Paper Menu >>
Journal Menu >>
 American Journal of Analytical Chemistry, 2010, 2, 41-46 doi:10.4236/ajac.2010.12006 Published Online August 2010 (http://www.SciRP.org/journal/ajac) Copyright © 2010 SciRes. AJAC Amperometric Determination of Serum Cholesterol with Pencil Graphite Rod Nidhi Chauhan, Jagriti Narang, Chandra S. Pundir* Department of Biochemistry, Maharshi Dayanand University, Rohtak, India E-mail: pundircs@rediffmail.com Received March 14, 2010; revised July 14, 2010; accepted July 20, 2010 Abstract A cholesterol oxidase from Streptomycin sp. was immobilized onto pencil graphite rod and employed for amperometric determination of serum cholesterol. The method has the advantage over earlier amperometric methods that it requires low potential to generate electrons from H2O2, which does not allow ionization of serum substances. The optimum working conditions of amperometric determination were pH 6.8, 25˚C and 30 s. The current measured was in proportion to cholesterol concentration ranging from 1.29×10-3 to 10.33×10-3 M. Minimum detection limit of the method was 0.09 ×10-3 M. Mean analytical recovery of added cholesterol (100 mg/dl and 200 mg/dl) in serum was 85.0% & 90.0% respectively. Within batch and between batch coefficients of variations were 1.59% & 4.15% respectively. A good correlation (r = 0.99) was ob- tained between serum cholesterol values by standard enzymic colorimetric method and the present method. No interference by metabolites was observed in the method. The enzyme electrode was reused 200 times over a period of 25 days, when stored at 4˚C. Keywords: Cholesterol, Cholesterol Oxidase, Pencil Graphite, Enzyme Electrode, Serum, Amperometric Biosensor 1. Introduction Cholesterol an important steroid in human body plays a vital role as a precursor to various hormones. Cholesterol determination in blood is known to be clinically impor- tant for diagnosis of various diseases like cardiac disor- ders, atherosclerosis, nephritis, diabetes mellitus, myxo- dema, obstructive jaundice and cerebral thrombosis [1]. Among various methods available for cholesterol deter- mination, biosensors are comparatively simpler, rapid, sensitive and specific [2-6]. Various amperometric cho- lesterol biosensors have been reported, employing cho- lesterol esterase, cholesterol oxidase and peroxidase im- mobilized onto nylon mesh over a platinum electrode [7], octyl-agarose gel activated with cyanogen bromide and placed in a reactor [8], pyrrole membrane through elec- tropolymerization and coupled with FIA for H2O2 analy- sis [9], carbon paste electrode modified with hydroxy- methyl ferrocene and hydrogen peroxide [10], poly (2- hydroxyethyl methacrylate) (p(HEMA))/polypyrrole me- mbrane [11], graphite-teflon composite matrix with in- corporated potassium ferrocyanide [12], layer of silica sol-gel matrix on the top of Prussian blue-modified glassy carbon electrode [13], photosensitive polymer on ultra-thin dialysis membrane [14], conducing polypyrrole (PPY) films using electrochemical entrapment technique [15], porous silicon [16], poly(vinylferrocenium) film [17]. Due to high electrochemical reactivity, good me- chanical rigidity, low cost and ease of modification, re- newal and miniaturization, pencil graphite electrode (PGE) received attention of workers for its use in work- ing electrode of biosensor [18]. Furthermore, PGE has a large active electrode surface area and therefore able to detect low concentrations and/or volume of the analyte [19]. The aim of this work was to develop a simple cho- lesterol biosensor based on PGE bound cholesterol oxi- dase. The electrode is better in the economic sense and a small amount of material used; hence it seems to be a better electrode than “high tech” electrodes described previously [20]. 2. Experimental 4-Amino-phenazone/4-aminoantipyrene, horseradish per- oxidase from Sigma Aldrich, USA, Triton X-100, Cho- lesterol, Cholesterol oxidase from Streptomycin sp. (500 units/10mg) from SRL, Mumbai. ‘HB’ lead pencil was from local market. The kit of enzymic colorimetric  42 N. CHAUHAN ET AL. method for cholesterol determination was from Erba Transasia, Daman, India. All other chemicals were of analytical reagent grade. 2.1. Assay of Free Cholesterol Oxidase Assay of free cholesterol oxidase was carried out in a 15 ml test tube wrapped with black paper according to Al- lain et al. (1974) with modification. The reaction mixture, consisting of 1.8 ml sodium phosphate buffer (0.05 M, pH 7.0) containing 0.4% Triton X-100, 0.1 ml of cho- lesterol solution (10mM) and 0.1 ml of cholesterol oxi- dase solution (13 Units) incubated for 5 min at 37˚C. Color reagent (1.0 ml) was added and kept at 37˚C for 10 min to develop the colour. A520 was read and the content of H2O2 was extrapolated from standard curve between H2O2 concentration and A520. Color reagent consisted 50 mg 4-aminophenazone, 100 mg phenol and 1 mg horse- radish peroxidase per 100 ml 0.4 M sodium phosphate buffer (pH 7.0). It was stored in amber colored bottle at 4˚C & prepared fresh after one weak. One enzyme unit is defined as the amount of enzyme required to generate 1.0 nmol of H2O2 per min per ml. 2.2. Immobilization of Enzyme onto Pencil Graphite Rod The wooden cover of a lead pencil was removed from its both the ends with a sharp blade upto 2 cm height. The one end of pencil graphite rod (0.15 diameter and 2 cm long) was dipped into 60% HCl at room temperature for 24 h and then washed thoroughly with 0.05 M sodium phosphate buffer (pH 7.4). It was dipped again into 70% HNO3. After keeping it for 24h at room temperature, the pencil rod was washed thoroughly with the same buffer and then put into 0.2% enzyme solution. After keeping it at 4˚C for 8h, the rod was taken off and washed thor- oughly with the reaction buffer & tested for cholesterol oxidase activity. The residual enzyme solution was tested for activity and protein by Lowry method. The pencil graphite rod containing immobilized enzyme acted as working electrode (PGE). 2.3. Construction and Response Measurement of Amperometric Cholesterol Biosensor An amperometric cholesterol biosensor was constructed by connecting pencil graphite electrode (PGE) as work- ing electrode, silver/silver chloride (Ag/AgCl) as refer- ence electrode and Cu wire as auxiliary electrode through electrometer/high resistance meter (Keithley 6517A, Japan). To test the activity of this biosensor, the electrode system was immersed into 1.8 ml 0.02 M so- dium phosphate solution pH 7.0 and 0.2 ml of cholesterol (12.9 mM) and polarized at a potential in the range 0-0.4 V versus Ag/AgCl. The current was maximum at 0.1 V. Hence in the subsequent amperometric studies; the sen- sor was polarized at 0.1 V to generate current. The elec- trochemical reactions involved in response meas- ure- ment are given in Figure 1. 2.4. Optimization of Cholesterol Biosensor The optimal working conditions of cholesterol biosensor were studied in terms of the current (mA) generated. To study optimum pH, the pH of reaction buffer was varied in the range pH 6.2 to pH 7.8 using the 0.02 M sodium phosphate buffer. Similarly for optimum temperature, the reaction mixture was incubated at temperature ranging from 20 to 50˚C at an interval of 5˚C. Time course was studied by incubating reaction mixture for different time ranging from 5 to 40 s at an interval of 5 s. To study effect of substrate concentration, the concentrations of cholesterol was varied from 1.29 to 12.9 mM. Km (Micha- elis Menten constant) and Imax (maximum current) were calculated from L.B. plot. 2.5. Electrochemical Determination of Cholesterol in Serum Blood samples (1.0 ml each) from apparently healthy male and female (10 each) and diseased persons (suffer- ing from coronary heart diseases, hypertension and atherosclerosis) were collected from local Pt BD Sharma Post Graduate Institute of Medical Sciences, Rohtak, and centrifuged at 5000 rpm for 5 min and their supernatant (serum) was collected. Cholesterol content in serum was determined by the present biosensor in the similar man- ner as described for its response measurement, under its optimal working conditions except that cholesterol was replaced by serum. The current (mA) was measured and the amount of cholesterol in serum extrapolated from standard curve between cholesterol concentrations and current (in mA) prepared under optimal working condi- tions (Figure 2). 2.6. Evaluation of Cholesterol Biosensor The biosensor was evaluated by studying analytical re- Figure 1. Chemical reactions involved in generation of electron in amperometric cholesterol biosensor based on pencil graphite electrode (PGE) bound cholesterol oxidase (ChOx). Copyright © 2010 SciRes. AJAC 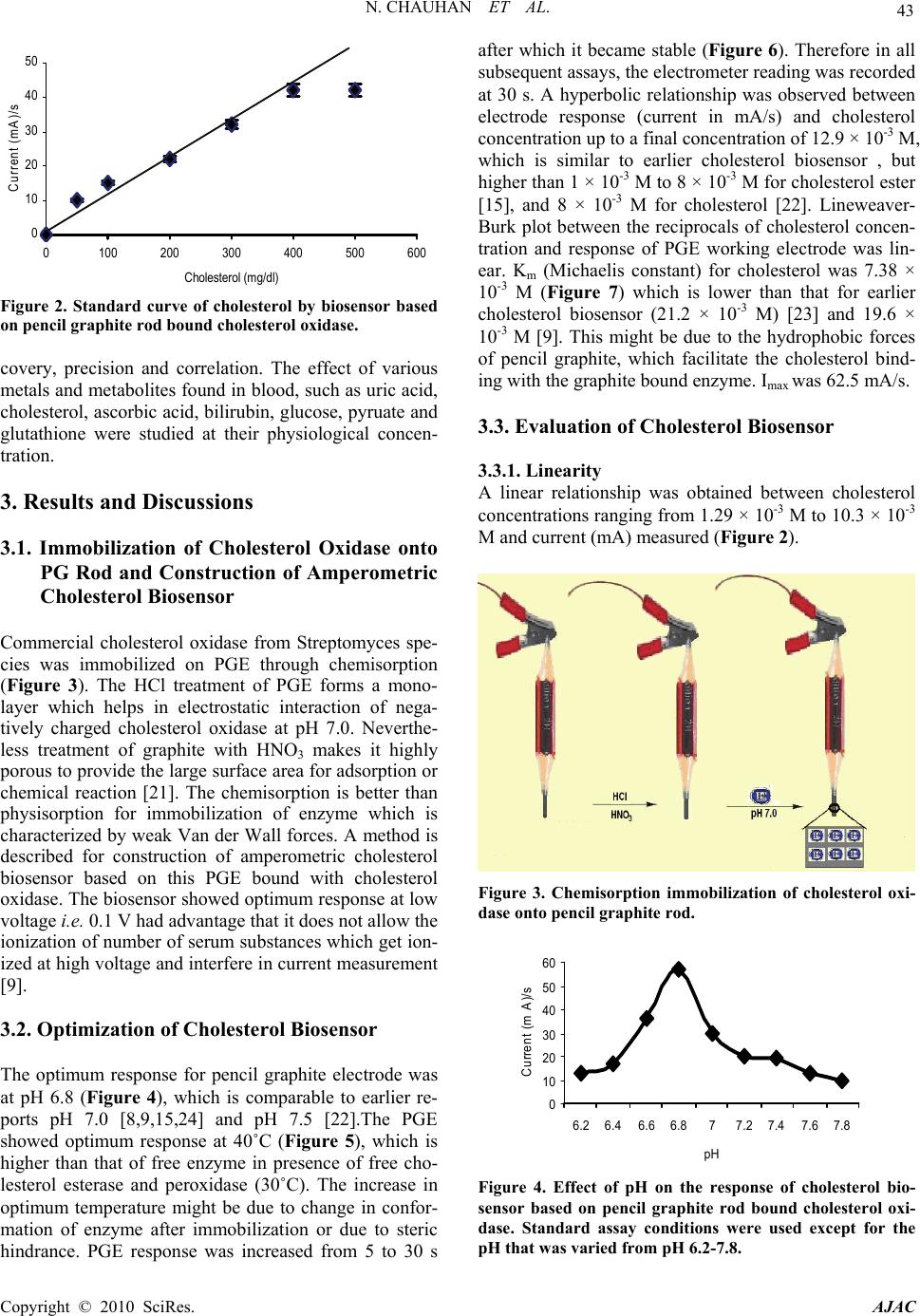 N. CHAUHAN ET AL. 43 0 10 20 30 40 50 0100 200 300 400 500 600 Cholesterol (mg/dl) Current (mA)/s Figure 2. Standard curve of cholesterol by biosensor based on pencil graphite rod bound cholesterol oxidase. covery, precision and correlation. The effect of various metals and metabolites found in blood, such as uric acid, cholesterol, ascorbic acid, bilirubin, glucose, pyruate and glutathione were studied at their physiological concen- tration. 3. Results and Discussions 3.1. Immobilization of Cholesterol Oxidase onto PG Rod and Construction of Amperometric Cholesterol Biosensor Commercial cholesterol oxidase from Streptomyces spe- cies was immobilized on PGE through chemisorption (Figure 3). The HCl treatment of PGE forms a mono- layer which helps in electrostatic interaction of nega- tively charged cholesterol oxidase at pH 7.0. Neverthe- less treatment of graphite with HNO3 makes it highly porous to provide the large surface area for adsorption or chemical reaction [21]. The chemisorption is better than physisorption for immobilization of enzyme which is characterized by weak Van der Wall forces. A method is described for construction of amperometric cholesterol biosensor based on this PGE bound with cholesterol oxidase. The biosensor showed optimum response at low voltage i.e. 0.1 V had advantage that it does not allow the ionization of number of serum substances which get ion- ized at high voltage and interfere in current measurement [9]. 3.2. Optimization of Cholesterol Biosensor The optimum response for pencil graphite electrode was at pH 6.8 (Figure 4), which is comparable to earlier re- ports pH 7.0 [8,9,15,24] and pH 7.5 [22].The PGE showed optimum response at 40˚C (Figure 5), which is higher than that of free enzyme in presence of free cho- lesterol esterase and peroxidase (30˚C). The increase in optimum temperature might be due to change in confor- mation of enzyme after immobilization or due to steric hindrance. PGE response was increased from 5 to 30 s after which it became stable (Figure 6). Therefore in all subsequent assays, the electrometer reading was recorded at 30 s. A hyperbolic relationship was observed between electrode response (current in mA/s) and cholesterol concentration up to a final concentration of 12.9 × 10-3 M, which is similar to earlier cholesterol biosensor , but higher than 1 × 10-3 M to 8 × 10-3 M for cholesterol ester [15], and 8 × 10-3 M for cholesterol [22]. Lineweaver- Burk plot between the reciprocals of cholesterol concen- tration and response of PGE working electrode was lin- ear. Km (Michaelis constant) for cholesterol was 7.38 × 10-3 M (Figure 7) which is lower than that for earlier cholesterol biosensor (21.2 × 10-3 M) [23] and 19.6 × 10-3 M [9]. This might be due to the hydrophobic forces of pencil graphite, which facilitate the cholesterol bind- ing with the graphite bound enzyme. Imax was 62.5 mA/s. 3.3. Evaluation of Cholesterol Biosensor 3.3.1. Linearity A linear relationship was obtained between cholesterol concentrations ranging from 1.29 × 10-3 M to 10.3 × 10-3 M and current (mA) measured (Figure 2). Figure 3. Chemisorption immobilization of cholesterol oxi- dase onto pencil graphite rod. 0 10 20 30 40 50 60 6.2 6.46.6 6.877.2 7.47.6 7.8 pH Current (m A)/s Figure 4. Effect of pH on the response of cholesterol bio- sensor based on pencil graphite rod bound cholesterol oxi- dase. Standard assay conditions were used except for the pH that was varied from pH 6.2-7.8. Copyright © 2010 SciRes. AJAC 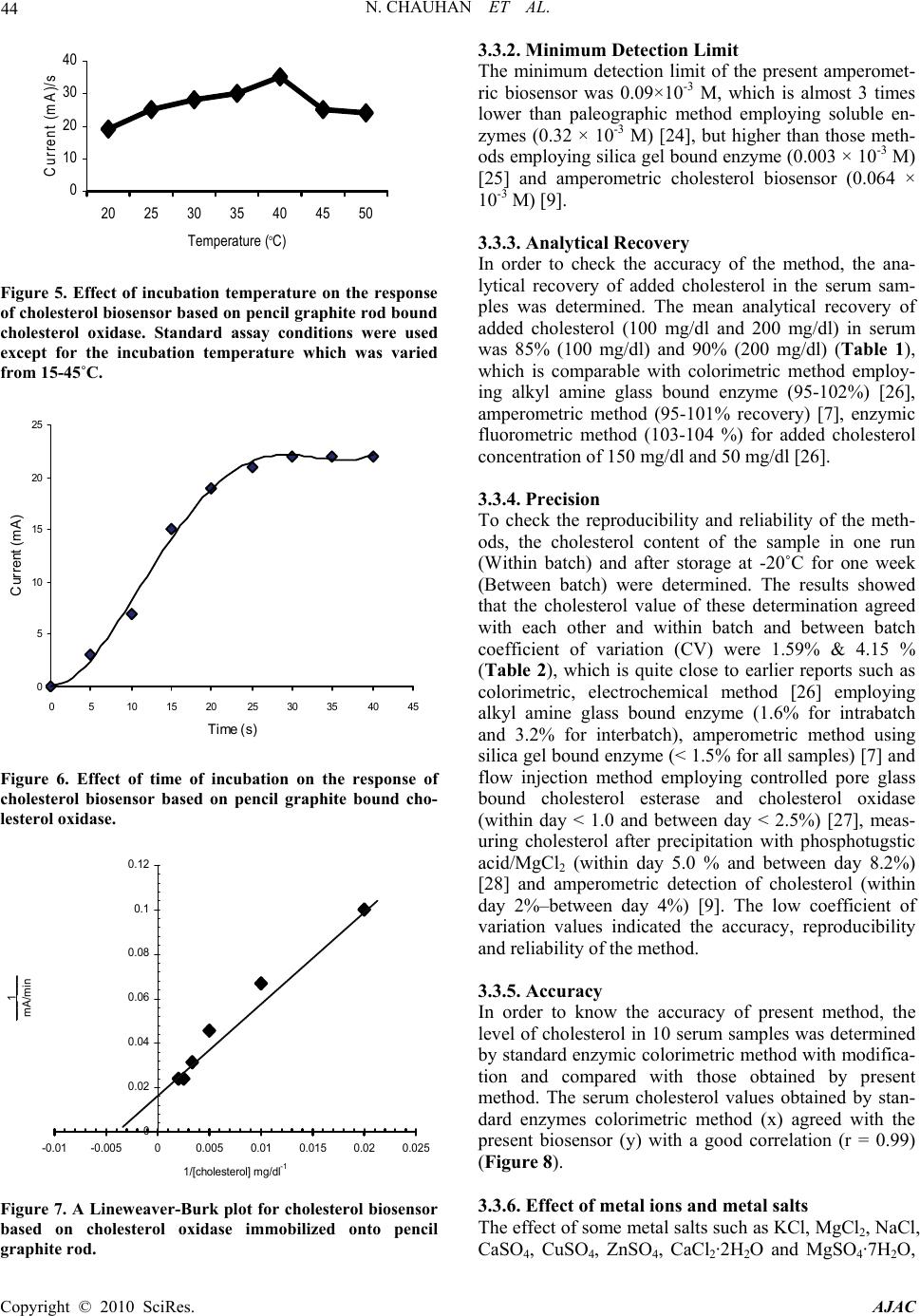 44 N. CHAUHAN ET AL. 0 10 20 30 40 20 25 30 35 40 45 50 Temperature (oC) Current (mA)/s Figure 5. Effect of incubation temperature on the response of cholesterol biosensor based on pencil graphite rod bound cholesterol oxidase. Standard assay conditions were used except for the incubation temperature which was varied from 15-45˚C. 0 5 10 15 20 25 0510 15 2025 30 3540 45 Time (s) Current (mA) Figure 6. Effect of time of incubation on the response of cholesterol biosensor based on pencil graphite bound cho- lesterol oxidase. 0 0.02 0.04 0.06 0.08 0.1 0.12 -0.01-0.00500.005 0.01 0.0150.02 0.025 1/[cholesterol] mg/dl -1 ___1___ mA/min Figure 7. A Lineweaver-Burk plot for cholesterol biosensor based on cholesterol oxidase immobilized onto pencil graphite rod. 3.3.2. Minimum Detection Limit The minimum detection limit of the present amperomet- ric biosensor was 0.09×10-3 M, which is almost 3 times lower than paleographic method employing soluble en- zymes (0.32 × 10-3 M) [24], but higher than those meth- ods employing silica gel bound enzyme (0.003 × 10-3 M) [25] and amperometric cholesterol biosensor (0.064 × 10-3 M) [9]. 3.3.3. Analytical Recovery In order to check the accuracy of the method, the ana- lytical recovery of added cholesterol in the serum sam- ples was determined. The mean analytical recovery of added cholesterol (100 mg/dl and 200 mg/dl) in serum was 85% (100 mg/dl) and 90% (200 mg/dl) (Table 1), which is comparable with colorimetric method employ- ing alkyl amine glass bound enzyme (95-102%) [26], amperometric method (95-101% recovery) [7], enzymic fluorometric method (103-104 %) for added cholesterol concentration of 150 mg/dl and 50 mg/dl [26]. 3.3.4. Precision To check the reproducibility and reliability of the meth- ods, the cholesterol content of the sample in one run (Within batch) and after storage at -20˚C for one week (Between batch) were determined. The results showed that the cholesterol value of these determination agreed with each other and within batch and between batch coefficient of variation (CV) were 1.59% & 4.15 % (Table 2), which is quite close to earlier reports such as colorimetric, electrochemical method [26] employing alkyl amine glass bound enzyme (1.6% for intrabatch and 3.2% for interbatch), amperometric method using silica gel bound enzyme (< 1.5% for all samples) [7] and flow injection method employing controlled pore glass bound cholesterol esterase and cholesterol oxidase (within day < 1.0 and between day < 2.5%) [27], meas- uring cholesterol after precipitation with phosphotugstic acid/MgCl2 (within day 5.0 % and between day 8.2%) [28] and amperometric detection of cholesterol (within day 2%–between day 4%) [9]. The low coefficient of variation values indicated the accuracy, reproducibility and reliability of the method. 3.3.5. Accuracy In order to know the accuracy of present method, the level of cholesterol in 10 serum samples was determined by standard enzymic colorimetric method with modifica- tion and compared with those obtained by present method. The serum cholesterol values obtained by stan- dard enzymes colorimetric method (x) agreed with the present biosensor (y) with a good correlation (r = 0.99) (Figure 8). 3.3.6. Effect of metal ions and metal salts The effect of some metal salts such as KCl, MgCl2, NaCl, CaSO4, CuSO4, ZnSO4, CaCl2·2H2O and MgSO4·7H2O, Copyright © 2010 SciRes. AJAC  N. CHAUHAN ET AL. 45 Figure 8. Correlation between serum cholesterol value as determined by enzo kit method employing free enzymes (x axis) and present biosensor method (y-axis) based on pencil graphite rod bound cholesterol oxidase. Table 1. Analytical recovery of added cholesterol in serum by biosensor based on pencil graphite electrode. Cholesterol added (mg/dl) Cholesterol found (mg/dl) % Recov- ery Nil 150 - 100 235 85 200 330 90 Serum cholesterol was measured by cholesterol biosensor as described in text. It was measured again after adding cholesterol into serum at 100 mg/dl & 200 mg/dl. % Recovery was calculated. Values are mean of six serum samples. Table 2. Precision measurement of serum cholesterol by a cholesterol biosensor based on pencil graphite electrode (PGE). Total number of samples (n = 6) Mean Cholesterol (mg/dl) % CV 6 (within assay) 54.25 1.59 6 (between assay)Ψ 54.2 4.15 Cholesterol was measured in six serum samples six times on the same day (Within assay) and after one week storage at -20oC (Between as- says) by cholesterol biosensor based on pencil graphite rod bound cholesterol oxidase. % Coefficient of variation (CV) was calculated. each at a final concentration of 1.0 mM was tested on the response of the working electrode. Only Mg2+ caused slight stimulation, while rest metals had practically no effect. 3.3.7. Effect of Serum Metabolites To study interference by serum metabolites glucose, uric acid, ascorbic acid, acetone and bilirubin were added into the reaction mixture at their normal physiological con- centration before addition of cholesterol. The results showed that there was practically no interference in presence of these metabolites. Earlier uric acid and ascorbic acid, at 0.6 V caused significant increase in the value of current [29], which were attributed to the fact that at high potentials for both uric acid & ascorbic acid got oxidized contributing to oxidation current. Some interference of endogenous electro reactive species like uric acid, glucose had been reported when their concen- tration was higher than their normal physiological con- centrations [15]. 3.4. Storage Stability and Reusability The PG electrode lost 50% of its initial activity after its regular use for 200 times over a period of 25 days, when stored in 0.05 M sodium phosphate buffer, pH 7.0 at 4˚C. 4. Conclusions A method is described for immobilization of cholesterol oxidase onto pencil graphite (PG) rod and its use in con- struction of a simple amperometric cholesterol biosensor. The biosensor had an advantage that it worked at low potential and thus had no interference by serum sub- stances. The sensor was evaluated. 5. References [1] S. Hirany, D. Li and I. Jialal, “A More Valid Measure- ment of Low Density Lipoprotein Cholesterol in Diabetic Patients”, American Journal of Medicine, Vol. 102, No. 1, 1997, pp. 48-53. [2] C. C. Allain, L.S. Poon, C. S. G. Chan, W. Richmond and P.C. Fu, “Enzymatic Determination of Serum Total Cho- lesterol,” Clinical Chemistry, Vol. 20, No. 4, 1974, pp. 470-475. [3] H. Osman and Kwee Yap, “Comparative Sensivity of Cholesterol Analysis Using GC, HPLC and Spectropho- tometric Methods,” Malaysian Journal of Food Science, Vol. 10, No. 2, 2006, pp. 205-210. [4] D. L. Witte, D. A. Barrett and D. A. Wycoff, “Evaluation of an Enzymatic Procedure for Determination of Serum Cholesterol with the Abbott ABA-100,” Clinical Chem- istry, Vol. 20, No. 10, 1974, pp. 1282-1286. [5] I. Karubeet, K. Hera, H. Matsuoka and S. Suzuki, “Am- perometric Determination of Total Cholesterol in Serum with Use of Immobilized Cholesteriol Esterase and Cho- lesterol Oxidase,” Analytical Chimica Acta, Vol. 139, No. 10, 1982, pp. 127-132. [6] B. Shahnaz, S. Tada, T. Kajikawa, T. Ishida and K. Ka- wanishi, “Automated Fluimetric Determination of Cellu- lar Cholesterol,” Annals of Clinical Biochemistry, Vol. 35, No. 6, 1998, p. 665. [7] M. J. Tabata and T. Murachi, “Automated Analysis of Total Cholesterol in Serum Using Coimmobilized Cho- lesterol Ester Hydrolase and Cholesterol Oxidase,” Jour- nal of Applied Biochemistry, Vol. 3, 1981, pp. 84-92. [8] Suman and C. S. Pundir, “Co-immobilization of Choles- terol Esterase, Cholesterol Oxidase & Peroxidase onto Alkylamine Glass Beads for Measurement of Total Cho- Copyright © 2010 SciRes. AJAC  N. CHAUHAN ET AL. Copyright © 2010 SciRes. AJAC 46 lesterol in Serum,” Current Applied Physics, Vol. 3, No. 2-3, 2003, pp. 129-133. [9] V. Hooda, A. Gahlautb, H. Kumar and C. S. Pundir, “Biosensor Based on Enzyme Coupled PVC Reaction Cell for Electrochemical Measurement of Serum Total Cholesterol,” Sensor and Actuator B, Vol. 136, No. 1, 2009, pp. 235-241. [10] L. Braco, J. A. Daros and M. de la. Guardia, “Enzymatic Flow Injection Analysis in Nonaqueous Media,” Analyti- cal Chemistry, Vol. 64, No. 2-3, 1992, pp. 129-133. [11] B. Sean, P. Narine, D. Singh and A. Guiseppi-Elie, “Amperometric with in Determination of Cholesterol in Serum Using A Biosensor of Cholesterol Oxidase Con- tained Polypyrol-Hydrogel Membrane,” Analytical Chimica Acta, Vol. 448, No. 1-2, 2001, pp. 27-36. [12] P. Nuria, Gloria Ruiz, A. Julio Reviejo and Jose M. Pin- garron, “Graphite Teflon Composite Bienzyme Elec- trodes for the Determination of Cholesterol in Reversed Micelles: Application to Food Samples,” Analytical Chemistry, Vol. 5, No. 6, 2001, pp. 1190-1195. [13] L. Jianping, T. Peng and Y. Peng, “Cholesterol Biosensor Based on Entrapment of Cholesterol Oxidase in a Silicic Sol-Gel Matrix at a Prussian Blue Modified,” Electro- analysis, Vol. 15, No. 12, 2003, pp. 1031-1033. [14] H. Endo, M. Masashi, T. Mio, R. Huifeng, H. Tetushito, U. Naoto and M. Kohji, “Enzyme Sensor System for De- termination of Total Cholesterol in Fish Plasma,” Fisher- ies Science, Vol. 69, No. 6, 2003, pp. 1194-1199. [15] S. Singh, A. Chaubey and B. D. Mahlotra, “Amperomet- ric Cholesterol Biosensor Based on Immobilized Choles- terol Esterase and Cholesterol Oxidase on Conducting Polypyrol Films,” Analytical Chimica Acta, Vol. 502, No. 2, pp. 229-234, 2004. [16] M. J. Song, D. H. Yun, J. H. Jin, N. K. Min and S. Hong, “Comparison of effective working electrode area on pla- nar and porous silicon substrates for cholesterol biosen- sor,“ Japanese Journal of Applied Physics, Vol. 45, No. 9A, 2006, pp. 7197-7202. [17] Ö. B. Cem, Ö. Haluk, Celebi, S. Serda and Y. Atilla, “Amperometric Enzyme Electrode for Free Cholesterol Determination Prepared with Cholesterol Oxidase Immo- bilized in Poly(Vinylferrocenium) Film,” Enzyme Micro- bial Technology, Vol. 40, No. 2, 2007, pp. 262-265. [18] W. Gao, J. Song and N. Wu, “Voltametric Behaviour and Square-Wave Voltametric Determination of Trepibutone at a Pencil Graphite Electrode,” Journal of Electroana- lytical Chemistry, Vol. 576, No. 1, 2005, pp. 1-7. [19] M. D. Vestergaard, K. Kerman and E. Tamiya, “An Elec- trochemical Approach for Detecting Copper-Chelating Properties of Flavonoids Using Disposable Pencil Graph- ite Electrodes: Possible Implications in Copper-Mediated Illnesses,” Analytical Chimica Acta, Vol. 538, No. 1-2, 2005, pp. 273-281. [20] A. M. Bond, J. Peter, Mahon, J. G. Schiewe and V. Vicente-Beckett, “An Inexpensive and Renewable Pencil Electrode for Use in Field Based Stripping Voltam- metry,” Analytical Chimica Acta, Vol. 345, No. 1-2, 1997, pp. 67- 74. [21] B Erable, N. Duteanu, S. M. Kumar, Y. Feng, M. M. Ghangrekar and K. Scott, “Nitric Acid Activation of Graphite Granules to Increase the Performance of the Non-Catalyzed Oxygen Reduction Reaction (ORR) for MFC Applications,” Electrochemistry Communication, Vol. 11, No. 7, 2009, pp. 1547-1549. [22] J. C. Vidal, J. Espuelas and J. R. Castillo, “Amperometric Cholesterol Biosensor Based on In Situ Reconstituted Cholesterol Oxidase on an Immobilized Monolayer of Flavin Adenine Dinucleotide Cofactor,” Analytical Bio- chemistry, Vol. 333, No. 1, 2004, pp. 88-98. [23] A. Kumar, R. R. Pandey and B. Brantley, “Tetraethylor- thosilicate Film Modified with Protein to Fabricate Cho- lesterol Biosensor,” Talanta, Vol. 69, No. 3, 2006, pp. 700-705. [24] A. Noma and K. Nakayama, “Polarographic Method for Rapid Micro Determination of Cholesterol with Choles- terol Esterase and Cholesterol Oxidase,” Clinical Chem- istry, Vol. 22, No. 3, 1976, pp. 336-40. [25] T. Yao, M. Sato, Y. Kobayashi and T. Wasa, “Am- perometric Assays of Total and Free Cholesterol in Se- rum by the Combined Use of Immobilized Cholesterol Esterase and Cholesterol Oxidase Reactors and Peroxi- dase Electrode in Flow Injection System,” Analytical Biochemistry, Vol. 149, 1985, pp. 387-391. [26] H. S. Huang, S. S. Kuan and G. G. Guiblault, “Am- perometric Determination of Total Cholesterol in Serum, with Use of Immobilized Cholesterol Ester Hydrolase and Cholesterol Oxidase,” Clinical Chemistry, Vol. 23, No. 1, 1977, pp. 671-676. [27] R. J. Fernandez, M. M. D. L. De Castro and M. Valcarcel, “Determination of Total Cholesterol in Serum by Flow Injection Analysis with Immobilized Enzyme,” Clinical Chimica Acta, Vol. 31, No. 2, 1987, pp. 97-104. [28] C. Heuck, I. Erbe and D. Mathias, “Cholesterol Determi- nation in Serum after Fractionation of Lipoproteins by Immunoprecipitation,” Clinical Chemistry, Vol. 31, No. 1-2, 1985, pp. 252-256. [29] S. Singh, P. R. Solanki and B. D. Malhotra, “Covalent Immobilization of Cholesterol Esterase and Cholesterol Oxidase on Polyaniline Films for Application to Choles- terol Biosensor,” Analytica Chimica Acta, Vol. 568, No. 1-2, 2006, pp. 126-132. |

