Paper Menu >>
Journal Menu >>
 American Journal of Analytical Chemistry, 2010, 2, 70-72 doi:10.4236/ajac.2010.12009 Published Online August 2010 (http://www.SciRP.org/journal/ajac) Copyright © 2010 SciRes. AJAC A New Sesquiterpene from Trichilia casarettii (Meliaceae) Ivo José Curcino Vieira1,2*, Elaine Rodrigues Figueiredo2, Virginia Rodrigues Freitas2, Leda Mathias1, Raimundo Braz-Filho3, Renata Mendonça Araújo4 1Laboratório de Ciências Químicas-CCT, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Brazil 2Laboratório de Tecnologia de Alimentos-CCTA, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Brazil 3Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Brazil 4Departamento de Química-CCET, Universidade Federal do Rio Grande do Norte, Natal, Brazil E-mail: curcino@uenf.br Received July 1, 2010; revised July 27, 2010; accepted August 2, 2010 Abstract The dichloromethane extract of the air-dried stems of Trichilia casarettii afforded a new sesquiterpene (1), lupeol, stigmasterol, campesterol and sitosterol. The structure of 1 was elucidated by extensive one-and two- dimensional nuclear magnetic resonance and mass spectrometry. Keywords: Trichilia Casarettii, Meliaceae, Sesquiterpene 1. Introduction The Meliaceae family has attracted much interest among bioproduction phytochemists because of its very com- plex and diverse chemical structures and its biological activity, mainly against insects [1-3]. The Trichilia genus (Meliaceae) includes about 230 species distributed throughout tropical America which are recognized for their significant economic importance and high commer- cial value. The genus is rich in terpenoids, including triterpenes, limonoids, steroids and other terpenes de- rivatives [3-5]. In previous the activity of aqueous extract of leaves and twigs from T. casarettii was evaluated on Spodop- tera frugiperda (J. E. Smith) development in laboratory conditions [6]. To the best of our knowledge, the litera- ture reports no chemical investigation evaluation of T. casarettii native of Americas [7]. This stimulated our interest in the present work, involving isolation and structural elucidation of the constituents of the stems of this species. Then, we report the isolation of new ses- quiterpene 1 (Figure 1) of T. casarettii DC. The stems also afforded lupeol, stigmasterol, campesterol, sitosterol and fatty acid esters. The stems from T. casarettii DC., was collected on November 2006, at Vale do Rio Doce Cia., Linhares City, Espírito Santo State, Brazil. A voucher specimen (No 449) was deposited at Vale do Rio Doce Cia. Her- barium. 2. Results and Discussion Comound 1 (Figure 1) was obtained as white powder (MeOH), mp 121-122C, and which is optically active with an = -18.1 (CHCl3, c 0.002). Its IR spectrum (KBr disk) obtained in spectrometer Shimadzu, model FTIR-8300, showed bands at max 3433 (O-H stretching), 3394 (O-H stretching) cm-1. Comparative analysis using spectrometer Brüker, DRX model [(operating at 400 (1H) and 100 (13C) MHz, respectively, in pyridine-d5)] of the {1H}- and DEPT 135°-13C NMR spectra (Table 1) re- vealed signals corresponding to 15 carbon atoms. These data allowed us to recognize the presence of signals cor- responding to three nonhydrogenated carbons [(C)3: all sp3 (including two bound to an oxygen atoms at C 71.51 and 71.58)], two methine [(CH)2: all sp3, two α to a carbinolic carbon atom at C 55.64/ H 1.58 and C 51.14/ H 1.60, correlated in the HMQC spectrum with 1H chemical 23 ][ D 15 14 13 12 11 10 9 8 7 6 5 4 3 21 OH H HOH Figure 1. Chemical structure of ambrosanoli-10, 11-diol (1). 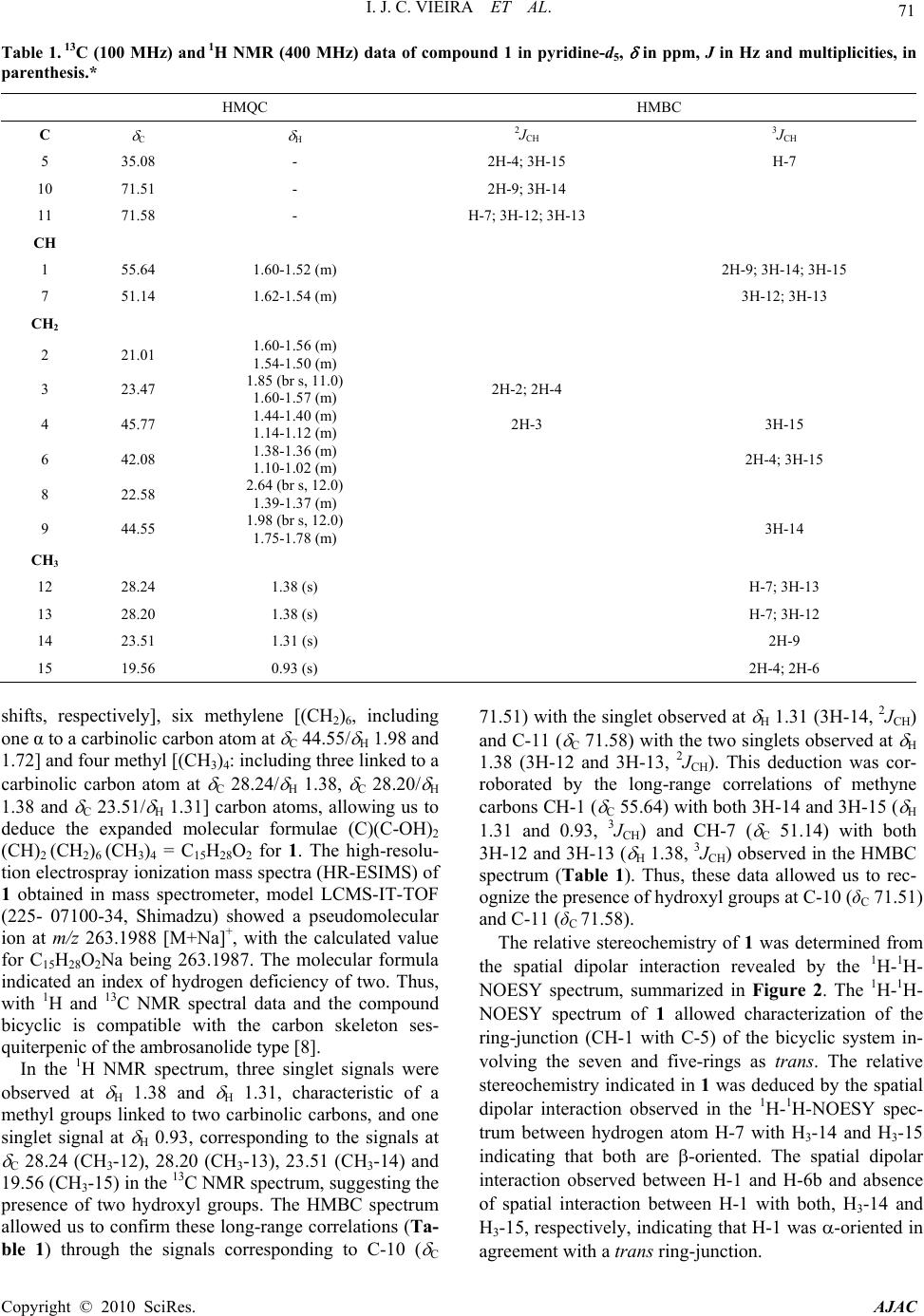 I. J. C. VIEIRA ET AL. Copyright © 2010 SciRes. AJAC 71 Table 1. 13C (100 MHz) and 1H NMR (400 MHz) data of compound 1 in pyridine-d5, in ppm, J in Hz and multiplicities, in parenthesis.* HMQC HMBC C C H 2JCH 3JCH 5 35.08 - 2H-4; 3H-15 H-7 10 71.51 - 2H-9; 3H-14 11 71.58 - H-7; 3H-12; 3H-13 CH 1 55.64 1.60-1.52 (m) 2H-9; 3H-14; 3H-15 7 51.14 1.62-1.54 (m) 3H-12; 3H-13 CH2 2 21.01 1.60-1.56 (m) 1.54-1.50 (m) 3 23.47 1.85 (br s, 11.0) 1.60-1.57 (m) 2H-2; 2H-4 4 45.77 1.44-1.40 (m) 1.14-1.12 (m) 2H-3 3H-15 6 42.08 1.38-1.36 (m) 1.10-1.02 (m) 2H-4; 3H-15 8 22.58 2.64 (br s, 12.0) 1.39-1.37 (m) 9 44.55 1.98 (br s, 12.0) 1.75-1.78 (m) 3H-14 CH3 12 28.24 1.38 (s) H-7; 3H-13 13 28.20 1.38 (s) H-7; 3H-12 14 23.51 1.31 (s) 2H-9 15 19.56 0.93 (s) 2H-4; 2H-6 shifts, respectively], six methylene [(CH2)6, including one α to a carbinolic carbon atom at C 44.55/ H 1.98 and 1.72] and four methyl [(CH3)4: including three linked to a carbinolic carbon atom at C 28.24/ H 1.38, C 28.20/ H 1.38 and C 23.51/ H 1.31] carbon atoms, allowing us to deduce the expanded molecular formulae (C)(C-OH)2 (CH)2 (CH2)6 (CH 3)4 = C15H28O2 for 1. The high-resolu- tion electrospray ionization mass spectra (HR-ESIMS) of 1 obtained in mass spectrometer, model LCMS-IT-TOF (225- 07100-34, Shimadzu) showed a pseudomolecular ion at m/z 263.1988 [M+Na]+, with the calculated value for C15H28O2Na being 263.1987. The molecular formula indicated an index of hydrogen deficiency of two. Thus, with 1H and 13C NMR spectral data and the compound bicyclic is compatible with the carbon skeleton ses- quiterpenic of the ambrosanolide type [8]. In the 1H NMR spectrum, three singlet signals were observed at H 1.38 and H 1.31, characteristic of a methyl groups linked to two carbinolic carbons, and one singlet signal at H 0.93, corresponding to the signals at C 28.24 (CH3-12), 28.20 (CH3-13), 23.51 (CH3-14) and 19.56 (CH3-15) in the 13C NMR spectrum, suggesting the presence of two hydroxyl groups. The HMBC spectrum allowed us to confirm these long-range correlations (Ta- ble 1) through the signals corresponding to C-10 ( C 71.51) with the singlet observed at H 1.31 (3H-14, 2JCH) and C-11 ( C 71.58) with the two singlets observed at H 1.38 (3H-12 and 3H-13, 2JCH). This deduction was cor- roborated by the long-range correlations of methyne carbons CH-1 ( C 55.64) with both 3H-14 and 3H-15 ( H 1.31 and 0.93, 3JCH) and CH-7 ( C 51.14) with both 3H-12 and 3H-13 ( H 1.38, 3JCH) observed in the HMBC spectrum (Table 1). Thus, these data allowed us to rec- ognize the presence of hydroxyl groups at C-10 (δC 71.51) and C-11 (δC 71.58). The relative stereochemistry of 1 was determined from the spatial dipolar interaction revealed by the 1H-1H- NOESY spectrum, summarized in Figure 2. The 1H-1H- NOESY spectrum of 1 allowed characterization of the ring-junction (CH-1 with C-5) of the bicyclic system in- volving the seven and five-rings as trans. The relative stereochemistry indicated in 1 was deduced by the spatial dipolar interaction observed in the 1H-1H-NOESY spec- trum between hydrogen atom H-7 with H3-14 and H3-15 indicating that both are -oriented. The spatial dipolar interaction observed between H-1 and H-6b and absence of spatial interaction between H-1 with both, H3-14 and H3-15, respectively, indicating that H-1 was -oriented in agreement with a trans ring-junction.  72 I. J. C. VIEIRA ET AL. H3C HOH H H H H H H CH3 H CH3 H3COH 10 14 7 1 45 15 12 13 2 6 Figure 2. Selected NOESY correlations and relative stereo- chemistry for compound 1. Arrows denote the main NOESY correlations. Additional spatial interaction is shown in Figure 2. Therefore, the structure of the new sesquiterpene (Figure 1) isolated from T. casarettii was defined as am- brosanoli-10,11-diol (1) (30 mg, 0.0433%). The structures of lupeol [9], -sitosterol [10] and stigmasterol [10] were deduced by comparison of their 1H and 13C NMR spectral data with those reported in the literature. 3. Acknowledgements The authors are grateful to Fundação de Amparo à Pes- quisa do Estado do Rio de Janeiro (FAPERJ) for grants and to Conselho Nacional de Desenvolvimento Científico (CNPq-Brazil) for a research fellowship and grants. 4. References [1] Y. S. Xie, M. B. Isman, P. Gunning, S. Mackinnon, J. T. Arnason, D. R. Taylor, P. Sánchez, C. Hasbun and G. H. N. Towers, “Biological Activity of Extracts of Trichilia Species and the Limonoid Hirtin against Lepidopteran Larvae,” Biochemical Systematics and Ecology, Vol. 22, No. 2, March 1994, pp. 129-136. [2] T. D. Pennington and B. D. Styles, “A Generic Mono- graph of Meliaceae,” Blumea, Vol. 22, No. 3, August- December 1975, pp. 419-540. [3] M. T. Pupo, P. C. Vieira, J. B. Fernandes, M. F. G. F. Silva and J. R. Pirani, “Terpenoids and Steroids from Tri- chilia Species,” Journal of the Brazilian Chemical Soci- ety, Vol. 13, No. 3, June 2002, pp. 382-388. [4] M. C. Ramírez, R. A. Toscano, J. Arnason, S. Omar, C. M. Cerda-Garcia-Rojas and R. Mata, “Structure, Con- formation and Absolute Configuration of New An- tifeedant Dolabellanes from Trichilia trifolia,” Tetrahe- dron, Vol. 56, No. 29, July 2000, pp. 5085-5091. [5] D. A. G. Cortez, J. B. Fernandes, P. C. Vieira, M. F. G. F. Silva, A. G. Ferreira, Q. B. Cass and J. R. Pirani, “Melia- cin Butenolides from Trichilia estipulata,” Phytochemis- try, Vol. 49, No. 8, December 1998, pp. 2493-2496. [6] P. C. Bogorni and J. D. Vendramim, “Sublethal Effect of Aqueous Extracts of Trichilia spp. on Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) De- velopment on Maize,” Neotropical Entomolology, Vol. 34, No. 2, March/April 2005, pp. 311-317. [7] P. C. Patrício and A. C. Cervi, “O Gênero Trichilia P. Browne (Meliaceae) no estado do Paraná, Brasil,” Acta Biologica Paranaense, Vol. 34, No. 1-4, January/April 2005, pp. 27-71. [8] A. U. Rahman and V. U. Ahmad, “13C-NMR of Natural Products: Monoterpenes and Sesquiterpenes,” Hussain Ebrahim Jamal Research Institute of Chemistry, Univer- sity of Karachi, Pakistan, Vol. 1, 1992. [9] V. U. Ahmad and A. U. Rahman, “Handbook of Natural Products Data, Pentacyclic Triterpenoids,” Elsevier Press, Karachi, Vol. 2, 1994. [10] N. P. Rai, B. B. Adhikari, A. Paudel, K. Masuda, R. D. Mckelvey and M. D. Madandhar, “Phytochemical Con- stituents of the Flowers of Sarcococca coriacea of Nep- alese Origin,” Journal of Nepal Chemical Society, Vol. 21, 2006, pp. 1-7. Copyright © 2010 SciRes. AJAC |

