young worms and 18% from the five old worms to the five worms selected which were exposed to 50% oil. Figure 2. C. elegans 4-5 days old (“young”), exposed to 50% oil, demonstrate the same condensation in chromatin (ar- row) as in senescent worms. Bar equals 0.1 µm. Figure 3. Synaptonemal complexes (arrow) from 4-5 day old (“young”) C. elegans exposed to 50% oil are altered in structure. The lateral elements and central element are not discernible. Nuclear Envelope (NE) Chromatin (C) Bar equals 0.2 µm. Nuclear volume also decreased 13% between those worms exposed to 5% versus 50% oil (Figure 6, Table 1). This was due to abnormal condensation, i.e. heterochromati- zation, of the chromosomes and altered protein structure. Heterochromatization is a normal process in senescent cells (those from C. elegans that are 8-9 days old) and results in decreased expression of genes. The fact that the same morphology is manifested in 4-5 day old cells (“young”) after exposure to 50% oil, is significant, as it indicates the deterioration and loss of function in the oo- cytes [17]. Disjunction Regulator Regions (DRR) appeared as de- condensed chromatin and were associated with the syn- aptonemal complex. In C. elegans, they have been shown 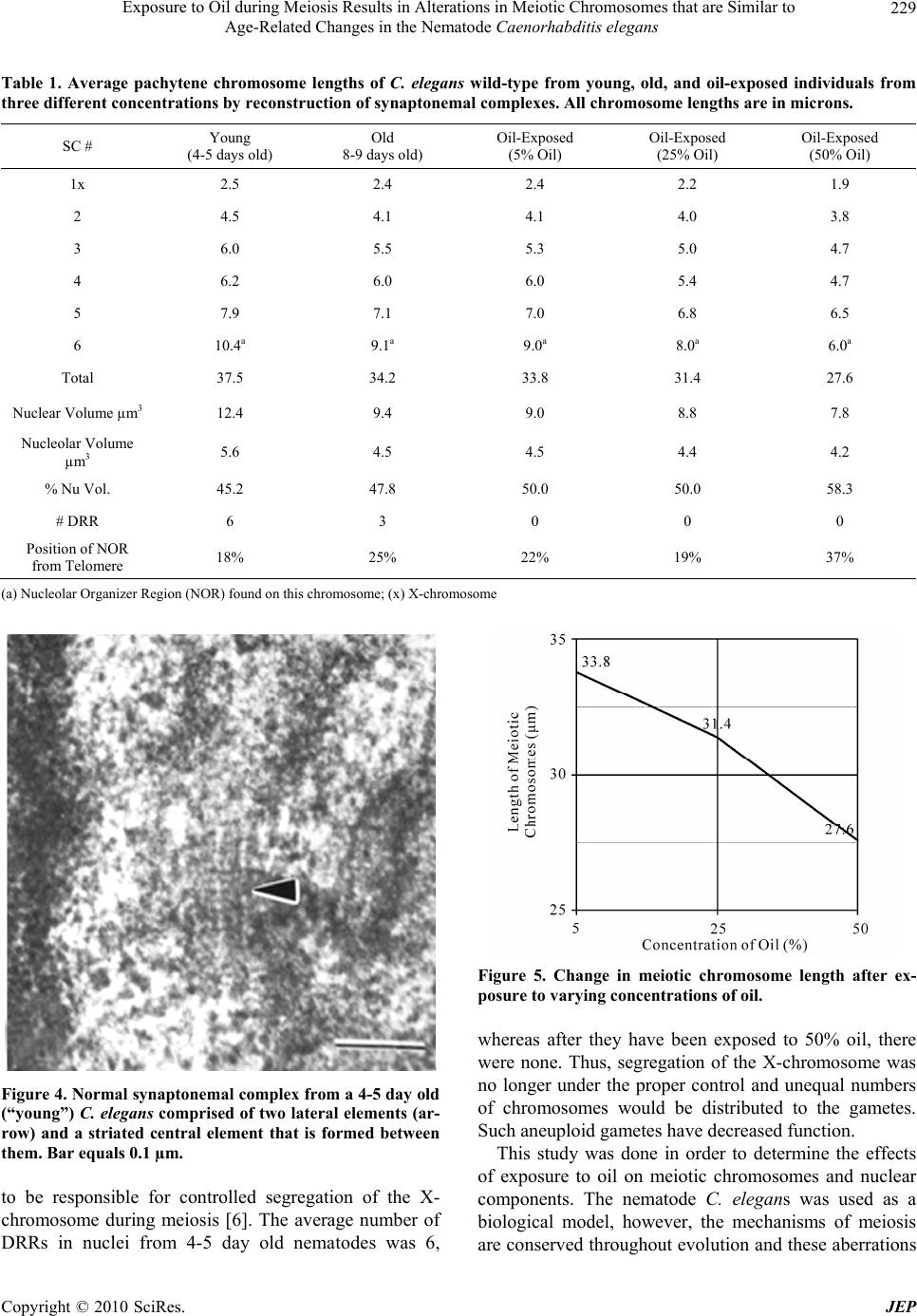 Exposure to Oil during Meiosis Results in Alterations in Meiotic Chromosomes that are Similar to Age-Related Changes in the Nematode Caenorhabditis elegans Copyright © 2010 SciRes. JEP 229 Table 1. Average pachytene chromosome lengths of C. elegans wild-type from young, old, and oil-exposed individuals from three different concentrations by reconstruction of synaptonemal complexes. All chromosome lengths are in microns. SC # Young (4-5 days old) Old 8-9 days old) Oil-Exposed (5% Oil) Oil-Exposed (25% Oil) Oil-Exposed (50% Oil) 1x 2.5 2.4 2.4 2.2 1.9 2 4.5 4.1 4.1 4.0 3.8 3 6.0 5.5 5.3 5.0 4.7 4 6.2 6.0 6.0 5.4 4.7 5 7.9 7.1 7.0 6.8 6.5 6 10.4a 9.1a 9.0a 8.0a 6.0a Total 37.5 34.2 33.8 31.4 27.6 Nuclear Volume µm3 12.4 9.4 9.0 8.8 7.8 Nucleolar Volume µm3 5.6 4.5 4.5 4.4 4.2 % Nu Vol. 45.2 47.8 50.0 50.0 58.3 # DRR 6 3 0 0 0 Position of NOR from Telomere 18% 25% 22% 19% 37% (a) Nucleolar Organizer Region (NOR) found on this chromosome; (x) X-chromosome Figure 4. Normal synaptonemal complex from a 4-5 day old (“young”) C. elegans comprised of two lateral elements (ar- row) and a striated central element that is formed between them. Bar equals 0.1 µm. to be responsible for controlled segregation of the X- chromosome during meiosis [6]. The average number of DRRs in nuclei from 4-5 day old nematodes was 6, Figure 5. Change in meiotic chromosome length after ex- posure to varying concentrations of oil. whereas after they have been exposed to 50% oil, there were none. Thus, segregation of the X-chromosome was no longer under the proper control and unequal numbers of chromosomes would be distributed to the gametes. Such aneuploid gametes have decreased function. This study was done in order to determine the effects of exposure to oil on meiotic chromosomes and nuclear components. The nematode C. elegans was used as a biological model, however, the mechanisms of meiosis are conserved throughout evolution and these aberrations 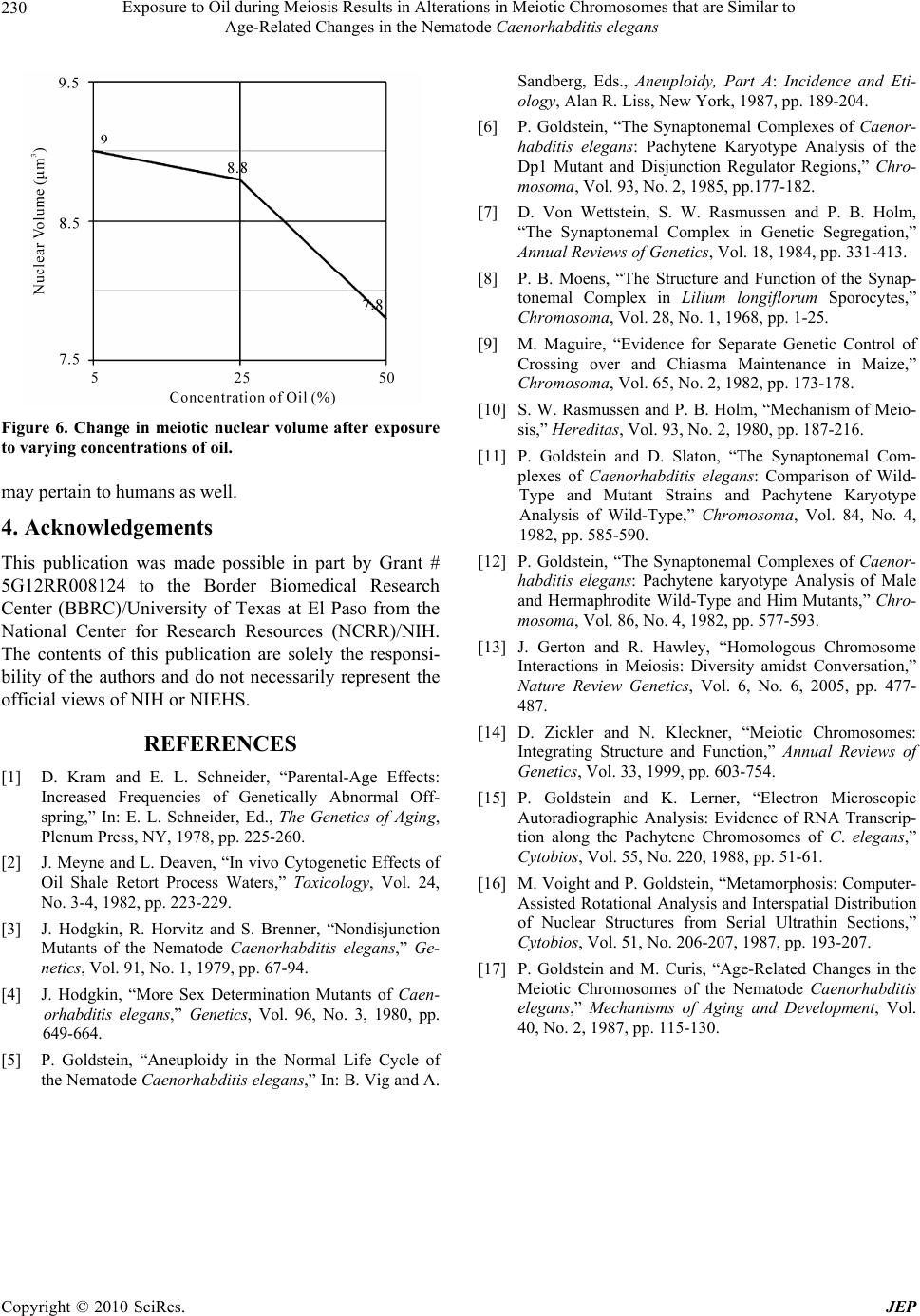 Exposure to Oil during Meiosis Results in Alterations in Meiotic Chromosomes that are Similar to Age-Related Changes in the Nematode Caenorhabditis elegans Copyright © 2010 SciRes. JEP 230 Figure 6. Change in meiotic nuclear volume after exposure to varying concentrations of oil. may pertain to humans as well. 4. Acknowledgements This publication was made possible in part by Grant # 5G12RR008124 to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso from the National Center for Research Resources (NCRR)/NIH. The contents of this publication are solely the responsi- bility of the authors and do not necessarily represent the official views of NIH or NIEHS. REFERENCES [1] D. Kram and E. L. Schneider, “Parental-Age Effects: Increased Frequencies of Genetically Abnormal Off- spring,” In: E. L. Schneider, Ed., The Genetics of Aging, Plenum Press, NY, 1978, pp. 225-260. [2] J. Meyne and L. Deaven, “In vivo Cytogenetic Effects of Oil Shale Retort Process Waters,” Toxicology, Vol. 24, No. 3-4, 1982, pp. 223-229. [3] J. Hodgkin, R. Horvitz and S. Brenner, “Nondisjunction Mutants of the Nematode Caenorhabditis elegans,” Ge- netics, Vol. 91, No. 1, 1979, pp. 67-94. [4] J. Hodgkin, “More Sex Determination Mutants of Caen- orhabditis elegans,” Genetics, Vol. 96, No. 3, 1980, pp. 649-664. [5] P. Goldstein, “Aneuploidy in the Normal Life Cycle of the Nematode Caenorhabditis elegans,” In: B. Vig and A. Sandberg, Eds., Aneuploidy, Part A: Incidence and Eti- ology, Alan R. Liss, New York, 1987, pp. 189-204. [6] P. Goldstein, “The Synaptonemal Complexes of Caenor- habditis elegans: Pachytene Karyotype Analysis of the Dp1 Mutant and Disjunction Regulator Regions,” Chro- mosoma, Vol. 93, No. 2, 1985, pp.177-182. [7] D. Von Wettstein, S. W. Rasmussen and P. B. Holm, “The Synaptonemal Complex in Genetic Segregation,” Annual Reviews of Genetics, Vol. 18, 1984, pp. 331-413. [8] P. B. Moens, “The Structure and Function of the Synap- tonemal Complex in Lilium longiflorum Sporocytes,” Chromosoma, Vol. 28, No. 1, 1968, pp. 1-25. [9] M. Maguire, “Evidence for Separate Genetic Control of Crossing over and Chiasma Maintenance in Maize,” Chromosoma, Vol. 65, No. 2, 1982, pp. 173-178. [10] S. W. Rasmussen and P. B. Holm, “Mechanism of Meio- sis,” Hereditas, Vol. 93, No. 2, 1980, pp. 187-216. [11] P. Goldstein and D. Slaton, “The Synaptonemal Com- plexes of Caenorhabditis elegans: Comparison of Wild- Type and Mutant Strains and Pachytene Karyotype Analysis of Wild-Type,” Chromosoma, Vol. 84, No. 4, 1982, pp. 585-590. [12] P. Goldstein, “The Synaptonemal Complexes of Caenor- habditis elegans: Pachytene karyotype Analysis of Male and Hermaphrodite Wild-Type and Him Mutants,” Chro- mosoma, Vol. 86, No. 4, 1982, pp. 577-593. [13] J. Gerton and R. Hawley, “Homologous Chromosome Interactions in Meiosis: Diversity amidst Conversation,” Nature Review Genetics, Vol. 6, No. 6, 2005, pp. 477- 487. [14] D. Zickler and N. Kleckner, “Meiotic Chromosomes: Integrating Structure and Function,” Annual Reviews of Genetics, Vol. 33, 1999, pp. 603-754. [15] P. Goldstein and K. Lerner, “Electron Microscopic Autoradiographic Analysis: Evidence of RNA Transcrip- tion along the Pachytene Chromosomes of C. elegans,” Cytobios, Vol. 55, No. 220, 1988, pp. 51-61. [16] M. Voight and P. Goldstein, “Metamorphosis: Computer- Assisted Rotational Analysis and Interspatial Distribution of Nuclear Structures from Serial Ultrathin Sections,” Cytobios, Vol. 51, No. 206-207, 1987, pp. 193-207. [17] P. Goldstein and M. Curis, “Age-Related Changes in the Meiotic Chromosomes of the Nematode Caenorhabditis elegans,” Mechanisms of Aging and Development, Vol. 40, No. 2, 1987, pp. 115-130. |

