P. PAL ET AL. 281
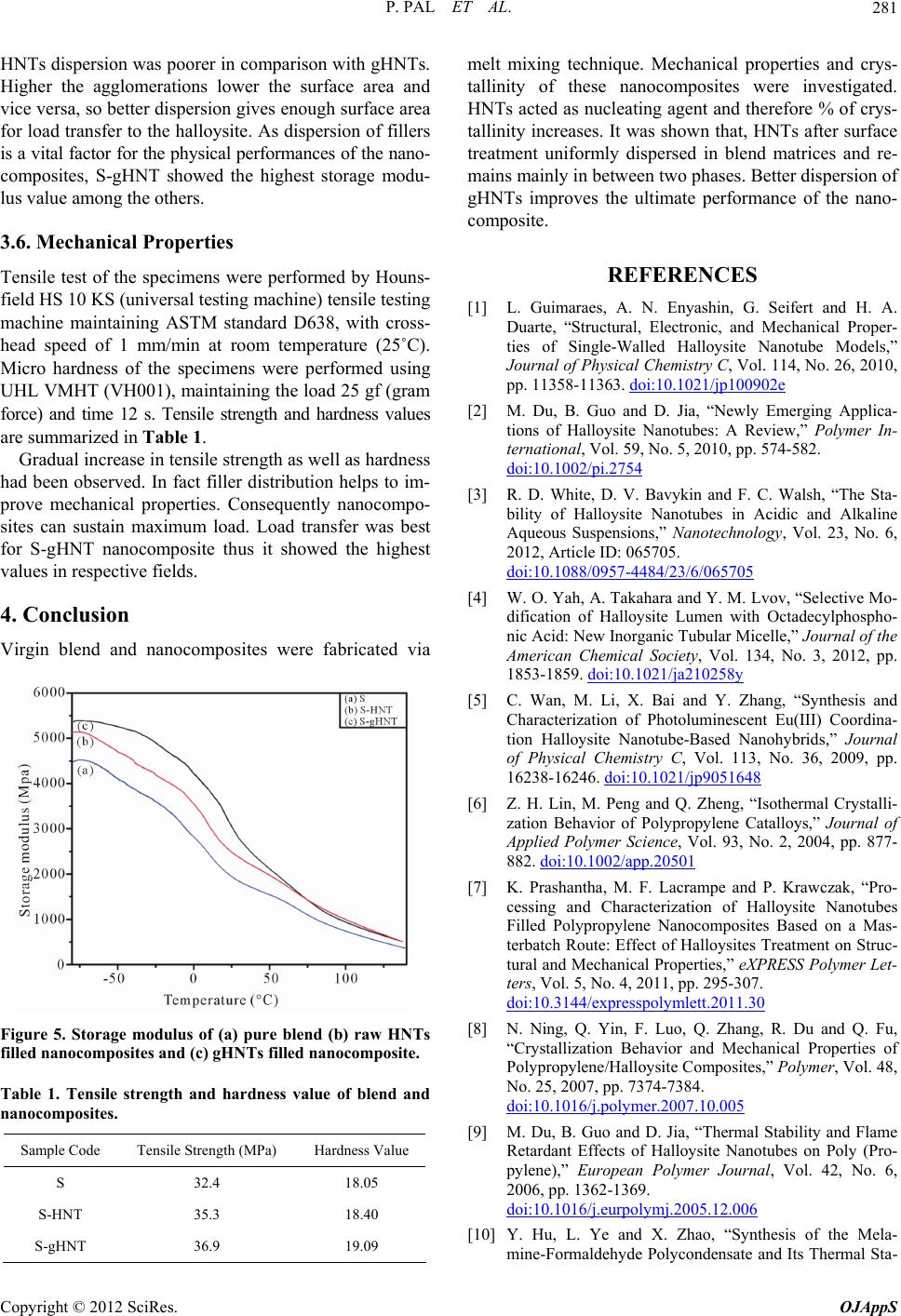
HNTs dispersion was poorer in comparison with gHNTs.
igher the agglomerations lower the surface areHa and
es
re performed by Houns-
g machine) tensile testing
ller distribution helps to im-
pr
nanocomposites were fabricated via
vice versa, so better dispersion gives enough surface area
for load transfer to the halloysite. As dispersion of fillers
is a vital factor for the physical performances of the nano-
composites, S-gHNT showed the highest storage modu-
lus value among the others.
3.6. Mechanical Properti
Tensile test of the specimens we
field HS 10 KS (universal testin
machine maintaining ASTM standard D638, with cross-
head speed of 1 mm/min at room temperature (25˚C).
Micro hardness of the specimens were performed using
UHL VMHT (VH001), maintaining the load 25 gf (gram
force) and time 12 s. Tensile strength and hardness values
are summarized in Table 1.
Gradual increase in tensile strength as well as hardness
had been observed. In fact fi
ove mechanical properties. Consequently nanocompo-
sites can sustain maximum load. Load transfer was best
for S-gHNT nanocomposite thus it showed the highest
values in respective fields.
4. Conclusion
Virgin blend and
Figure 5. Storage modulus of (a) pure blend (b) raw HNTs
filled nanocomposites and (c) gHNTs filled nanocomposite.
anocomposites.
Table 1. Tensile strength and hardness value of blend and
n
Sample Code Tensile Strength (MPa) Hardness Value
S 32.4 18.05
S-T
S-gHNT 36.9 19.09
HN35.3 18.40
melt mixing technique. Mechanical properties and crys-
tallinity of these nanocomposites were investigated.
HNT as nucleatingt and therefore crys-
tallieases. It wasn that, HNTs after surface
“Structural, Electronic, and Mechanical Proper-
ties of Single-Walled Halloysite Nanotube Models,”
Journal of Phy 114, No. 26, 2010,
pp. 11358-1132e
s acted agen % of
nity incr show
treatment uniformly dispersed in blend matrices and re-
mains mainly in between two phases. Better dispersion of
gHNTs improves the ultimate performance of the nano-
composite.
REFERENCES
[1] L. Guimaraes, A. N. Enyashin, G. Seifert and H. A.
Duarte,
sical Chemistry C, Vol.
63. doi:10.1021/jp10090
[2] M. Du, B. Guo and D. Jia, “Newly Emerging Applica-
tions of Halloysite Nanotubes: A Review,” Polymer In-
ternational, Vol. 59, No. 5, 2010, pp. 574-582.
doi:10.1002/pi.2754
[3] R. D. White, D. V. Bavykin and F. C. Walsh, “The Sta-
4/23/6/065705
bility of Halloysite Nanotubes in Acidic and Alkaline
Aqueous Suspensions,” Nanotechnology, Vol. 23, No. 6,
2012, Article ID: 065705.
doi:10.1088/0957-448
, Vol. 134, No. 3, 2012, pp.
[4] W. O. Yah, A. Takahara and Y. M. Lvov, “Selective Mo-
dification of Halloysite Lumen with Octadecylphospho-
nic Acid: New Inorganic Tubular Micelle,” Journal of the
American Chemical Society
1853-1859. doi:10.1021/ja210258y
[5] C. Wan, M. Li, X. Bai and Y. Zhang, “Synthesis and
Characterization of Photoluminescent Eu(III) Coordina-
tion Halloysite Nanotube-Based Nanohybrids,” Journal
of Physical Chemistry C, Vol. 113, No. 36, 2009, pp.
16238-16246. doi:10.1021/jp9051648
[6] Z. H. Lin, M. Peng and Q. Zheng, “Isothermal Crystalli-
zation Behavior of Polypropylene Catalloys,” Journal of
Applied Polymer Science, Vol. 93, No. 2, 2004, pp. 877-
882. doi:10.1002/app.20501
[7] K. Prashantha, M. F. Lacrampe and P. Krawczak, “Pro-
ies,” eXPRESS Polymer Let-
cessing and Characterization of Halloysite Nanotubes
Filled Polypropylene Nanocomposites Based on a Mas-
terbatch Route: Effect of Halloysites Treatment on Struc-
tural and Mechanical Propert
ters, Vol. 5, No. 4, 2011, pp. 295-307.
doi:10.3144/expresspolymlett.2011.30
[8] N. Ning, Q. Yin, F. Luo, Q. Zhang, R. Du and Q. Fu,
“Crystallization Behavior and Mechanical Properties of
Polypropylene/Halloysite Composites,” Polymer, Vol. 48,
No. 25, 2007, pp. 7374-7384.
doi:10.1016/j.polymer.2007.10.005
[9] M. Du, B. Guo and D. Jia, “Thermal Stability and Flame
Retardant Effects of Halloysite Nanotubes on Poly (Pro-
pylene),” European Polymer Journal, Vol. 42, No. 6,
2006, pp. 1362-1369.
doi:10.1016/j.eurpolymj.2005.12.006
mine-Formaldehyde Polycondensate and Its Thermal Sta-
[10] Y. Hu, L. Ye and X. Zhao, “Synthesis of the Mela-
Copyright © 2012 SciRes. OJAppS