 Journal of Cancer Therapy, 2012, 3, 1151-1158 http://dx.doi.org/10.4236/jct.2012.36150 Published Online December 2012 (http://www.SciRP.org/journal/jct) 1151 Hypofractioned Radiation Therapy in the Treatment of Partial Breast: 30 Gy in Five Consecutive Fractions Sara Terenzi, Rosaria Barbarino, Maria Daniela Falco, Daniela di Cristino, Luana Di Murro, Dania Janniello, Gianluca Ingrosso, Alessandra Murgia, Grazia Tortorelli, Barbara Tolu, Riccardo Santoni Department of Diagnostic Imaging, Molecular Imaging, Interventional Radiology and Radiotherapy, Tor Vergata University General Hospital, Viale Oxford, Rome, Italy. Email: sara_terenzi@fastwebnet.it Received September 13th, 2012; revised October 12th, 2012; accepted October 24th, 2012 ABSTRACT Background and Purpose: Recent prospective studies have explored the partial breast irradiation (PBI) for patients with early-stage breast cancer using different technical approaches. The purpose of this study is to explore feasibility, tumor control and acute and late toxicity of a specific hypo-fractionated 3D-CRT when treating postmenopausal pa- tients with early breast cancer with partial breast irradiation, using five fractions in five consecutive days. Materials and Methods: Ten patients, aged 70 underwent breast conservative surgery for invasive breast carcinoma with a complete microscopic resectio n; no lymphovascu lar invasion was found and negative axillary node status was assessed. Metal clips were positioned in the surgical bed at the time of surgery. All of the patients provided an informed consent for breast irradiation. Seven patients received Tamoxifen. Of the ten patients, five were treated for left breast disease, and five for right breast disease. The dose fractionation schedule was 3000 cGy delivered to the isocenter in 5 fractions (600 cGy/fr) using 6 MV photons. According to the lin ear quadratic model and a / ratio of 4 Gy this prescription is equivalent to 50 Gy in a standard 2-Gy fractionation schedule. Patients were treated in the supine position. A comer- cial breast board was used as immobilization device in order to keep the arms of the patient raised. The clinical target volume (CTV) was drawn with a uniform 1-cm three-dimensional margin around the surg ical clips. The CTV was lim- ited to 3 mm from the skin surface and 3 mm from the lung-chest wall interface. A three-dimensional margin was added to the CTV to obtain the planning target volume (PTV). The ipsilateral and controlateral breast, the ipsilateral and con- trolateral lung, heart and spinal cord were contoured as organs at risk (OAR). The treatment was developed using Pre- cise Plan Treatment Planning System and four no-coplanar fields. The constraints used have been: uninvolved breast (ipsilateral breast-PTV): V15 50%; heart: V3 10%; ipsilateral lung: V10 20%; controlateral lung: V5 10% and controlateral breast: maximum dose 1 Gy. We required PTV coverage of 90%. Patient set-up was verif ied ever y day before treatment using portal images. No tumour bed boost was delivered. Clinical assessments of early normal tissue reaction were carried out every day during radiotherapy and 10 days after the end of the treatment. After radiotherapy, we visited all patients every 3 months during the first 2 years and ever y six month thereaf ter. Fron tal and lateral p ictur es of the breast were taken on the first day of treatment (baseline), at the end of treatment, 10 days after the end of treat- ment and at the first follow-up. Any change in breast appearance compared with the baseline picture was scored on a four-point RTOG for acute and late radiation morbidity scoring scale. Results: No local or distant recurrences were ob- served and then confirmed by mammograms performed every year and breast ultrasound performed every six months. For acute and late toxicity, only 2 patients developed acute effects at the end of the treatment. Conclusion: The clinical outcomes observed in ten patients demon strate a good feasibility of the schedu le adopted both in terms of tumour con- trol and acute and late toxicity, with good co smetics results. Long term follow-up and a large number of patien ts will be needed for full evaluation. Keywords: Breast Cancer; Partial Breast I rradiation; Hy pofractioned 1. Introduction In the past decades, a major change has occurred in the local management of breast cancer, from a mutilating therapeutic approach to a conservative approach with osmetic and functional aims. The use of conservative surgery combined with whole-breast irradiation has been established as a valid alternative to mastectomy. The Copyright © 2012 SciRes. JCT  Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions 1152 conservative approach consists in the removal of the tu- mor, followed by 5 - 7 weeks of daily whole breast irra- diation (total dose of 50 Gy delivered to the entire breast and 10 - 16 Gy boost delivered to the tu mor bed). A dis- advantage of this approach is the increase of the non- breast-cancer-related morbidity due to irradiation of non- target tissue [1,2] and the prolonged duration of treat- ment. Observation from earlier studies demonstrated that distant recurrences, in quadrants other than that origin- nally involved by the tumour, occur infrequently (range, 0.6% - 6%) [3-13]. A strategy that aims at improving the therapeutic ratio and at reducing treatment duration, in women with rela- tively low risk of local tumour relapse, involves limited high-radiation doses to the index quadrant and reduces doses to breast tissue remote from the tumour bed [14,15]. Radiobiological analysis of clinical data has shown that breast adenocarcinomas have an / ratio of 4 Gy, like late reacting normal tissues. Consequently, hypo-fractionation in breast cancer may have a reason- able radiobiological support. Recent prospective studies have thus explored the techniques of only treating the tumor bed of the breast, i.e. partial breast irradiation (PBI), for patients with early-stage breast cancer using different technical approaches [16-24]. These studies have investigated the use of low-dose-rate and high-dose- rate brachytherapy and the use of External-Beam Radio- therapy (EBRT) for partial breast irradiation. The purpose of this study is to evaluate feasibility, tumor control and acute and late toxicity of a specific hypo-fractionated 3D-CRT in the treatment of partial breast in postmenopausal patients with early breast can- cer, using five consecutive 6 Gy fractions. 2. Material and Methods 2.1. Patients Sarting on January 2008 ten patients, out of all those who underwent breast conservative surgery for invasive breast carcinoma, received postoperative radiotherapy delivered to the index quadrant only after having provided full written informed consent. The in clusion criteria are listed in Table 1. All of the patients enro lled in the study were in postmenopausal status, age ranged from 70 to 84 years (median 76 years). Eight patients had Stage I invasive ductal carcinoma and two patients had Stage I invasive lobular carcinoma. Tumour size ranged between 10 mm and 20 mm, with a median of 14 mm. Seven patients had positive estrogenic receptors and received Tamoxifen, no patients received chemotherapy. All patients underwent lumpectomy with negative surgical margins. The sur- geons were requested to place clips at the borders of the surgical bed, using a minimum of six clips. The presence of surgical clips represented a selection criteria to avoid geographic misses. Of the ten patients, five were treated for left breast disease, and five for right breast disease. The main patients’ characteristics are listed in Table 2. Clinical assessments of early normal tissue reaction were carried out every day during radiotherapy and after 10 days from the end of the treatment. After radiotherapy, all of the patients underwen t a clinical examination every 3 months during the first two years and every six months subsequently. Median follow-up from the end of irradia- tion was 21.1 months (range, 10 - 48 m ont h s ). Bilateral mammogram, and bilateral breast ultrasound were obtained once a year during follow-up. An echo- cardiogram was obtained in patients with left breast can- cer. Frontal and lateral pictures (depending on the tumour Table 1. Inclusion criteria. Age > 70 aa Patological stage pT1 pN0 Surgical margin negative (>2 mm) Clips placed in tumor bed Full informed consent from patient No lymphovascular invasion Unifocal Intraductal component < 25% ER and PgR positive ER = Esrogen Receptor; PgR = Progesteron Receptor Table 2. Baseline patient characteristics (n = 10). Characteristics Patients Breast side Right 5 Left 5 Tumor estrogen receptor status Positive 10 Negative 0 Tumor progesterone receptor status Positive 10 Negative 0 Tumor Her-2 status Score 0 3 Score 1 2 Score 2 2 Score 3 1 Unknown 2 Copyright © 2012 SciRes. JCT 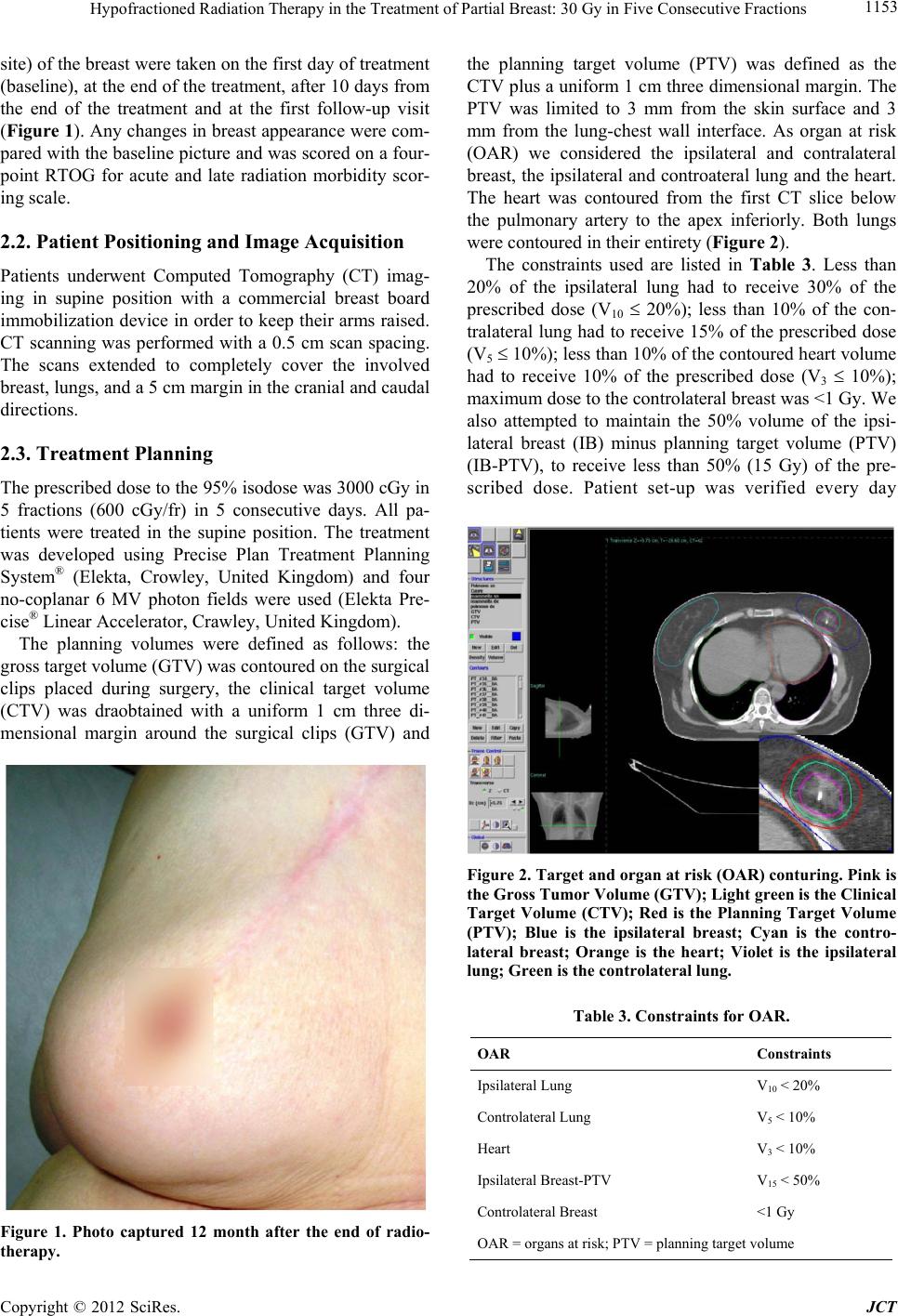 Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions 1153 site) of the breast were taken on the first day of treatment (baseline), at the end of the treatment, after 10 days from the end of the treatment and at the first follow-up visit (Figure 1). Any changes in breast appearance were com- pared with the baseline picture and was scored on a four- point RTOG for acute and late radiation morbidity scor- ing scale. 2.2. Patient Positioning and Image Acquisition Patients underwent Computed Tomography (CT) imag- ing in supine position with a commercial breast board immobilization device in order to keep th eir arms raised. CT scanning was performed with a 0.5 cm scan spacing. The scans extended to completely cover the involved breast, lungs, and a 5 cm margin in the cranial and caudal directions. 2.3. Treatment Planning The prescribed do se to the 95% isodose was 3000 cG y in 5 fractions (600 cGy/fr) in 5 consecutive days. All pa- tients were treated in the supine position. The treatment was developed using Precise Plan Treatment Planning System® (Elekta, Crowley, United Kingdom) and four no-coplanar 6 MV photon fields were used (Elekta Pre- cise® Linear Accelerator, Crawley, United Kingdom). The planning volumes were defined as follows: the gross targ et volume (GTV) was contou r ed on th e su rgical clips placed during surgery, the clinical target volume (CTV) was draobtained with a uniform 1 cm three di- mensional margin around the surgical clips (GTV) and Figure 1. Photo captured 12 month after the end of radio- therapy. the planning target volume (PTV) was defined as the CTV plus a uniform 1 cm three dimensional margin. The PTV was limited to 3 mm from the skin surface and 3 mm from the lung-chest wall interface. As organ at risk (OAR) we considered the ipsilateral and contralateral breast, the ipsilateral and controateral lung and the heart. The heart was contoured from the first CT slice below the pulmonary artery to the apex inferiorly. Both lungs were contoured in their entirety (Figure 2). The constraints used are listed in Table 3. Less than 20% of the ipsilateral lung had to receive 30% of the prescribed dose (V10 20%); less than 10% of the con- tralateral lung had to receive 15% of the prescribed dose (V5 10%); less than 10% o f the con toured h eart volu me had to receive 10% of the prescribed dose (V3 10%); maximum dose to the controlateral breast was <1 Gy. We also attempted to maintain the 50% volume of the ipsi- lateral breast (IB) minus planning target volume (PTV) (IB-PTV), to receive less than 50% (15 Gy) of the pre- scribed dose. Patient set-up was verified every day Figure 2. Target and organ at risk (OAR) conturing. Pink is the Gross Tumor Volume (GTV); Light green is the Clinical Target Volume (CTV); Red is the Planning Target Volume (PTV); Blue is the ipsilateral breast; Cyan is the contro- lateral breast; Orange is the heart; Violet is the ipsilateral lung; Green is the controlater al lung. Table 3. Constraints for OAR. OAR Constraints Ipsilateral Lung V10 < 20% Controlateral Lung V5 < 10% Heart V3 < 10% Ipsilateral Breast-PTV V15 < 50% Controlateral Breast <1 Gy OAR = organs at risk; PTV = planning target volume Copyright © 2012 SciRes. JCT  Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions Copyright © 2012 SciRes. JCT 1154 before treatment, using orthogonal portal images (Gantry 0˚ and 90˚, coach 0˚) and 2 portal images of the treatment beams. 3. Results The target coverage was acceptable for all patients. The dose-volume constraints of OARs were always respected. Only in 1 patient the uninvolved breast dose-volume constraint was not respected given that the 82% of unin- volved breast volume received more than 15 Gy (Table 4). This was probably due to the anatomic positio n of the tumour (supero-internal quadrant) and to the small vol- ume of the breast (435 cc). We observed grade 1 acute skin toxicity (Radiation Therapy Oncology Group scale) developing during the first week after the end of treatment in 2 patients (20%). No patients had late skin toxicity. No difference was ob- served between patient who received or not Tamoxifen. No patients experienced a reduction in left ventricular ejection fraction or in forced expiratory volume. To date no local recurrence was observed. 4. Discussion In the far past years the treatment for breast cancer was mastectomy while actually the gold standard for patients with early-stage breast cancer is conservative with a cosmetic and functional surgical approach followed by radiotherapy to increase local control and overall survival [25]. The standard radiation therapy treatment has a du- ration of 5 - 7 weeks and the delivered dose is 50 Gy in 25 daily fractions delivered to the entire breast plus 10 Gy boost to the tumour bed. This approach has the dis- advantage of prolonged duration, which can be a serious inconvenience for patients that have to travel every day for a prolonged period to the radiation therapy centre, especially for the elderly ones. Several clinical trials have demonstrated that shorter radiation schedules, justified by radiobiological models, delivering larger doses per fraction in shorter periods of time [26-30] offer equiva- lent local control and same acute and late toxicity com- pared to the standard radiation therapy courses. Whelan et al. [27] examined whether a 22-day radiation therapy fractionation schedule was as effective, on the local con- trol, as the traditional 35-day schedule in 1934 women with invasive breast cancer who underwent BCS with pathologically clear resection margins and negative axil- lary lymph nodes. Patients were randomly assigned to receive 42.5 Gy in 16 fraction over 22 days or 50 Gy in 25 fraction over 35 days to the whole breast. With a me- dian follow-up of 12 years no differences in local recu- rences, disease free or overall survival rates and cosmetic results were recorded. They concluded that the more convenient 22-d ay fractionation schedule app ear to be an acceptable alternative to the 35-day schedule. The START A (Standardization of Breast Radiotherapy) from the UK trial has shown that 41.6 Gy in 13 fractions or 39 Gy in 13 fractions are similar to the standard treatment (50 Gy in 25 fractions) in terms of local-regional tumour control and late normal tissue effects [28]; this results are consistent with those of the START B trial, which has shown that a radiation schedule of 40 Gy in 15 fractions offers equivalent results to the standard schedule of 50 Gy in 25 fractions [29]. Livi et al. [30] evaluated the in- cidence of loco-regional recurrence and the cosmetic results in a group of 539 patients with breast cancer treated with a hypo-fractionated schedule after conserva- tive surgery. The dose delivered was 44 Gy (2.75 daily fraction) and the tumour bed boost was 10 Gy (Electron beam). They obtained a low local relapse and good tol- erance (76.4% patients showing grade 0 - 1 late toxicity, 20.9% patients grade 2 and 2.5% patients grade 3; no patients with grade 4 toxicity was observed). All this fraction regimen do not represent the limits of hypofrac- tionation for whole breast radiotherapy. The UK FAST trial [31] randomized 915 women 50 years old or older with node-negative tumours, following breast conserva- tive surgery, to receive whole breast radiotherapy deliv- ered using 3D dosimetry to a total dose of 50 Gy in 25 fractions (control) versus 28.5 Gy or 30 Gy in 5 once- weekly fractions of 5.7 Gy or 6.0 Gy with no tumour bed boost. The first analysis showed good results in terms of late normal tissue responses and tumour control. A schedule of 30 Gy in 5 fractions over 15 days to the Table 4. DVH analysis: OAR doses. OAR Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 Patient 6 Patient 7 Patient 8 Patient 9 Patient 10 Hearth: dose to 10% volume 0.5 Gy 1 Gy 0.5 Gy1 Gy 2 Gy 0.5 Gy1 Gy 0.1 Gy 1 Gy 1 Gy Ipsilateral Lung: dose to 20% volume 1 Gy 4 Gy 2 Gy 1.5 Gy4 Gy 1 Gy 2 Gy 1 Gy 1 Gy 2 Gy Controlateral Lung: dose to 10% volume 0.1 Gy 0.4 Gy0.1 Gy0.1 Gy1 Gy 0.1 Gy0.1 Gy 0.1 Gy 0.1 Gy0.1 Gy Controlateral Breast: dose to whole organ 0.2 Gy 0.6 Gy0.5 Gy0.1 Gy0.9 Gy0.1 Gy0.1 Gy 0.1 Gy 0.1 Gy0.3 Gy Ipsilateral Breast-PTV: dose to 50% volume 4.5 Gy 4 Gy 27 Gy10 Gy7 Gy 12 Gy14 Gy 5 Gy 6 Gy 10 Gy DVH = dose-volume histogram; OAR = organ at risk.  Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions 1155 whole breast, using 3D dosimetry, reported very mild acute reactions and acceptable 2-year outcome in terms of change in breast appearance compared to a matched sample of patients treated to 50 Gy in 25 fractions [32]. Observations that th e vast majority of ipsilateral breast recurrences occur in close proximity to the lumpectomy cavity have led to question the opportunity of elective partial breast irradiation (PBI), treating only the tumor bed. Baglan KL et al. [21] presented a 3D-CRT tech- nique for partial breast irradiation in supin e position. The prescribed dose was 34 Gy in 5 patients and 38.5 Gy in 4 patients, delivered in 10 fractions twice daily over 5 consecutive days. They reported an excellent patient tol- erance with minimal acute toxicity. No skin changes were noted during treatment, and at the in itial 4 - 8-week follow-up examination, only mild localized hyperpig- mentation and/or erythema were observed. Formenti S. et al. [22] reported the clinical and dose-volume histogram results in 47 patients accrued to a 3D-CRT accelerated partial breast irradiation (APBI) protocol in the prone position. The prescribed do se was 30 Gy at 6 Gy/fraction delivered in 5 fractions within 10 days. The lun g and the heart were spared by treating in the prone position. Acute toxicity was mild (Grade 1 - 2 erythema). With a median follow-up of 18 month only grade 1 late toxicity occurred, and no patient developed local recurrence. Livi L. et al. [23] compared, in a randomized phase III clinical trial, conventional (tangential field) fractionated whole breast treatment (Arm A, 128 patients) with accelerated partial breast irradiation plus intensity-modulated radiotherapy (Arm B, 131 patients). For patients in Arm B (PBI) the prescribed dose was 30 Gy in 5 fractions, 6 Gy/fraction. The rate of Grade 1 and Grade 2 acute skin toxicity was respectively 22% and 19% in Arm A (Radiation Therapy Oncology Group scale). The tolerance in Arm B was excellent with only 5% Grade 1 and 0.8% Grade 2 acute skin toxicity. With a median 9.6 years of follow-up An- tonucci et al. [24] compared a group of patients treated with APBI vs a similar group of patients treated with whole breast irradiation to determine the potential dif- ferences in local recurrence rates according to the vol- ume breast tissue irradiated. The cumulative incidence of ipsilateral breast tumour recurrences at 10 years was 5%. On matched-pair analysis, the rate of ipsilateral breast tumour recurrences was not significantly statistically different between the patient groups. These data suggest the potential efficacy of APBI in selected low-risk pa- tients. Different studies [33-38] demonstrate that breast cancer has the same radiobiological behaviour of late reacting normal tissue ( / ratio of 4 Gy), late effects (fibrosis and telangiectasia) have / ratio of 2 Gy and 4 Gy respectively, and acute reaction (erythema and des- quamation) 8 Gy and 11 Gy respectiv ely. To co mpare th e fractionation schedule of 30 Gy delivered in 5 consecu- tive days with the conventional fractionation of 50 Gy delivered in 32 days, the Biologically Effective Dose (BEDs) has to be calculated assuming cell repopulation during treatment. The BEDs formula taking into account cell repopulation is the fo llow: BED1ln 2. pot k nddTT T were n is the number of fraction, d is the dose per frac- tion, / is a tissue-specific and effect-specific parameter associated with the linear-quadratic model, T is the ov er- all time of radiotherapy (days, with first day counted as day 0), Tk is the Kick-off time of repopulation in the tis- sue of interest (21 days) [26,39,40], is the radiosensi- tivity coefficient of non recoverable damage (0.35) [34, 41] and Tpot is the potential doubling time of cancer re- population cells (3 days) [4 2,43]. This correction for cell proliferation causes the tumour standard treatment BED values to decrease by 3 Gy (from 75 Gy to 72 Gy). The BED values of PBI schedule were calculated with the standard equation: BED 1.nd d considering that the treatment is accomplished within a period that is shorter than th e lag period. Table 5 lists the BEDs for tumour control, the early responses (erythema and desquamation), and the late responses (telangiectasia and fibrosis). The BEDs for normal tissue acute effects were generally lower for the 30 Gy hypo-fractionated schedule than for the standard 50 Gy treatment, indicat- ing that the risk of radiation-induced complications should be lower in the PBI schedule. According with the literature experience [22,23,31,32] and to our very preliminary results we want to increase our experience of a 30 Gy (6 Gy/fraction) fractionation schedule delivered in 5 consecutive days, with three- dimensional conformal radiotherapy (3D-CRT), without considering an intermediate time period in order to have a complete cellular recovery between fractions (>24 h). The proposal of such a simpler and less expensive tech- nique, compared to Intensity Modulated Radiotherapy Table 5. Biologically effective doses (BED). α/β (Gy) Standard (50 Gy) Hypofractionated (30 Gy) Erythema 8 63 53 Desquamation11 59 46 Teleangectasia4 75 75 Fibrosis 2 100 120 Tumor 4 75 75 Tumor* 4 72 75 *Taking into account cell proliferation during course of treatment. Copyright © 2012 SciRes. JCT  Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions 1156 (IMRT), with an excellent coverage of the target volume and excellent results in term of dose-volume histogram for OARs for all patients (Table 4), seems feseable but deserves more experience and long term results before beeing delivered to a larg er population of patients. 5. Conclusion The clinical results observed in ten patients demonstrated a good feasibility of the schedule adopted both in terms of tumour control rate and acute and late toxicity, with good cosmetics results. Encouraged by the protocol study of the University of Florence [23] (where the age inclu- sion criteria is >40 y), we propose to go on with this study delivering this sch edu le to patients younger than 70 years in order to achieve a larger number experience. REFERENCES [1] M. Clarke, R. Collins, S. Darby, C. Davies, P. Elphin- stone, E. Evans, et al., “Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Years Survival: An Overview of the Randomised Trials,” Lancet, Vol. 366, No. 9503, 2005, pp. 2087-2106. [2] S. C. Darby, P. McGale, C. W. Taylor and R. Peto, “Long-Term Mortality from Heart Disease and Lung Cancer after Radiotherapy for Early Breast Cancer: Pro- spective Cohort Study of about 300,000 Women in US SEER Cancer Registries,” Lancet Oncology, Vol. 6, 2005, pp. 557-565. doi:10.1016/S1470-2045(05)70251-5 [3] A. Fourquet, F. Campana, B. Zafrani, V. Mosseri, P. Vielh, J. C. Durand, et al., “Prognostic Factors of Breast Recurrence in the Conservative Management of Early Breast Cancer: A 25-Year Follow-Up,” International Journal of Radiation Oncology*Biology*Physics, Vol. 17, No. 4, 1989, pp. 719-725. doi:10.1016/0360-3016(89)90057-6 [4] J. Boyages, A. Recht, J. L. Connolly, S. J. Schnitt, R. Gelman, H. Kooy, et al., “Early Breast Cancer: Predictors of Breast Recurrence for Patients Treated with Conserva- tive Surgery and Radiation Therapy,” Radiotherapy & Oncology, Vol. 19, No. 1, 1990, pp. 29-41. doi:10.1016/0167-8140(90)90163-Q [5] J. M. Kurtz, J. M. Spitalier, R. Amalric, H. Brandone, Y. Ayme, J. Jacquemier, et al., “The Prognostic Significance of Late Local Recurrence after Breast-Conserving Ther- apy,” International Journal of Radiation Oncology*Bio- logy*Physics, Vol. 18, No. 1, 1990, pp. 87-93. doi:10.1016/0360-3016(90)90271-K [6] B. Fowble, L. J. Solin, D. J. Schultz, J. Rubenstein, R. L. Goodman et al., “Breast Recurrence Following Conserva- tive Surgery and Radiation: Pattern of Failure, Prognosis, and Pathologic Findings from Mastectomy Specimens with Implications for Treatment,” International Journal of Radiation Oncology*Biology*Physics, Vol. 19, 1990, pp. 833- 842. [7] R. M. Clark, P. B. McCulloc h, M. N. Levine, M. Lipa, R. H. Wilkinson, L. J. Mahoney, et al., “Randomized Clini- cal Trial to Assess the Effectiveness of Breast Irradiation Following Lumpectomy and Axillary Dissection for Node Negative Breast Cancer,” Journal of the National Cancer Institute, Vol. 84, No. 9, 1992, pp. 683-689. doi:10.1093/jnci/84.9.683 [8] I. Gage, A. Recht, R. Gelman, A. J. Nixon, B. Silver, B. A. Bornstein, et al., “Long-Term Outcome Following Breast-Conserving Surgery and Radiation Therapy,” In- ternational Journal of Radiation Oncology*Biology* Physics, Vol. 33, No. 2, 1995, pp. 245-251. doi:10.1016/0360-3016(95)02001-R [9] G. Liljegren, L. Holmberg, J. Bergh, A. Lindgren, L. Tabár, H. Nordgren, et al., “10-Year Results after Sector Resection with or without Postoperative Radiotherapy for Stage I Breast Cancer: A Randomized Trial,” Journal of Clinical Oncology, Vol. 17, No. 8, 1999, pp. 2326-2333. [10] E. Touboul, L. Buffat, Y. Belkacémi, J. P. Lefranc, S. Uzan, P. Lhuillie r, et al., “Local Recurrences and Distant Metastases after Breast-Conserving Surgery and Radia- tion Therapy for Early Breast Cancer,” International Journal of Radiation Oncology*Biology*Physics, Vol. 43, No. 1, 1999, pp. 25-38. doi:10.1016/S0360-3016(98)00365-4 [11] T. E. Smith, D. Lee, B. C. Turner, D. Carter and B. G. Haffty, “True Recurrence vs New Primary Ipsilateral Breast Tumor Relapse: An Analysis of Clinical and Pathologic Differences and Their Implications in Natural History, Prognosis, and Therapeutic Management,” In- ternational Journal of Radiation Oncology*Biology* Physics, Vol. 48, No. 5, 2000, pp. 1281-1289. doi:10.1016/S0360-3016(00)01378-X [12] U. Veronesi, E. Marubini, L. Mariani, V. Galimberti, A. Luini, P. Veronesi, et al., “Radiotherapy after Breast- Conserving Surgery in Small Breast Carcinoma: Long- Term Results of Randomized Trial,” Annals of Oncology, Vol. 12, No. 7, 2001, pp. 997-1003. doi:10.1023/A:1011136326943 [13] E. Huang, T. A. Buchholz, F. Meric, S. Krishnamurthy, N. Q. Mirza, F. C. Ames, et al., “Classifying Local Disease Recurrences after Breast Conservation Therapy Based on Location and Histology: New Primary Tumors Have More Favorable Outcomes than True Local Disease Re- currences,” Cancer, Vol. 95, No. 10, 2002, pp. 2059-2067. doi:10.1002/cncr.10952 [14] J. M. Kurtz, R. Amalric, H. Brandone, Y. Ayme, J. Jacquemier, J. C. Pietra, et al., “Local Recurrence after Breast-Conserving Surgery and Radiotherapy. Frequency, Time Course, and Prognosis,” Cancer, Vol. 63, No. 10, 1989, pp. 1912-1917. doi:10.1002/1097-0142(19890515)63:10<1912::AID-CN CR2820631007>3.0.CO;2-Y [15] T. E. Smith, D. Lee, B. C. Turner, D. Carter, B. G. Haffty, “True Recurrence vs New Primary Ipsilateral Breast Tu- mor Relapse: An Analysis of Clinical and Pathologic Differences and Their Implications in Natural History, Prognoses, and Therapeutic Management,” International Journal of Radiation Oncology*Biology*Physics, Vol. 48, 2000, pp. 1281-1289. doi:10.1016/S0360-3016(00)01378-X Copyright © 2012 SciRes. JCT  Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions 1157 [16] L. Cionini, P. Pacini and S. Marzano, “Exclusive Brachy- therapy after Conservative Surgery in Cancer of the Breast,” Lyon Chirurgical, Vol. 89, 1993, p. 128. [17] L. Krishnan, W. R. Jewell, O. W. Tawfik and E. C. Krish- nan, “Breast Conservation Therapy with Tumor Bed Irra- diation Alone in a Selected Group of Patients with Stage I Breast Cancer,” Breast Journal, Vol. 7, No. 2, 2001, pp. 91-96. doi:10.1046/j.1524-4741.2001.007002091.x [18] F. A. Vicini, K. L. Baglan, L. L. Kestin, C. Mitchell, P. Y. Chen, R. C. Frazier, et al., “Accelerated Treatment of Breast Cancer,” Journal of Clinical Oncology, Vol. 19, No. 7, 2001, pp. 1993-2001. [19] D. W. Arthur, D. Koo, R. D. Zwicker, S. Tong, H. D. Bear, B. J. Kaplan, et al., “Partial Breast Brachytherapy after Lumpectomy: Low-Dose-Rate and High-Dose-Rate Experience,” International Journal of Radiation Oncol- ogy*Biology*Physics, Vol. 56, No. 3, 2003, pp. 681-689. doi:10.1016/S0360-3016(03)00120-2 [20] C. Leonard, D. Carter, J. Kercher, K. Howell, P. Hen- kenberns, M. Tallhamer, et al., “Prospective Trial of Ac- celerated Partial Breast Intensity-Modulated Radiothe- ratpy,” International Journal of Radiation Oncology*Bio- logy*Physics, Vol. 67, No. 5, 2007, pp. 1291-1298. doi:10.1016/j.ijrobp.2006.11.016 [21] K. L. Baglan, M. B. Sharpe, D. Jaffray, R. C. Frazier, J. Fayad, L. L. Kestin, et al., “Accelerated Partial Breast Ir- radiation Using 3D Conformal Radiation Therapy (3D- CRT),” International Journal of Radiation Oncology* Biology*Physics, Vol. 55, No. 4, 2003, pp. 302-311. doi:10.1016/S0360-3016(02)03811-7 [22] S. C. Formenti, M. T. Truong, J. D. Goldberg, V. Mukhi, B. Rosenstein, D. Roses, et al., “Prone Accelera ted Part ial Breast Irradiation after Breast-Conserving Surgery: Pre- liminary Clinical Results and Dose-Volume Histogram Analysis,” International Journal of Radiation Oncology* Biology*Physics, Vol. 60, No. 2, 2004, pp. 493-504. doi:10.1016/j.ijrobp.2004.04.036 [23] L. Livi, F. B. Buonamici, G. Simontacchi, V. Scotti, M. Fambrini, A. Compagnucci, et al., “Accelerated Partial Breast Irradiation with IMRT: New Tecnical Approach and Interim Analysis of Acute Toxicity in a Phase III Randomized Clinical Trial,” International Journal of Ra- diation Oncology*Biology*Physics, Vol. 77, No. 2, 2010, pp. 509-515. doi:10.1016/j.ijrobp.2009.04.070 [24] J. V. Antonucci, M. Wallace, N. S. Goldstein, L. Kestin, P. Chen, P. Benitez, et al., “Differences in Patterns of Failure in Patients Treated with Accelerated Partial Breast Irradiation versus Whole-Breast Irradiation: A Matched- Pair Analysis with 10-Year Follow-Up,” International Journal of Radiation Oncology*Biology*Physics, Vol. 74, No. 2, 2009, pp. 447-452. doi:10.1016/j.ijrobp.2008.08.025 [25] B. Fisher, S. Anderson, J. Bryant, R. G. Margolese, M. Deutsch, E. R. Fisher, et al., “Twenty-Year Follow-Up of a Randomized Trial Comparing Total Mastectomy, Lum- pectomy, and Lumpectomy plus Irradiation for the Treat- ment of Invasive Breast Cancer,” New England Journal of Medici ne, Vol. 347, No. 16, 2002, pp. 1233-1241. doi:10.1056/NEJMoa022152 [26] J. Fowler, “The Linear-Quadratic Formula and Progress in Fractionated Radiotherapy,” British Journal of Radio- logy, Vol. 62, No. 740, 1989, pp. 679-694. doi:10.1259/0007-1285-62-740-679 [27] T. J. Whelan, J. P. Pignol, M. N. Levine, J. A. Julian, R. MacKenzie, S. Parpia, et al., “Long-Term Results of Hy- pofractionated Radiation Therapy for Breast Cancer,” New England Journal of Medicine, Vol. 362, No. 6, 2010, pp. 513-520. doi:10.1056/NEJMoa0906260 [28] START Trialists’ Group, S. M. Bentzen, R. K. Agrawal, E. G. Aird, J. M. Barrett, P. J. Barrett-Lee, J. M. Bliss, J. Brown, J. A. Dewar, H. J. Dobbs, J. S. Haviland, P. J. Hoskin, P. Hopwood, P. A. Lawton, B. J. Magee, J. Mills, D. A. Morgan, J. R. Owe n, S. Simmons, G. Sumo, M. A. Sydenham, K. Venables and J. R. Yarnold, “The UK Standardization of Breast Radiotherapy (START) Trial a of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomized Trial,” Lancet Oncology, Vol. 9, No. 4, 2008, pp. 331-341. doi:10.1016/S1470-2045(08)70077-9 [29] START Trialists’ Group, S. M. Bentzen, R. K. Agrawal, E. G. Aird, J. M. Barrett, P. J. Barrett-Lee, S. M. Bentzen, J. M. Bliss, J. Brown, J. A. Dewar, H. J. Dobbs, J. S. Haviland, P. J. Hoskin, P. Hopwood, P. A. Lawton, B. J. Magee, J. Mills, D. A. Morgan, J. R. Owen, S. Simmons, G. Sumo, M. A. Sydenham, K. Venables, J. R. Yarnold, “The UK Standardization of Breast Radiotherapy (START) Trial B of Radiotherapy Hypofractionation for Treatment of Early Breast Cancer: A Randomized Trial,” Lancet, Vol. 371, No. 9618, 2008, pp. 1098-1107. doi:10.1016/S0140-6736(08)60348-7 [30] L. Livi, M. Stefanacci, S. Scoccianti, D. Dicosmo, S. Borghesi, F. Nosi, et al., “Adjuvant Hypofractionated Radiation Therapy for Breast Cancer after Conserving Surgery,” Clinical oncology (Royal College of Radiolo- gists), Vol. 19, No. 2, 2007, pp. 120-124. doi:10.1016/j.clon.2006.11.006 [31] FAST Trialists Group, R. K. Agrawal, A. Alhasso, P. J. Barrett-Lee, J. M. Bliss, P. Bliss, D. Bloomfield, J. Bowen, A. M. Brunt, E. Donovan, M. Emson, A. Good- man, A. Harnett, J. S. Haviland, R. Kaggwa, J. P. Morden, A. Robinson, S. Simmons, A. Stewart, M. A. Sydenham, I. Syndikus, J. Tremlett, Y. Tsang, D. Wheatley, K. Venables and J. R. Yarnold, “First Results of the Ran- domised UK FAST Trial of Radiotherapy Hypofractiona- tion for Treatment of Early Breast Cancer (CRUKE/ 04/015),” Radiotherapy and Oncology, Vol. 100, No. 1, 2011, pp. 93-100. doi:10.1016/j.radonc.2011.06.026 [32] S. Martin, M. Mannino, A. Rostom, D. Tait, E. Donovan, S. Eagle, et al. , “Acute Toxicity and 2-Years Adverse Ef- fects of 30 Gy in Five Fractions over 15 Days to Whole Breast after Local Excision of Early Breast Cancer,” Clinical oncology (Royal College of Radiologists), Vol. 20, No. 7, 2008, pp. 502-505. doi:10.1016/j.clon.2008.04.020 [33] G. W. Barendsen, “Dose Fractionation, Dose Rate and Iso-Effect Relationship for Normal Tissue Responses,” International Journal of Radiation Oncology*Biology* Physics, Vol. 8, 1982, pp. 1981-1997. doi:10.1016/0360-3016(82)90459-X Copyright © 2012 SciRes. JCT  Hypofractioned Radiation Therapy in the Treatm ent of Partial Breast: 30 Gy in Five Consecutive Fractions Copyright © 2012 SciRes. JCT 1158 [34] G. G. Steel, J. M. Deacon, G. M. Duchesne, A. Horwich, L. R. Kelland and J. H. Peacock, “The Dose-Rate Effect in Human Tumout Cells,” Radiotherapy and Oncology, Vol. 9, No. 4, 1987, pp. 299-310. doi:10.1016/S0167-8140(87)80151-2 [35] Y. Yamada, I. Ackerman, E. Franssen, R. G. MacKenzie and G. Thomas, “Does the Dose Fractionation Schedule Influence Local Control of Adjuvant Radiotherapy for Early-Sta ge Breast Cancer?” International Journal of Ra- diation Oncology*Biology*Physics, Vol. 44, No. 1, 1999, pp. 99-104. doi:10.1016/S0360-3016(98)00507-0 [36] H. D. Thames, S. M. Bentzen, I. Turesson, M. Overgaard and W. Van den Bogaert, “Time-Dose Factors in Radio- therapy: A Review of the Human Data,” Radiotherapy and Oncology, Vol. 19, No. 3, 1990, pp. 219-235. doi:10.1016/0167-8140(90)90149-Q [37] I. Turesson and H. D. Thames, “Repair Capacity and Kinetics of Human Skin during Fractionated Radiother- apy: Erythema, Desquamation, and Telangiectasia after 3 and 5 Year’s Follow-Up,” Radiotherapy and Oncology, Vol. 15, No. 2, 1989, pp. 169-188. doi:10.1016/0167-8140(89)90131-X [38] J. O. Archambeau, R. Pezner and T. Wasserman, “Patho- physiology of Irradiated Skin and Breast,” International Journal of Radiation Oncology*Biology*Physics, Vol. 31, No. 5, 1995, pp. 1171-1185. doi:10.1016/0360-3016(94)00423-I [39] E. L. Travis and S. L. Tucker, “Isoeffect Models and Fractionated Radiation Therapy,” International Journal of Radiation Oncology*Biology*Physics, Vol. 13, No. 2, 1987, pp. 283-287. doi:10.1016/0360-3016(87)90141-6 [40] R. G. Dale, “Time-Dependent Tumour Repopulation Factors in Linear-Quadratic Equation: Implication for Treatment Strategies,” Radiotherapy and Oncology, Vol. 15, No. 4, 1989, pp. 371-381. doi:10.1016/0167-8140(89)90084-4 [41] J. H. Matthews, B. E. Meeker and J. D. Chapman, “Re- sponse of Human Tumor Cell Lines in Vitro for Fraction- ated Radiation,” International Journal of Radiation On- colo gy*B io logy *Ph ysi cs, Vol. 16, 1989, pp. 133-138. doi:10.1016/0360-3016(89)90020-5 [42] P. D. Stanton, T. G. Cooke, G. Forster, D. Smith and J. J. Going, “Cell Kinetics in Vivo of Human Breast Cancer,” British Journal of Surgery, Vol. 83, No. 1, 1996, pp. 98-102. doi:10.1002/bjs.1800830130 [43] K. Haustermans, J. Fowler, K. Geboes, M. R. Christiaens, A. Lerut and E. van der Schueren, “Relationshio between Potential Doubling Time (Tpot), Labeling Index and Du- ration of DNA Synthesis in 60 Esophageal and 35 Breast Tumors: Is It Worthwhile to Measure Tpot?” Radio- therapy and Oncology, Vol. 46, No. 2, 1989, pp. 157-167. doi:10.1016/S0167-8140(97)00164-3
|