Paper Menu >>
Journal Menu >>
 J. Mod. Phys., 2010, 1, 171-174 doi:10.4236/jmp.2010.13025 Published Online August 2010 (http://www.scirp.org/journal/jmp) Copyright © 2010 SciRes. JMP Statistical Modeling of the Influence of Electron Degeneracy on the Interatomic Interactions Anatoly M. Dolgonosov Vernadsky Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences, Moscow, Russia E-mail: amdolgo@mail.ru Received April 15, 2010; revised July 2, 2010; accepted July 30, 2010 Abstract It is shown that electrons forming simple and multiple covalent bonds may have different contributions to the interatomic interactions due to the degeneracy of electron states. A simple relationship between the length of covalent bond, its order and atomic numbers of the interacting atoms is deduced. Keywords: Interatomic Interactions, Covalent Bond, Degeneracy of Electron States, Theory of Generalized Charges, Bond Length Ratio 1. Introduction In the semiempirical methods describing interatomic interactions, the contribution to the interaction energy of -bond is assumed to be larger than that of -bond [1,2 ]. The ratio of - to -electron weight factors equal to 1.41 was empirically determined in [3,4] and checked many times for the adsorption of unsaturated hydrocarbons. We will show that this ratio can be evaluated from the characteristics of covalent bonds (and vice versa). It follows from the theoretical and empirical equations found by London, Heitler, Lennard-Jon es, an d others th at the parameters of atoms symmetrically enter the expres- sion for interatomic bond energy [5]. A quasi-classical method for describing the self-consistent field of multi- component electron gas was developed in [6]. This method enables us to express the interatomic interaction energy. In particular it is shown that this energy is asso- ciated with the volume V of each atom through a sym- metrical operati on – v ol umes product 1212 12 121 212 ,; ij ij EfXr X VVv v (1) where 12 r is the internuclear distance; v is a “volume” of one electron, or the elementary volume, which is equal to unity for a nondegenerate electron; V is the electronic volume of the atom equal to the sum of elementary vol- umes of its electrons; the indices enumerate all electrons participating in bonding; and the figures at the summa- tion symbol indicate summation over every electron of the corresponding atom. According to [6], the shielding radius, which is in- versely proportional to the square root of the height of the potential barrier that the electron overcomes, can be used as a criterion of the participation of the electron in interatomic interactions. A sphere centered at the atomic nucleus with radius equal to the shielding radius of elec- trons (the shielding sphere) bounds the electrons that participate in bonding. An electron contributes to the electronic volume of its atom only if the nucleus of the atom with which the interaction is considered is situated within the electron shielding sphere. It is very important to distinguish between the elec- tronic volume, the key concept of the theory of general- ized charges developed in [6], and the corresponding number of electrons, though these characteristics often quantitatively coincide. It will be shown below that the elementary volume of a degenerate electron larger than that of a nondegenerate one, i.e. larger than unity. 2. Theory Let us consider the sums in (1) in detail 12 12 12 1 2 ij ij ij ijij ij ijij VVv v vv vvvv (2) where 1 ij vv . The summation is over all possible pairs of electrons. The coefficient ½ appears because there are no limitations on the permutation of indices. Let us apply (2) to pairs of - and -bond electrons. For a -bond, the orbital moment projection (m) of its electrons onto the internuclear axis has a single (zero) value. A pair of -electrons has therefore one state only 212 12 11 2ij ji vvvvv .  A. M. DOLGONOSOV Copyright © 2010 SciRes. JMP 172 For -bond electrons, the orbital moment projections onto the same axis take equal values, either +1 or –1 (depending on whether the right- or left-handed coordi- nate system is used; here we use atomic units of mo- ment). We therefore have four terms for -bond electrons 21212 12 111 12 1 1[ 2 ]2 ijji ij mmm jim vvvvv vv vv This gives 2v . It follows that the n-fold state of degeneracy corresponds to the n-fold increase in the sum of pair electronic products, that enhances the elementary volume of the pair of bond electrons, e vn. It is ne- cessary to bear in mind that the electron balance condi- tion imposes the following restriction: if aa VZ , then 1 e v and, vice versa, if at least one e v value is larger than 1, then aa VZ . The role of the product of volumes in describing the interaction of atoms is clarified by the following identity: 2 12121 2 1 2 VVVVV V (3) The appropriately normalized electronic volume of an atom corresponds to the probability of that its electrons belong to the bond under consideration. In terms of probability, the expression in brack ets is the excess value, which appears as a result of bond formation. On the other hand, the covalent bond energy is a func- tion of the excess electron density in the internuclear space 12 121 2 1 21212 ** *, 1 *2 lr l rZZd d ZZrr A A (4) where 12 , Z Z are the charges of nuclei in the elemen- tary charge units; d is the space volume element; l is the interatomic distance; and the bars denote the averag- ing over the scale indicated in parentheses, which coin- cides with one of the arguments of the functional relation. The first integral equals the probability for an atomic electron to occur between the planes passing through the nuclei normally to the interatomic axis at distance 12 r from each other. The second integral gives the same probability at infinite interatomic distance. The integra- tion in (4) is performed taking into account th at the elec- tronic wave function in the internuclear space depends on bond length. When atoms are infinitely separated, the excess electron density is zero, and exactly one-half of all the electrons occur in the internuclear space. We can therefore write 12 12 EFr 12 12 , f Xr that gives 121 2 rgVV (5) Comparing (3) and (4) by their sense and taking into account (5), we obtain * 1 212121212 1 2 VVZ Zrrr (6) According to (6), the excess density in the intern uclear space is proportional to the geometric mean of the prob- abilities for atomic electrons to take part in the bond un- der consideration. To simplify (6), let us make the substitution 12 121 12212 12 12 11 22 ZZ rrr ZZ ZZ (7) where 12 exp; 1,2 aa a ria . We obtain: ** 12 12121212 1cos 2 VV ZZ (8) Let us clear the combinatorial meaning of : its square is the relative part of the cases when a pair of th e particles belonging to two nonoverlapping sets of 1 Z and 2 Z particles occurs among the set of 12 VV par- ticles, where 1 V particles belong to the first set and 2 V particles, to the second one. This interpretation suffers from the shortcoming that the electronic volume is a more complex concept that the number of electrons. The above-mentioned quantity 12aa r is the characteristic of ato m depending on bond length . In such a case the a phase is a function of the scalar product of the wave vector of atomic electrons (ka) and the in- ternuclear vector '' ; ,'1,2;';0,1, 2,... i aa aaia aa i A aaaa i krkr (9) The presence of even exponents in expansion (9) is inessential because of their zero contribution to the dif- ference of phases in (8) when the atoms are identical. For the same reason, at least one of the odd constants Ai in (9) is nonzero. When the internuclear distance in its tending to zero falls beneath a certain value, the number of elec- trons forming the bond becomes nonzero. Negative ex- ponents are therefore absent in (9). The absolute term (i = 0) in (8) is annulled and therefore does not play any role. Thus, we can keep in (9) only the linear term, and set, without loss of generality, the constant А1 equal to  A. M. DOLGONOSOV Copyright © 2010 SciRes. JMP 173 unity 'aaaa kr ; 12 1212 rk k (10) Rewrite expression (8) taking into acco unt (10): 12 1212 coscos r rk rk k, (11) where r k is the sum of projections of the wave vec- tors of bond electron s on the bond axis. Finding this sum from (11), w e get 1 12 arccos r kr (12) The remarkable feature of r k is its independence of -electrons of the bond, because the limit value of -electron moment projection on the bond axis corre- sponds to the zero projection of its wave vector in this direction 12 ,0 rrr r kk kk (13) Combining (11) with (12) and (13), we find the gen- eral expression for a covalent bond 12 12 12 coscos arccos r r rk r (14) where primed and unprimed values relate to different cases of the bond between the same atoms. Note that (14) is valid for both double and triple bonds. Similar to (8), we can write 12 12 VV Z Z , where V differs from the single bond property V by the replacement of one or two electrons by -electrons of double or trip le bond , corresp onding ly. Thus, th e number of electrons forming a covalent bond is independent of its order and, due to the above-introduced normalization of electronic volume, is equal to the electronic volume of atom for the case of single bond (a V). Taking into ac- count the value 2v obtained above, we find 21 1 aa VV n (15) where 1,2, 3n is the bond order. In particular, it fol- lows from (14) that for homo-nuclear bonds 12 12 cos arccos rV VZ rZ (16) It is quite natural to assume that a V can differ from a Z by the number of closed shell electrons (for which the shielding radius is smaller than that for outer shell electrons), equal to two for the second-period atoms that yields 2 aa VZ. This expression in combination with (15) and (16) gives after simple transformations (see Appendix) 12 12 22111 arcsinarcsin (17) 2 21 1 1(18) 2 n r ZZ r n The last result valid for large atomic numbers is valu- able due to its independence of the kind of atoms, dis- playing only the dependence of the bond orde r. The calculation using (18) gives 12 12 0.890 r r for double bond and 12 12 0.765 r r for triple bond. Formula (18) is approximately valid for hetero-nuclear com- pounds as well. The comparison of the theoretical result obtained with experimental data is given in the Tabl e. For some possible compounds there is no information on bond length. This lack of knowledge can be elimi- nated by theoretical forecast. For example, the triple bond of boron with carbon or nitrogen is possible in principle. Its length will be about 24% shorter than the corresponding single bond. 3. Conclusions Thus, there is good compliance between experimental and theoretical values that confirms the necessity to dis- tinguish in interatomic interactions the contributions of differently degenerated electrons. The contribution of -electron to such an additive property of the interactive atom as its electronic volume is in 2 times larger than that of -electron. This effect can be explained by dif- ferent symmetries of the states with different degeneracy of - and -electrons. In present work, the simple ex- pression (17, 18) for covalent bond length ratio which shows strong influence of bond orders and weak de- pendence on atomic numbers is obtained. Table. Ratios of the bond lengths for compounds of the second-period elements: reference data [7,8] versus calcula- tion results. Atoms bonded Bond length ratio: double to single Bond length ratio: triple to single C-C 0.865 0.778 N-N 0.862 0.757 O-O 0.813(O2); 0.861(O3) 0.766(O2+)[8] C-N 0.910 0.786 C-O 0.852 0.791 N-O 0.897 0.779(NO+)[8] Theory: formula (18) 0.890 0.765 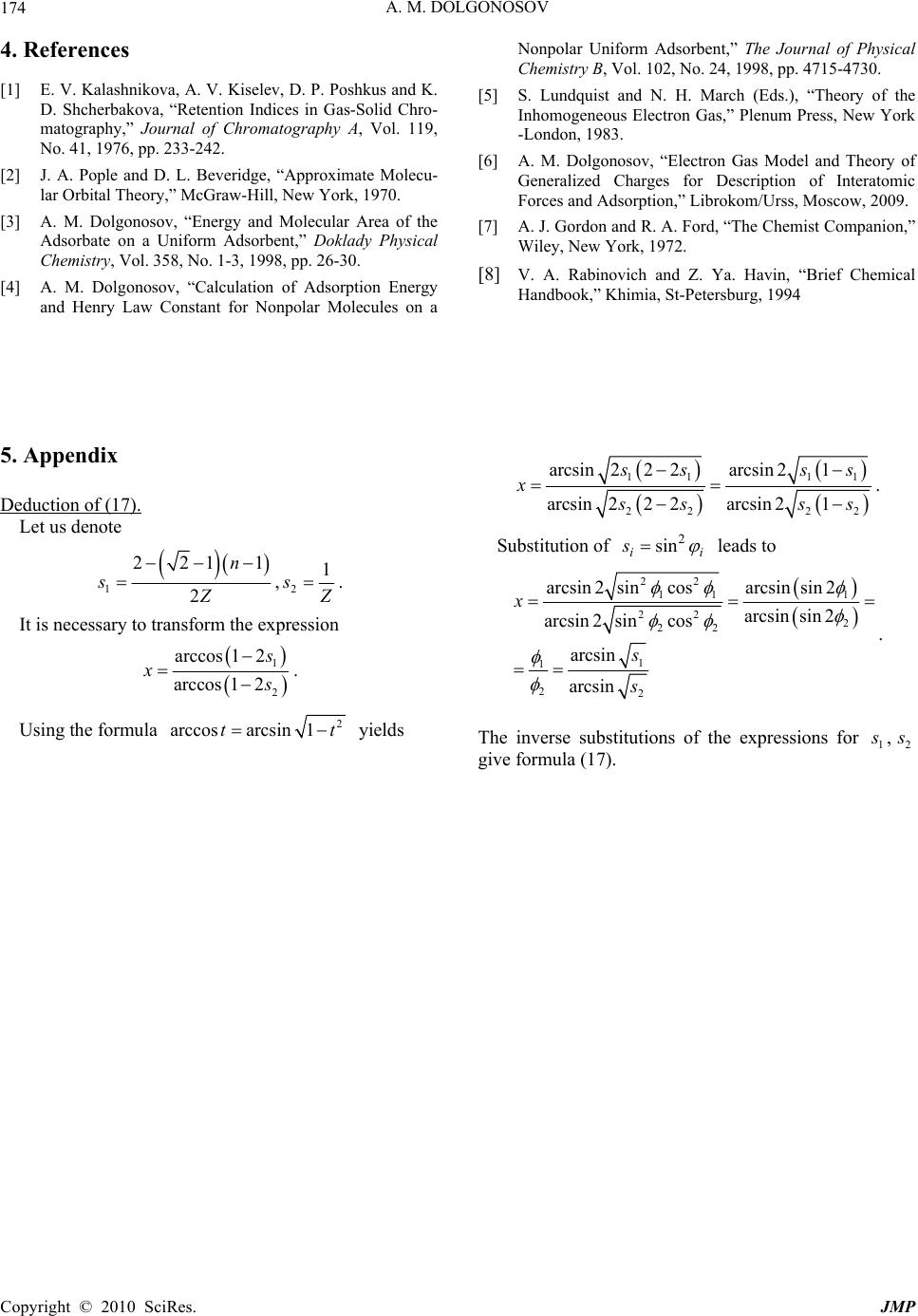 A. M. DOLGONOSOV Copyright © 2010 SciRes. JMP 174 4. References [1] E. V. Kalashnikova, A. V. Kiselev, D. P. Poshkus and K. D. Shcherbakova, “Retention Indices in Gas-Solid Chro- matography,” Journal of Chromatography A, Vol. 119, No. 41, 1976, pp. 233-242. [2] J. A. Pople and D. L. Beveridge, “Approximate Molecu- lar Orbital Theory,” McGraw-Hill, New York, 1970. [3] A. M. Dolgonosov, “Energy and Molecular Area of the Adsorbate on a Uniform Adsorbent,” Doklady Physical Chemistry, Vol. 358, No. 1-3, 1998, pp. 26-30. [4] A. M. Dolgonosov, “Calculation of Adsorption Energy and Henry Law Constant for Nonpolar Molecules on a Nonpolar Uniform Adsorbent,” The Journal of Physical Chemistry B, Vol. 102, No. 24, 1998, pp. 4715-4730. [5] S. Lundquist and N. H. March (Eds.), “Theory of the Inhomogeneous Electron Gas,” Plenum Press, New York -London, 1983. [6] A. M. Dolgonosov, “Electron Gas Model and Theory of Generalized Charges for Description of Interatomic Forces and Adsorption,” Librokom/Urss, Moscow, 2009. [7] A. J. Gordon and R. A. Ford, “The Chemist Companion,” Wiley, New York, 1972. [8] V. A. Rabinovich and Z. Ya. Havin, “Brief Chemical Handbook,” Khimia, St-Petersburg, 1994 5. Appendix Deduction of (17). Let us denote 1 2211 2 n s Z ,21 s Z . It is necessary to transform the expression 1 2 arccos 12 arccos 12 s x s . Using the formula 2 arccosarcsin 1tt yields 11 11 22 22 arcsin222arcsin 21 arcsin222arcsin 21 s sss x s sss . Substitution of ii s 2 sin leads to 22 11 1 22 2 22 1 1 22 arcsin 2sincosarcsinsin 2 arcsinsin 2 arcsin 2sincos arcsin arcsin x s s . The inverse substitutions of the expressions for 12 , s s give formula (17). |

