Paper Menu >>
Journal Menu >>
 Vol.1, No.2, 119-133 (2010) doi:10.4236/jbpc.2010.12015 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/JBPC/ Journal of Biophysical Chemistry Overtone spectra of porphyrins and its substituted forms: an algebraic approach Srinivasa Rao Karumuri1*, Ambati Siva Rama Prasad2, Nirmal Kumar Sarkar3, Joydeep Choudhury4, Ramendu Bhattacharjee4 1Faculty of Sciences & Humanities, Sri Viveka Institute of Technology (SVIT), Vijayawada, India; *Corresponding Author: drsrkarumuri@rediffmail.com 2Faculty, Department of Mathematics, P.B. Siddhartha College of Arts & Sciences, Vijayawada, India 3Faculty, Department of Physics, Karimganj College, Karimganj, India 4Faculty, Department of Physics, Assam (A Central) University, Silchar, India Received 8 June 2010; revised 13 July 2010; accepted 18 July 2010. ABSTRACT We introduce an algebraic model to vibrations of polyatomic Bio-molecules and present, as an example, the vibrational analysis of Cm-H, Cm-C, Cm-D, Cb-Cb, pyrrol breathing and Cb-C, stretch- ing modes of Metalloporphyrins and its substi- tuted forms. The excited energy levels of Cb-C, pyrrol breathing stretching modes of Ni(OEP) and Ni(OEP)-d4 are calculated by using U(2) al- gebraic mode Hamiltonian. The higher excited energy levels of Cm-H, Cm-C, Cm-D and Cb-Cb vibrational modes of Porphyrin and its substi- tuted forms are predicted upto second overtone. It shows that the energy levels are clustering at the higher overtones. The results obtained by this method are accuracy with experimental data. Keywords: Algebraic Model; Vibrational Spectra; Energy Levels; Metalloporphyrins 1. INTRODUCTION Recently measurement of highly-excited overtone-com- bination spectra of molecules have renewed in a theo- retical description and understanding of the observed spectral properties. Two approaches have been mostly used so far in an analysis of experimental data: 1) the familiar Dunham like expansion of energy levels in terms of rotations-vibrations quantum numbers and 2) the solution of Schrodinger equation with potentials ob- tained either by appropriately modifying ab-initio calcu- lations or by more phenomenological methods. In this article, we begin a systematic analysis of overtone- combination spectra of molecules in terms of novel ap- proach: 3) Vibron model [1-4]. This model is a formula- tion of the molecular spectral problem in terms of ele- ments of Lie algebra and it contains the same physical information of the Dunham and potential approach. However, by making use of the powerful methods of group theory, one is able to obtain the desired results in a much faster and straightforward way. In recent years, these polyatomic bio-molecules (i.e Metalloporphyrins) have numerous importances in the field of Chemical Physics. In case of polyatomic bio- molecules the parameters play major role in the Vibron model. Of course, we have explicitly described with few parameters, the vibrational bands of the triatomic linear molecules HCN, OCS, HCP [5-7] and tetratomic mole- cules HCCF, HCCD by using an algebraic approach [8]. We have also reported the vibrational bands of tetrahe- dral molecules CCl4, SnBr4 and Propadiene [9-11] and polyatomic bio-molecules like Nickel Octaethyl porphy- rin, Nickel porphyrin molecules using U (2) Vibron model respectively [12-18]. The advantage of the alge- braic approach, as compared to that of Dunham or pho- nological potential models, is that typically it requires few parameters to obtain the same level of accuracy. It also provides a simultaneous description of bending and stretching modes [19-25]. In Section 2, review the theory of algebraic model to polyatomic molecules is described. In Section 3, the calculation procedure of Vibron number and the fitting algebraic parameters corresponding to various Porphy- rins and its substitute form molecule results are dis- cussed. Finally, the conclusions are presented in Section 4. 2. THEORY: AN ALGEBRAIC APPROACH A complete description of the theoretical foundations  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 120 Openly accessible at needed to formulate the algebraic model for a vibrating molecule. We apply the one-dimensional algebraic model, consisting of a formal replacement of the interatomic, bond coordinates with unitary algebras. To say it in dif- ferent words, the second-quantization picture suited to describe anharmonic vibrational modes, is specialized through an extended use of Lie group theory and dy- namical symmetries. By means of this formalism, one can attain algebraic expressions for eigenvalues and ei- genvectors of even complex Hamiltonian operators, in- cluding intermode coupling terms as well expectation values of any operator of interest (such as electric dipole and quadrupole interactions). Algebraic model are not ab-initio methods, as the Hamiltonian operator depends on a certain number of a priori undetermined parameters. As a consequence, algebraic techniques can be more convincingly compared with semi-empirical approaches making use of expansions over power and products of vibrational quantum numbers, such as a Dunham-like series. However, two noticeable advantages of algebraic expansions over conventional ones are that 1) algebraic modes lead to a (local) Hamiltonian formulation of the physical problem at issue(thus permitting a direct calcu- lation of eigenvectors in this same local basis) and 2) algebraic expansions are intrinsically anharmonic at their zero-order approximation. This fact allows one to reduce drastically the number of arbitrary parameters in com- parison to harmonic series, especially when facing me- dium-or large- size molecules. However, it should also be noticed that, as a possible drawback of purely local Hamiltonian formulations (either algebraic or not) com- pared with traditional perturbative approaches, the actual eigenvectors of the physical system. Yet, for very local situations, the aforementioned disadvantage is not a se- rious one. A further point of import here is found in the ease of accounting for proper symmetry adaptation of vibrational wave functions. This can be a great help in the systematic study of highly excited overtones of not-so-small molecules, such as the present one. Last but not least, the local mode picture of a molecule is en- hanced from the very beginning within the algebraic framework. This is an aspect perfectly lined up with the current tendencies of privileging local over normal mode pictures in the description of most topical situations. 2.1. Hamiltonian Operators We address here the explicit problem of the construction of the vibrational Hamiltonian operator for the Metal- loporphyrin molecules. According to the general alge- braic description for one-dimensional degrees of free- dom, a dynamically-symmetric Hamiltonian operator for n-interacting (not necessarily equivalent) oscillators can be written as H = E0 + 1 n i AiCi + n ij Aij Cij + ij Mij (1) n ij In this expresssion, one finds three different classes of effective contributions. The first one, AiCi is devo- ted to the description of n independent, anharmonic sequences of vibrational levels (associted wih n inde- pendent, local oscillator) in terms of the operators Ci. The second one, 1 n i n ij Aij C ij leads to cross-anhar- monicities between pairs of distinct local oscillators in terms of the operators Cij. The third one, n ij ij M ij, describes anharmonic, non-diagonal interactions involving pairs of local oscillators in terms of the operators Mij. The Ci, Cij operators are invariant (Casimir) operators of certain Lie algebras, whilst the Mij are invariant (Ma- jorana) operators associated with coupling schemes involving algebras naturally arising from a systematic study of the algebraic formulation of the one-dimen- sional model for n interacting oscillators. We work in the local (uncoupled oscillaators) vibrational basis written as 123 ....... n In which the aforementioned operators have the following matrix elements 4( ) iii CN i 4( )() iji iij ij CNN !! !(2) iij j ijiijji j MNN !! !1 11 [(1)()(1)] iij j ijiii jjj MNN /2 !! !1 11 [(1)() (1)] iij j ijjjj iii MNN /2 We note, in particular, th the expressions above de- pe ral Hamiltonian operator (1) can be adapted to at nd on the numbers Ni (Vibron numbers). Such num- bers have to be seen as predetermined parameters of well-defined physical meaning, as they relate to the in- trinsic anharmonicity of a single, uncoupled oscillator through the simple relation. We report in Table 4 & Ta- ble 5 the values of the Vibron numbers used in the pre- sent study. The gene describe the internal, vibrational degrees of freedom of any polyatomic molecule in two distinct steps. First, we associate three mutually perpendicular one-dimen- sional anharmonic oscillators to each atom. This proce- dure eventually leads to a redundant picture of the whole molecule, as it will include spurious (i.e transla- tional/rotational) degrees of freedom. However, it is possible to remove easily such spurious modes through  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 121 m-H/Cm-D/Cm-N st Openly accessible at different techniques. One is thus left with a Hamiltonian operator dealing only with true vibrations. Such modes are given in terms of coupled oscillators in the local ba- sis (3). The coupling is induced by the Majorana opera- tors. A sensible use of these operators is such that the correct symmetries of vibrational wave functions are properly taken into account. As a second step, the alge- braic parameters Ai, Aij, λij of Eq.1 need to be calibrated to reproduce the observed spectrum. Let us clarify the actual meaning of these two steps by considering explic- itly the Cm-H/Cm-D/Cm-N stretches manifold of the Nickel Metalloporphyrin molecule. We limit ourselves to in-plane C retching motions i.e ., without including possible cou- pling terms with ring deformation. So, we can write for these remaining four degrees of freedom the Hamilto- nian operator, 44 4 ! 1 CHi iijijijij iijij H ACA CM The algebraic theory of polyatomic molecules consists in For the stretching vibrations of polyatomic olecules co p(–βs)] (2) For re the separate quantization of rotations and vibrations in terms of vector coordinates r1, r2, r3,……. quantized through the algebra 1 ( 2)GU23 (2)(2) ..........U U m rrespond to the quantization of anharmonic Morse oscillators, with classical Hamiltonian H(ps, s) = ps 2/2μ + D[1 – ex2 each oscillator i, states are characterized by rep- sentations of (2) (2) ii ii UO Nm (3) With m = N, N – 2,…..,1 or 0 (N - odd or even). The M H = ε0i + A C, (4) where C is the invar ε =ε0i+Ai (m2 – N2). Introducing th number ν = (N A (N ν – ν2 ). (5) For non-interac iiii orse Hamiltonian (2) can be written, in the algebraic approach, simply as ii i iant operator of Oi(2), with eigen i values ii i e vibrational quantumi i-mi)/2, [26] one has εi =ε0i – 4iiii ting oscillators the total Hamiltonian is H = i i H , With eigenvalues E = i i = E0 – i 4A (N ν –ν2). (6) 2.2. Hamiltonian for Stretching Vibrations –αj sj)], (7) which kij si sj . Interaction of thetaken into account in ii ii The interaction potential can be written as V(si, sj)=kij´[1 – exp(–αi si)][1 – exp( reduces to the usual harmonic force field when the displacements are small V(si, sj) ≈ type Eq.7 can be the algebraic approach by introducing two terms [26]. One of these terms is the Casimir operator, Cij, of the combined (2) (2) ij OO algebra. The matrix elements of this operator ins Eq. 3 are given by Ni, νi; Nj, νjCij Ni, νi; Nj, νj = 4[(νi + ν the basi j)2 – (8) The operator C tu ν)2 – (9) The op νi; Nj, νjMijNi, νi; Nj,. νj = (Niνj + Njνi – 2νiνj) = –10) Th of total Hamiltonian for n stretching vibrations is H = E0 + (νi + νj)(Ni + Nj)] ij is diagonal and the vibrational quan- m numbers νi have been used instead of mi. In practical calculations, it is sometime convenient to substract from Cij a contribution that can be absorbed in the Casimir operators of the individual modes i and j, thus consider- ing an operator Cij´ whose matrix elements are Ni, νi; Nj, νjCij Ni, νi; Nj, νj = 4[(νi + j (νi + νj)(Ni + Nj)] + [(Ni + Nj)/Ni]4 (Niνi – νi 2) + [(Ni + Nj)/Nj]4(Njνj – νj 2). second term is the Majorana operator, Mij. This erator has both diagonal and off-diagonal matrix ele- ments Ni, Ni, νi +1 ; Nj, νj – 1MijNi, νi; Nj,. νj = – [νj(νi + 1)(Ni – νi)(Nj – νj + 1)]1/2 Ni, νi – 1; Nj, νj + 1MijNi, νi; Nj,.νj [νi(νj + 1)(Nj – νj)(Ni – νi + 1)]1/2 ( e Majorana operators Mij annihilâtes one quantum vibration in bond i and create one in bond j, or vice versa. The 1i n n ij A C + i iAij Cij + ij Mij (11) If λij =0 the vibrations have local behavior. As the λij s in ymmetry- Adapted Operators int group n ij crease, one goes more and more into normal vibra- tions. 2.3. S In polyatomic molecules, the geometric po symmetry of the molecule plays an important role. States must transform according to representations of the point symmetry group. In the absence of the Majorana opera- tors Mij, states are degenerate. The introduction of the Majorana operators has two effects: 1) it splits the de- generacies of figure and 2) in addition it generates states with the appropriate transformation properties under the point group. In order to achieve this result the λij must be chosen in an appropriate way that reflects the geometric symmetry of the molecule. The total Majorana operator 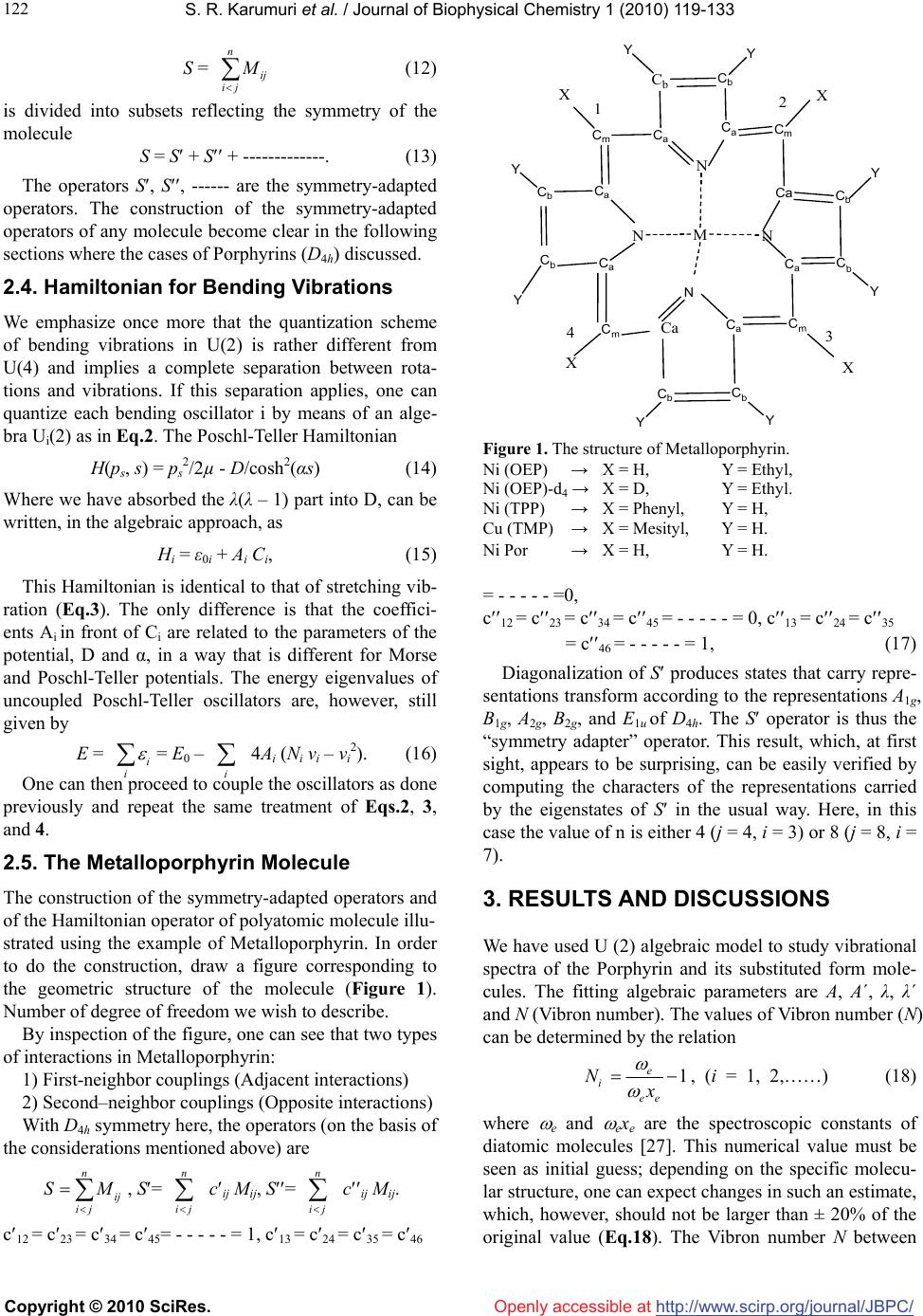 S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 122 Openly accessible at S = n ij ij M (12) is divided into subsets reflecting the symmetry of the S = S + S + -------------. (13) The operators op e (14) Where we (15) This Hamiltonian rat E = molecule S, S, ------ are the symmetry-adapted erators. The construction of the symmetry-adapted operators of any molecule become clear in the following sections where the cases of Porphyrins (D4h) discussed. 2.4. Hamiltonian for Bending Vibrations We emphasize once more that the quantization schem of bending vibrations in U(2) is rather different from U(4) and implies a complete separation between rota- tions and vibrations. If this separation applies, one can quantize each bending oscillator i by means of an alge- bra Ui(2) as in Eq.2. The Poschl-Teller Hamiltonian H(ps, s) = ps 2/2µ - D/cosh2(αs) have absorbed the λ(λ – 1) part into D, can be written, in the algebraic approach, as Hi = ε0i + Ai Ci, is identical to that of stretching vib- ion (Eq.3). The only difference is that the coeffici- ents Ai in front of Ci are related to the parameters of the potential, D and α, in a way that is different for Morse and Poschl-Teller potentials. The energy eigenvalues of uncoupled Poschl-Teller oscillators are, however, still given by i i n pro = E0 – 4Ai (Ni νi – νi 2). (16) One ca pr e Metalloporphyrin Molecule rators and types of ent interactions) s) th ij ijij Mij. c12 = c23 = c34 = c45= - - - - - = 1, c13 = c24 = c35 = c46 i coupn theceed to le the oscillators as done eviously and repeat the same treatment of Eqs.2, 3, and 4. 2.5. Th The construction of the symmetry-adapted ope of the Hamiltonian operator of polyatomic molecule illu- strated using the example of Metalloporphyrin. In order to do the construction, draw a figure corresponding to the geometric structure of the molecule (Figure 1). Number of degree of freedom we wish to describe. By inspection of the figure, one can see that two interactions in Metalloporphyrin: 1) First-neighbor couplings (Adjac 2) Second–neighbor couplings (Opposite interaction With D4h symmetry here, the operators (on the basis of e considerations mentioned above) are n S= n M, S= n ij ij SM , ij c ij c C b C b C a Y Y C a C m C m N Ca C b C b C a N C m C a C b C b M C a C b C b C a N C m Y Y Y Y Y Y Ca XX X X N 12 3 4 Figure 1. The structure of Metalloporphyrin. yl - - - - - =0, 34 = c45 = - - - - - = 0, c13 = c24 = c35 7) Diagona se . RESULTS AND DISCUSSIONS e have used U (2) algebraic model to study vibrational Ni (OEP) → X = H, Y = Ethyl, Ni (OEP)-d4 → X = D, Y = Ethyl. Ni (TPP) → X = Phen, Y = H, Cu (TMP) → X = Mesityl, Y = H. Ni Por → X = H, Y = H. = c12 = c23 = c = c46 = - - - - - = 1, (1 lization of S produces states that carry repre- ntations transform according to the representations A1g, B1g, A2g, B2g, and E1u of D4h. The S operator is thus the “symmetry adapter” operator. This result, which, at first sight, appears to be surprising, can be easily verified by computing the characters of the representations carried by the eigenstates of S in the usual way. Here, in this case the value of n is either 4 (j = 4, i = 3) or 8 (j = 8, i = 7). 3 W spectra of the Porphyrin and its substituted form mole- cules. The fitting algebraic parameters are A, A΄, λ, λ΄ and N (Vibron number). The values of Vibron number (N) can be determined by the relation 1 e i ee Nx , (i = 1, 2,……) (18) where e and exe are the spectroscopic constants of diatomic molecules [27]. This numerical value must be seen as initial guess; depending on the specific molecu- lar structure, one can expect changes in such an estimate, which, however, should not be larger than ± 20% of the original value (Eq.18). The Vibron number N between  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 123 rting guess for the pa (N – 1). (19) In the pre co the diatomic molecule C-C, C-H and C-D are 140, 44 and 59 respectively. From the figure 1, it is noticed that some of the bonds are equivalent. It may be noted that during the calculation of the vibrational frequencies of Porphyrins and substituted forms, the value of N is kept fixed and not used as free parameter. The second step is to obtain a sta rameter A. As such, the expression for the single- oscillator fundamental mode as E (ν = 1) = –4 A sent case we have three different energies, rresponding to symmetric and antisymmetric combi- nations of the different local modes. A possible strategy is to use the center of gravity of these modes, so the guess for Openly accessible at 4(1 ) E AN (20) The third step is to obtain an initial guess for λ. Its role is to split the initially degenerate local modes, placed here at the common value E used in Eq.19. Such an estimate is obtained by considering the simple matrix structure, we can find 1 2 EuA g EE N (21) & 21 ! 4 Bg Ag EE N (22) Finally a numerical fitting procedure is to be carried to are calcu- la study the hi 4. CONCLUSIONS nted a systematic analysis of st f the method is that it allows one to do MENTS e to thank Prof. Thom- adjust (in a least- square sense, for example) the para- meters A and λ starting from values Eq.20 and Eq.21, and A΄ (whose initial guess can be zero). Using the Eqs.20, 21 and 22, A, and ted [4,5-7,27] using the available data points. We have taken = 0 (In this case, the next nearest neighbor couplings are omitted). As one can see from Table 1 & Table 2, the agreement with experiment is good and thus we think that the parameter set of Table 4 & Table 5 can be used reliably to compute energies of highly excited overtones. We note that in Table 2 & Table 3, there are many predicted overtones that have not been studied experimentally. We have explicit calculations up to the second overtone (energy up to ≈ 10000 cm-1). We have used the algebraic Hamiltonian to ghly excited vibrational levels of the molecule Ni (TPP), Cu (OEP), Mg (OEP), Cu (TPP), Cu (TMP), Ni Porphyrin, Ni (OEP) and its substitution form Ni (OEP)-d4. Eight bands are studied, which can be labeled the Cm-H, Cb-Cb and only for Ni (OEP)-d4 the bands labeled are Cm-D, Cb-Cb respectively. The highly excited vibrational levels, calculated by using the algebraic Hamiltonian Eq .11, are shown in Figures 2, 3, 4, and 5 (The detail calculated vibrational energy levels are listed in Tables 3). Figures 2 and 3 gives the levels corre- sponding to the Cm-H, Cb-Cb of Ni (TPP). Figures 4 and 5 gives the levels corresponding to the Cm-H, Cb-Cb of Cu (TPP). Figures 6 and 7 gives the levels correspond- ing to the Cm-H, Cb-Cb of Cu (TMP). Figures 8 and 9 gives the levels corresponding to the Cm-D, Cb-Cb of Ni (OEP)-d4. When the quantum number ν increases in a fixed band, the numbers of energy levels increase rapidly. Usually, the degeneracy or quanti-degeneracy of energy levels is called clustering. It may be seen from Figures 2, 3, 4, 5, 6, 7, 8 and 9 that the vibrational energy levels of Porphyrin and its substituted form make up clusters. In this paper, we have prese vibrational spectra of Porphyrin and its substituted forms in the algebraic framework making use of the one-dimensional Vibron model i.e. U (2) Vibron model. Using the U (2) algebraic model Hamiltonian, the retching frequencies of Cb-C and Pyrrol breathing up to Second overtone ( = 2), the combinational bands of Nickel Octaethyl Porphyrin [Ni(OEP)] and its substitu- ted form Ni(OEP)-d4 molecules are given in Table 2. However, due to lack of sufficient data base, we could not compare the calculated vibrational frequencies with that of observed data of Nickel Metalloporphyrin and its substituted forms at higher overtones. This study is use- ful to the experimentalist to analyze the predicted vibra- tional frequencies with the observed data. The model pre sented here describes the splitting of local stretch- ing/bending modes due to residual interbond interactions. The splitting pattern determines the nature of interaction (Parameter ׳). Once we get the parameter, we predict the splitting pattern of overtones. It is worth to point out that most applications of previous algebraic models available in literature [28-33] are restricted to vibrations of Bio-molecules. The importance o a global analysis of all molecular species in terms of few algebraic parameters. In turn provides a way to make assignments of unknown levels or to check as- signments of known levels. The study of vibrational ex- citations of these bio-molecules (proteins) has numerous importance not only in human life but also in scientific research. 5. ACKNOWKEDGE The author Dr. Srinivasa Rao Karumuri would lik son G Spiro for providing the necessary literature for this study. The authors Dr.Srinivasa Rao Karumuri and Prof. Ramendu Bhattacharjee are grateful to the DST, New Delhi for supporting this work. The au- thor is very much grateful to the anonymous referee of this paper for his valuable suggestions and comments, which greatly helped to im- prove the quality of the paper. 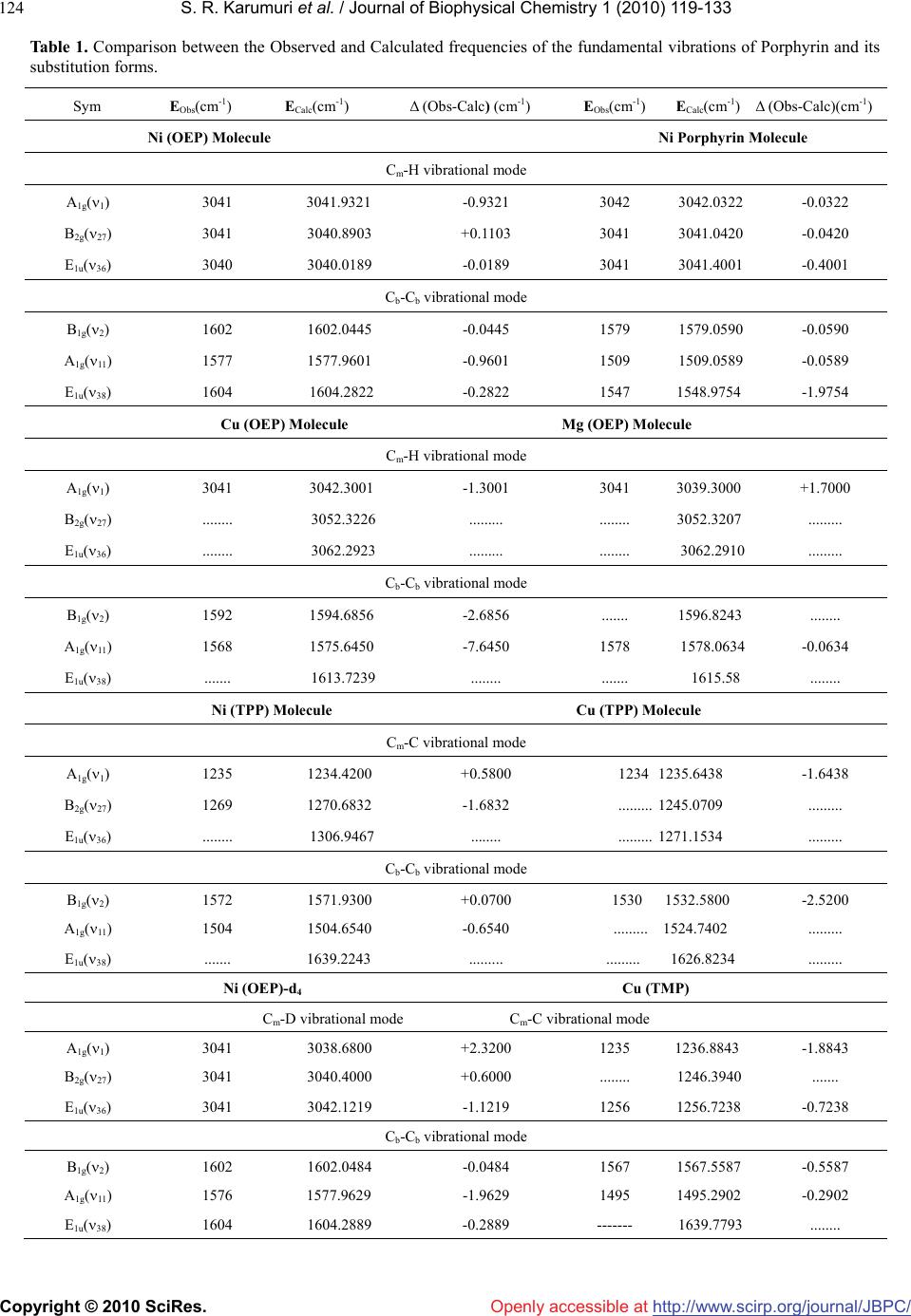 S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/Openly accessible at 124 etween the Observed and Calculated frequencies of the fundamental vibrations of Porphyrin and its Obs(cm-1) ECalc(cm-1) Δ (Obs-Calc) (cm-1) EObs(cm-1) ECalc(cm-1) Δ (Obs-Calc)(cm-1) Table 1. Comparison b substitution forms. Sym E Ni (OEP) Molecule Ni Porphyrin Molecule Cm-H vibrationamode l A1g(1) 3041 3041.9321 3042 3042.0322 -0.0322 -0.9321 B2g(27) 3041 3040.8903 +0.1103 3041 3041.0420 -0.0420 E1u(36) 3040 3040.0189 -0.0189 3041 3041.4001 -0.4001 Cb-Cb vibrate ional mod B1g(2) 1602 1602.0445 1579 1579.0590 -0.0590 -0.0445 A1g(11) 1577 1577.9601 -0.9601 1509 1509.0589 -0.0589 E1u(38) 1604 1604.2822 -0.2822 1547 1548.9754 -1.9754 Cu (OEP) M Mg (Oolecolecule EP) Mule Cm-H vibrational mode A1g(1) 3041 3042.3001 3041 3039.3000 +1.7000 -1.3001 B2g(27) ........ 3052.3226 ......... ........ 3052.3207 ......... E1u(36) ........ 3062.2923 ......... ........ 3062.2910 ......... Cb-Cb vibrational mode B1g(2) 1592 1594.6856 ....... 1596.8243 ........ -2.6856 A1g(11) 1568 1575.6450 -7.6450 1578 1578.0634 - PP) Mole Cu Molecu 0.0634 E1u(38) ....... 1613.7239 ........ ....... 1615.58 ........ Ni (Tcule (TPP)le Cm-C vibrational mode A1g(1) 1235 1234.4200 1234 1235.6438 -1.6438 +0.5800 B2g(27) 1269 1270.6832 -1.6832 ......... 1245.0709 ......... E1u(36) ........ 1306.9467 ........ ......... 1271.1534 ......... Cb-Cb vibrational mode B1g(2) 1572 1571.9300 1530 1532.5800 -2.5200 +0.0700 A1g(11) 1504 1504.6540 -0.6540 ......... 1524.7402 ......... E1u(38) ....... 1639.2243 ......... . (OEP)-d4 ........ 1626.8234 ......... Ni Cu (TMP) Cm-D vibrational mode Cm-C vibrational mode A1g(1) 3041 1236.8843 -1.8843 3038.6800 +2.3200 1235 B2g(27) 3041 3040.4000 +0.6000 ........ 1246.3940 ....... E1u(36) 3041 3042.1219 -1.1219 1256 1256.7238 - Cb-Cb vibrate 0.7238 ional mod B1g(2) 1602 1602.0484 1567 1567.5587 -0.5587 -0.0484 A1g(11) 1576 1577.9629 -1.9629 1495 1495.2902 -0.2902 E1u(38) 1604 1604.2889 -0.2889 ------- 1639.7793 ........ 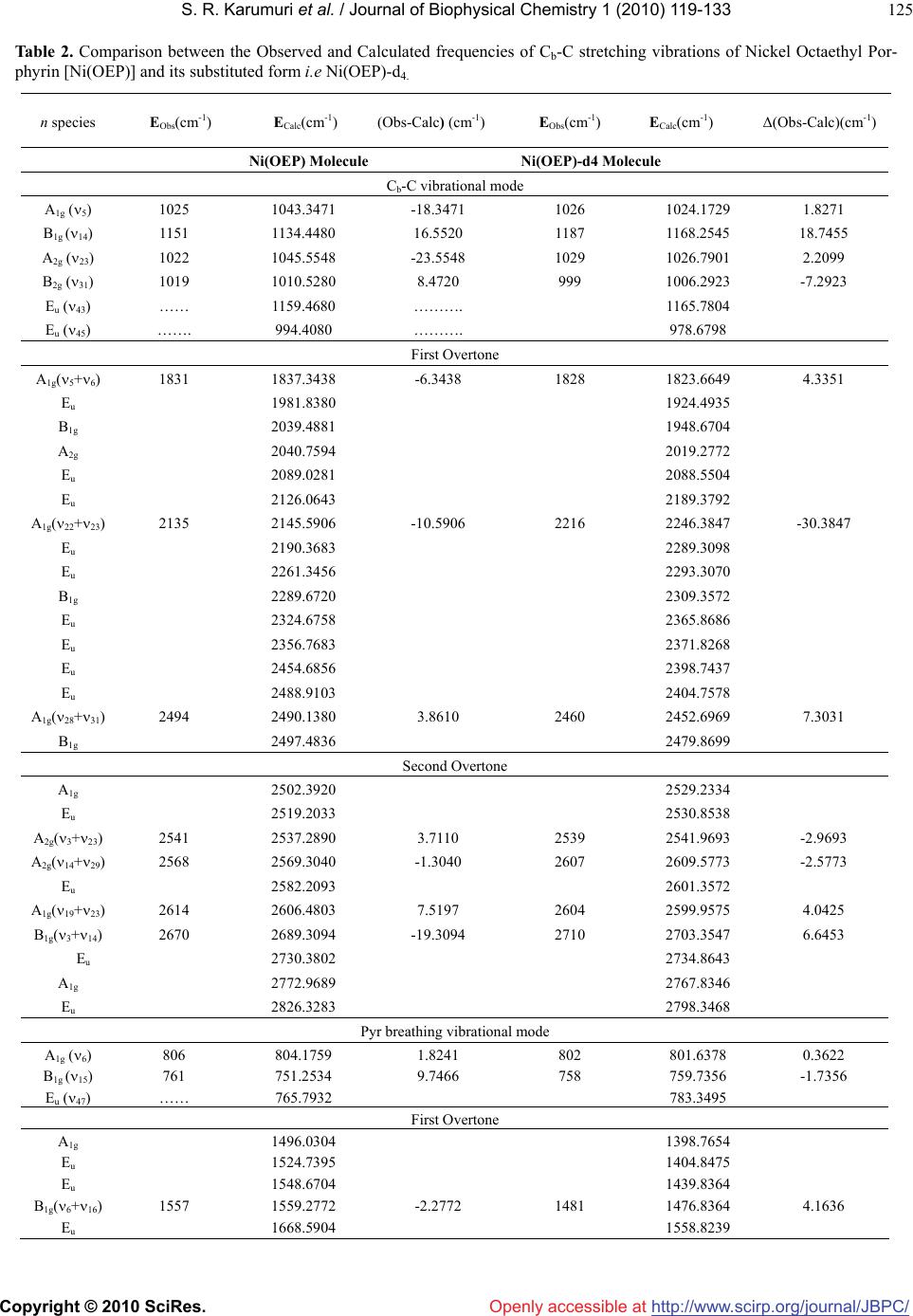 S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/Openly accessible at 125 Table arison betwe Observculated freqb-C stretching vibrickel Oyl Por- phyrin [Ni(OEP)] and its substited form i.e 4. 2. Compeen th tu ed and Cal Ni(OEP)-d uencies of Cations of Nctaeth n species EObs(cm-1) ECalc(cm-1) Δ (Obs-C -1 -1 -1 -1 alc) (cm) EObs(cm) ECalc(cm) Δ(Obs-Calc)(cm ) Ni(OPculei(Eolecu E) Mole NOP)-d4 Mle C-C vibrational bmode A1g (5) 1025 1729 1.8271 1043.3471 -18.3471 1026 1024. B1g (14) 1151 1134.4480 1187 1168.2545 18.7455 tone 16.5520 A2g (23) 1022 1045.5548 -23.5548 1029 1026.7901 2.2099 B2g (31) 1019 1010.5280 8.4720 999 1006.2923 -7.2923 Eu (43) …… 1159.4680 ………. 1165.7804 Eu (45) ……. 994.4080 ………. 978.6798 First Over A 1831 1828 4.3351 1g(5+6)1837.3438 -6.3438 1823.6649 Eu 1981.8380 1924.4935 A1g(23) 2135 -10.06 2216 -30.47 A1g(31) 2494 3.8610 2460 7.3031 Second Overtone B1g 2039.4881 1948.6704 A2g 2040.7594 2019.2772 Eu 2089.0281 2088.5504 Eu 2126.0643 2189.3792 22+2145.5906 592246.3847 38 Eu 2190.3683 2289.3098 Eu 2261.3456 2293.3070 B1g 2289.6720 2309.3572 Eu 2324.6758 2365.8686 Eu 2356.7683 2371.8268 Eu 2454.6856 2398.7437 Eu 2488.9103 2404.7578 28+2490.1380 2452.6969 B1g 2497.4836 2479.8699 A1g 2502.3920 2529.2334 Eu 2519.2033 2530.8538 A2g 23) 2541 3.72539 -2.9693 A2g(29) 2568 -1.3040 2607 -2.5773 B1g(14) 2670 -19.94 2710 6.6453 E Pyr breathing vibrational mode (3+2537.2890 110 2541.9693 14+2569.3040 2609.5773 Eu 2582.2093 2601.3572 A1g(19+23) 2614 2606.4803 7.5197 2604 2599.9575 4.0425 3+2689.3094 302703.3547 Eu 2730.3802 2734.8643 A1g 2772.9689 2767.8346 u 2826.3283 2798.3468 A1 g ( 6 ) 806 1.82418020.3622804.1759 801.6378 B1 g (15) 761 751.2534 758759.7356 -1.73569.7466 E u (47) …… 765.7932 783.3495 First Overtone A1 g 1496.0304 1398.7654 E u 1524.7395 1404.8475 E u 1548.6704 1439.8364 B1 g (16) 1557 -2.277214814.1636 6+1559.2772 1476.8364 Eu 1668.5904 1558.8239 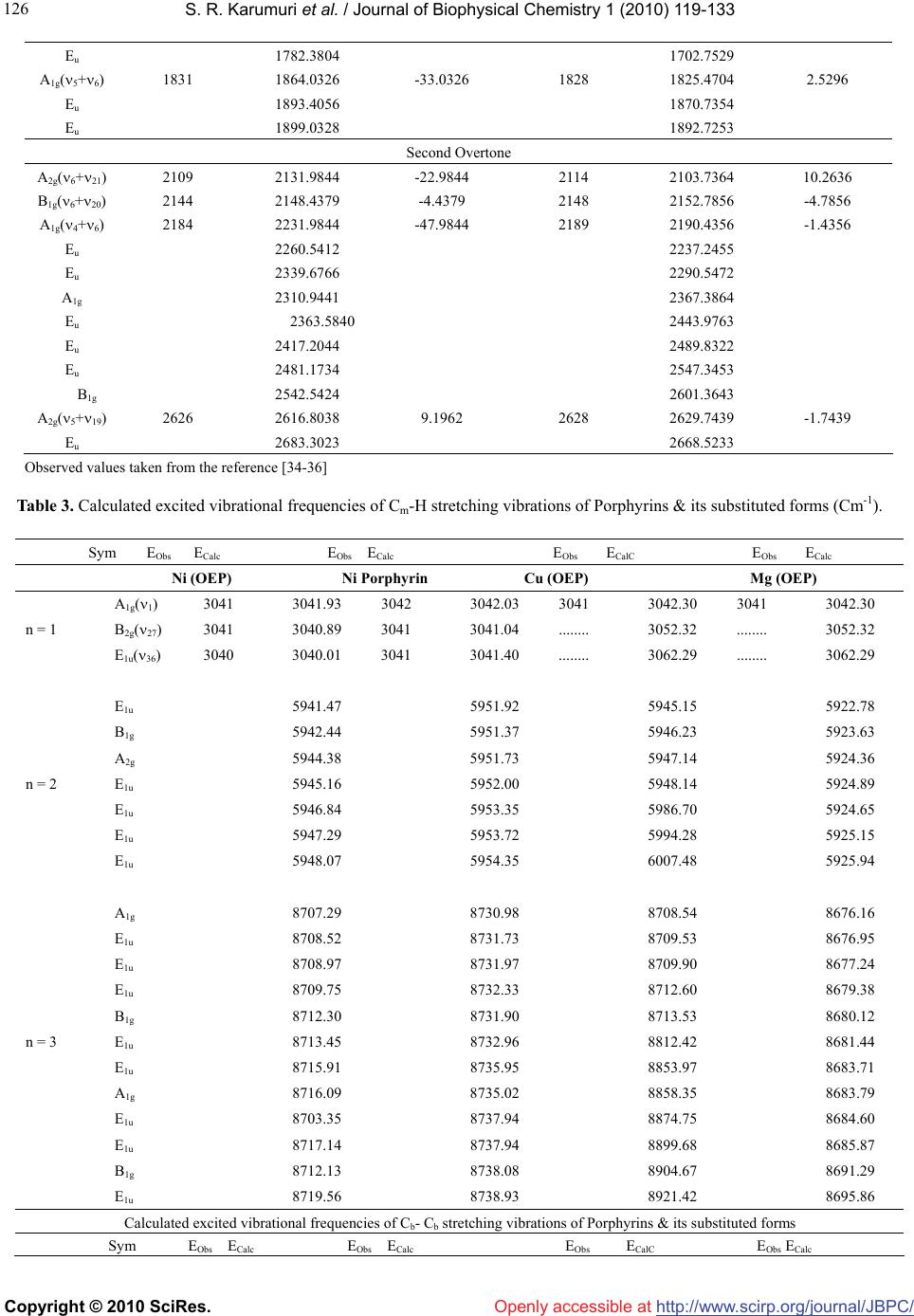 S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/Openly accessible at 126 Eu 1782.3804 1702.7529 A1g 6) 1831 -33.26 1828 2.5296 Second Overtone (5+1864.0326 031825.4704 Eu 1893.4056 1870.7354 Eu 1899.0328 1892.7253 A2g(21) 2109 -22.44 214 10.6 6+2131.9844 9812103.7364 263 B1g(6+20) 2144 2148.4379 2148 2152.7856 -4.7856 0 24 1g A2g(19) 2626 9.1962 2628 -1.7439 E -4.4379 A1g(4+6) 2184 2231.9844 -47.9844 2189 2190.4356 -1.4356 Eu 2260.5412 2237.2455 Eu 2339.6766 2290.5472 A1g 2310.9441 2367.3864 Eu 2363.584 2443.9763 Eu 2417.2044 2489.8322 Eu 81.1734 2547.3453 B2542.5424 2601.3643 5+2616.8038 2629.7439 u 2683.3023 2668.5233 Os takeneferenc Tcies of Cm-H stretching vibrations of Porphyrins & its substituted forms (Cm-1). bserved value from the re [34-36] able 3. Calculated excited vibrational frequen Sym EObs E Calc E Obs E Calc E Obs E CalC E Obs E Calc Ni (OEP) Ni Porphyrin Cu (OEP) Mg (OEP) 12.30 A 1g(1) 3041 304.93 3042 3042.03 3041 3042.30 3041 304 n = 1 3052.32 1g 942.44 951.37 946.23 923.63 = 2 1u 708.52 731.73 709.53 676.95 = 3 lculated eited vibrationcies of C B2g(27) 3041 3040.89 3041 3041.04 ........ 3052.32 ........ E 1u(36) 3040 3040.01 3041 3041.40 ........ 3062.29 ........ 3062.29 E 1u 5941.47 5951.92 5945.15 5922.78 B 5 5 5 5 A 2g 5944.38 5951.73 5947.14 5924.36 n E1u 5945.16 5952.00 5948.14 5924.89 E 1u 5946.84 5953.35 5986.70 5924.65 E 1u 5947.29 5953.72 5994.28 5925.15 E 1u 5948.07 5954.35 6007.48 5925.94 A 1g 8707.29 8730.98 8708.54 8676.16 E 8 8 8 8 E 1u 8708.97 8731.97 8709.90 8677.24 E 1u 8709.75 8732.33 8712.60 8679.38 B 1g 8712.30 8731.90 8713.53 8680.12 n E1u 8713.45 8732.96 8812.42 8681.44 E 1u 8715.91 8735.95 8853.97 8683.71 A 1g 8716.09 8735.02 8858.35 8683.79 E 1u 8703.35 8737.94 8874.75 8684.60 E 1u 8717.14 8737.94 8899.68 8685.87 B 1g 8712.13 8738.08 8904.67 8691.29 E 1u 8719.56 8738.93 8921.42 8695.86 Ca xcnal freque b - C b stretratioof Porphyrinbstitd forms ching vibns s & its suute Sym E E Calc Obs E Calc E Obs E Ca E Obs ECalc Obs E lC  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/Openly accessible at 127 P) Ni (OEP) Ni Por Cu (OEP) Mg (OE 1596.82 B 1g(2) 1602 1602.04 1579 1579.05 1592 1594.68 n = 1 578.06 1u 159.17 033.88 157.61 142.67 = 2 1u 720.62 559.73 707.83 695.26 = 3 Calculated excited vibrational s of -C & Cm-D vibrons of Porphs substituted forms A1g(11) 1577 1577.96 1509 1509.05 1568 1575.64 1578 1 E 1u(38) 1604 1604.28 1547 1548.97 1613.72 1615.58 A 2g 3143.88 3021.25 3138.57 3137.07 E 3 3 3 3 B 2g 3170.90 3061.13 3164.55 3144.71 n E1u 3183.36 3161.24 3176.65 3148.27 E 1u 3192.04 3081.13 3190.09 3174.59 E 1u 3200.82 3231.24 3195.69 3181.43 E 1u 3216.12 3068.45 3215.85 3193.35 A 1g 4696.54 4539.77 4688.79 4689.66 E 4 4 4 4 E 1u 4729.40 4567.02 4714.77 4697.30 E 1u 4744.70 4579.69 4766.28 4712.45 B 1g 4794.54 4621.02 4783.99 4717.66 n E1u 4816.94 4639.59 4869.11 4820.98 E 1u 4865.10 4679.51 4878.12 4827.54 A 1g 4868.71 4682.52 4894.87 4839.74 E 1u 4817.61 5183.77 4925.89 4862.25 E 1u 4889.18 4699.48 4927.63 4867.88 B 1g 4762.34 4889.77 4933.51 4872.57 E 1u 4895.60 4939.95 4874.32 frequencie Cmstretching atiyrins & it Sym EOb E Calc E Ob Calc E s E CalC E s E Calc s s E Ob Ob ) Ni (TPP) Cu (TPP) Cu (TMP Ni (OEP)-d 4 (Cm - D 3038.68 A 1g(1) 1235 1234.42 1234 1235.64 1235 1236.88 3041 n = 1 2710.40 1g 495.39 427.57 474.51 953.77 = 2 1u 713.46 554.51 692.22 776.62 = 3 B2g(27) 1269 10.68 245.07 1246.39 3041 304 E 1u(36) 1306.94 1271.15 1256 1256.72 3041 3042.12 E 1u 2459.13 2417.34 2464.18 5952.05 B 2 2 2 5 A 2g 2508.62 2436.20 2475.39 5953.91 n E1u 2531.65 2452.85 2484.84 5955.49 E 1u 2777.49 2459.33 2524.26 6007.76 E 1u 2835.59 2470.61 2535.23 6017.93 E 1u 2936.67 2445.64 2554.31 6035.62 A 1g 3674.12 3545.08 3681.89 8774.90 E 3 3 3 8 E 1u 3710.38 3557.95 3695.99 8777.25 E 1u 3821.69 3583.47 3702.55 8778.34 B 1g 4321.98 3633.87 3723.94 8781.91 n E1u 3855.42 3669.38 3804.16 8888.28 E 1u 4470.02 3675.60 3832.11 8914.19 A 1g 4788.38 3687.14 3892.19 8969.91  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/Openly accessible at 128 E 1u 4812.25 3704.90 3902.71 8979.66 E 1u 4947.56 3708.45 3922.24 8997.77 B 1g 5138.57 3617.35 3952.28 9025.63 E 1u 5187.30 3718.93 3958.29 9031.21 Calculated eited vibrationcies of Cb-Cb stretationof Porphyrinstitud forms xc nal freque ching vibrs s & its subte Sym EObs Calc E Obs E Calc E s E CalC E Obs E Calc E Ob Ni (TPP) Cu (TPP) Cu (TMP) Ni (OEP)-d 4 (1602.04 A 1g 1) 1572 1571.93 1530 1532.58 1567 1567.55 1602 n = 1 B2g(27 11577.96 1g 062.66 027.25 229.30 159.17 1u 571.45 478.84 810.31 720.62 = 3 ) 1504 504.65 1524.74 1495 1495.29 1576 E 1u(36) 1639.22 1626.82 1639.77 1604 1604.28 E 1u 3035.20 3018.75 3201.84 3143.88 B 3 3 3 3 A 2g 3065.00 3034.43 3231.64 3170.90 E 1u 3090.13 3042.27 3256.77 3183.36 E 1u 3169.76 3120.83 3346.37 3192.04 E 1u 3194.32 3139.46 3372.75 3200.82 E 1u 3237.05 3171.88 3418.64 3216.12 A 1g 4543.99 4471.00 4782.85 4696.54 E 4 4 4 4 E 1u 4581.48 4481.70 4820.34 4729.40 E 1u 4598.92 4502.90 4837.78 4744.70 B 1g 4655.78 4678.74 4894.64 4794.54 E 1u 4817.83 4726.22 5076.98 4816.94 n E1u 4880.41 4828.30 5144.19 4865.10 A 1g 5014.97 4846.17 5288.72 4868.71 E 1u 5038.52 4879.35 5314.01 4817.61 E 1u 5082.26 4930.39 5360.99 4889.18 B 1g 5149.54 4940.60 5433.26 4762.34 E 1u 5163.00 4945.56 5447.71 4895.60 TVa lueof Algebraic Parame in the calculatio, CStretchin of rphyrins ansti- tuted forms. (C m -D) able 4. s (a ) ters Usedn of Cm-H m-D g ModesPod its sub Ni(OEP) Cu(OEP) Mg(OEP) Ni(TPP) Cu(TPP) Cu(TMP) Ni Por Ni(OEP)-d 4 N 44 44 44 44 44 44 44 59 A -12 -10 -14 -2 -70 -23 - -13 0 7.680 7.6827.61.213.174.21717.653.04 A΄ -0.24 -0.25 -0.28 -0.9985 -0.4302 -1.0182 -1.3108 -0.2782 λ 0.014 .011360.009 0.1295 0.1072 0.0369 0.0113 0.0146 λ΄ 0.011 0.012 0.012 0.5685 0.2018 0.1073 0.0021 0.2361 (a) All values in cm-1 except N, which is dimensionless.  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 129 Table Va lu e s (a) ofbbtretching Modes of Porphyriforms. Ni(OEP) Cu(OEP) Mg(OEP) Ni(TPP) Cu(TPP) Cu(TMP) Ni Por Ni(OEP)-d 4 5. Algebraic Parameters Used in the calculation of C-C Sns and its substituted N 140 140 140 140 140 140 140 140 A -2.83 -2.825 -2.835 -2.7205 -2.7502 -2.8825 -2.691 -2.83 A΄ -1.223 -1.286 -0.452 -1.986 1.0921 -1.223 -3.216 -1.223 λ 0.086 0.068 0.067 0.2403 0.028 0.2581 0.0713 0.086 λ΄ 0.047 0.092 0.020 0.0981 0.1823 0.0981 0.25 0.047 (a) All values in cm-1 except N, which nsionles is dimes. Figure 2. Cm-H band vibrational energy level of Ni(TPP). Figure 3. Cb-Cb band vibrational energy level of Ni(TPP). Openly accessible at 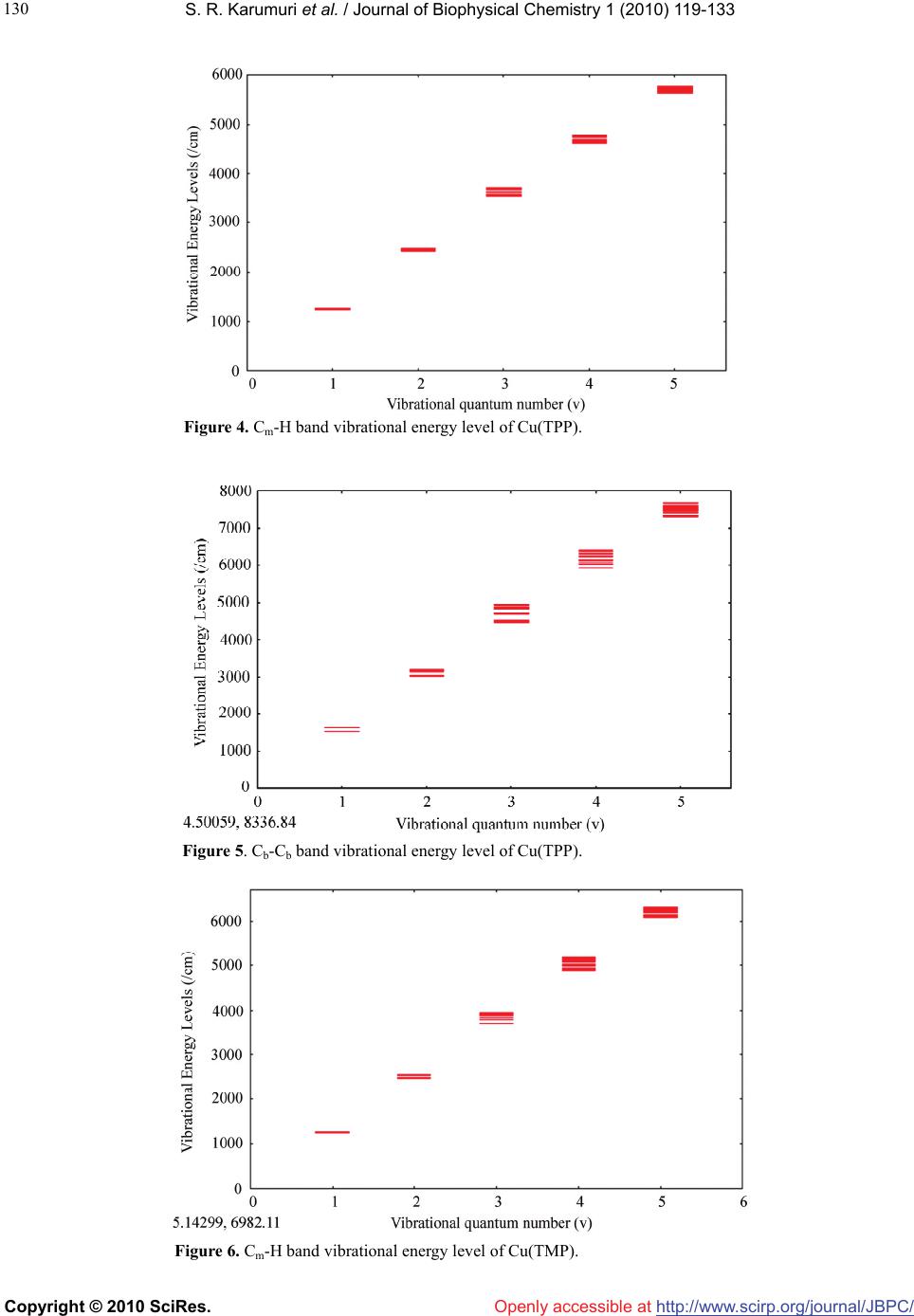 S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 130 Figure 4. Cm-H band vibrational energy level of Cu(TPP). Figure 5. Cb-Cb band vibrational energy level of Cu(TPP). Figure 6. Cm-H band vibrational energy level of Cu(TMP). Openly accessible at  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 131 Figure 7. Cb-Cb band vibrational energy level of Cu(TMP). F igure 8. Cm-H band vibrational energy level of Ni(OEP)-d4. Figure 9. Cb-Cb band vibrational energy level of Ni(OEP)-d4. Openly accessible at  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/ 132 REFERENCES [1] Iachello, F. (1981) Algebraic methods for molecular rota- tion-vibration spectra. Chemical Physics Letters, 78(3), 581-585. [2] Iachello, F. and Levine, R.D. (1982) Algebraic approach to molecular rotation-vibration spectra. I. Diatomic mole- cules. Journal of Chemical Physics, 77(6), 3046-3055. [3] Van Roosmalen, O.S., Dieperink, A.E.L. and Iachello, F. (1982) A dynamic algebra for rotation-vibration spectra of complex molecules. Chemical Physics Letters, 85(1), 32-36. [4] Van Roosmalen, O.S., Iachello, F., Levine R.D. and Die- perink, A.E.L. (1983) Algebraic approach to molecular rotation–vibration spectra. II. Triatomic molecules. Jour- nal of Chemical Physics, 79(6), 2515-2536. [5] Sarkar, N.K., Choudhury, J. and Bhattacharjee, R. (2006) An algebraic approach to the study of the vibrational spectra of HCN. [6] Sarkar, N.K., Chou Algebraic approach: Study of vibrational spectra of some olecules. Indian Journal of Physics tra of OCS and HCP using the Lie algebraic - ury, J., Karumuri, S.R., Sarkar, N.K. and Bhat- . (2008) (2), 020308. evelopment, aic approach. ing and bending modes of Nickel Tetra Phenyl Porphyrin: An algebraic approach. Chinese Physics Letters, 26(9), 093301. [16] Karumuri, S.R., Sarkar, N.K., Choudhury, J. and Bhat- tacharjee, R. (2009) U(2) algebraic model applied to stretching vibrational spectra of Metalloporphyrins. Jour- nal of Molecular Spectroscopy, 255(2), 183-188. [17] Karumuri, S.R. and Prasad, A.S.R. (2009) Analysis of vibrational spectra of Copper Octaethyl Porphyrin [Cu (OEP)] using U(2) algebraic technique. International Journal of Computational Mathematical Ideas, 1(3), 68. [18] Karumuri, S.R., Choudhury, J., Sarkar, N.K. and Bhat- tacharjee, R. (2010) Vibrational spectroscopy of Cm-C/ Cb-Cb stretching vibrations of Copper Tetramesityl Por- phyrin Cu (TMP): An algebraic approach. Pramana - Journal of Physics, 74(1), 57-66. [19] Iachello, F. and Oss, S. (1990) Overtone frequencies and intensities of bent XY2 molecules in the vibron model. Journal of Molecular Spectroscopy, 142(1), 85-107. [20] Iachello, F., Oss, S. and Lemus, R. (1991) Vibrational ear triatomic molecules in the vibron model. olecular Spectroscopy, 146(1), 56-78. nal Openly accessible at Molecular Physics, 104(19), 3051-3055. dhury, J. and Bhattacharjee, R. (2008) spectra of lin linear triatomic m 82(6), 767-772. , [2 [7] Sarkar, N.K., Choudhury, J., Karumuri, S.R. and Bhat- tacharjee, R. (2009) A comparative study of the vibra- tional spec method. European Physical Journal D, 53(2), 163-171. [8] Sarkar, N.K., Choudhury, J., Karumuri, S.R. and Bhat- tacharjee, R. (2008) An algebraic approach to the com parative study of the vibrational spectra of monofluo- roacetylene (HCCF) and deuterated acetylene (HCCD). Molecular Physics, 106(5), 693-702. [9] Choudh tacharjee, R. (2008) Vibrational spectroscopy of SnBr4 and CCl4 using Lie algebraic approach. Pramana - Journal of Physics, 71(3), 439-445. [10] Choudhury, J., Sarkar, N.K. and Bhattacharjee, R Algebraic approach to analyze the vibrational spectra of tetrahedral molecules. Indian Journal of Physics, 82(5), 561-565. [11] Choudhury, J., Karumuri, S.R., Sarkar, N.K. and Bhat- tacharjee, R. (2009) Vibrational spectroscopy of CH/CD stretches in propadiene: An algebraic approach. Chinese Physics Letters, 26 [12] Karumuri, S.R., Sarkar, N.K., Choudhury, J. and Bhat- tacharjee, R. (2008) Vibrational spectroscopy of Cm-H, Cb-Cb stretching vibrations of Nickel Metalloporphyrins: An algebraic approach. Molecular Physics, 106( 14), 1733- [28 1738. [13] [Karumuri, S.R., Choudhury, J., Sarkar, N.K. and Bhat- tacharjee, R. (2008) Analysis of resonance raman spectra of Nickel Octaethyl Porphyrin using Lie algebra. Journal of Environmental Research and D3(1), benzene in the algebraic model. Chemical Physics Let- ters, 187(5), 500-505. [30] Alhassid, Y., Gursey, F. and Iachello, F. (1983) Group theory approach to scattering. Annual Physics (New York), 148(2), 346-380. 250-256. [14] Karumuri, S.R., Sarkar, N.K., Choudhury, J. and Bhat- tacharjee, R. (2009) Study of vibrational spectra of Nickel Metalloporphyrins: An algebrPra- mana - Journal of Physics, 72(3), 517-525. [15] Karumuri, S.R., Sarkar, N.K., Choudhury, J. and Bhat- tacharjee, R. (2009) Vibrational spectroscopy of stretch- Journal of M 1] Iachello, F., Oss, S. and Lemus, R. (1991) Linear four-atomic molecules in the vibron model. Journal of Molecular Spectroscopy, 149(1), 132-151. [22] Wang, M.S., Ding, S.L., Feng, D.T. and Liu, H.Y. (2002) Lie-algebraic approach to vibrational spectra of a linear symmetrical tetratomic molecule: C2H2. Physical Review A, 66, (022506)1-10. [23] Van Roosmalen, O.S., Levine, R.D. and Dieperink, A.E.L. (1983) The geometrical-classical limit of algebraic Ham- iltonians for molecular vibrotational spectra. Chemical Physics Letters, 101(6), 512-517. [24] Benjamin, I., Van Roosmalen, O.S. and Levine, R.D. (1984) A model algebraic Hamiltonian for interacting nonequivalent local modes with application to HCCD and H12C13CD. Journal of Chemical Physics, 81(7), 3352-3353. [25] Van Roosmalen, O.S., Benjamin, I. and Levine, R.D. (1984) A unified algebraic model description for inter- acting vibrational modes in ABA molecules. Journal of Chemical Physics, 81(12), 5986-5997. [26] Halonen, L. and Child, M.S. (1983) Model stretching overtone eigenvalues for SF6, WF6, and UF6. Journal of Chemical Physics, 79(2), 559-570. [27] Bowman, J.M., Wierzbicki, A. and Zuniga, J. (1988) Exact vibrational energies of non-rotating H2O and D2O using an accurate ab initio potential. Chemical Physics Letters, 150(3-4), 269-274. ] Iachello. F and Oss, S. (1991) Model of n coupled an- harmonic oscillators and applications to octahedral molecules. Physical Review Letters, 66(23), 2976-2979. [29] Iachello. F and Oss, S. (1991) Stretching vibrations of [31] Alhassid, Y., Gursey, F. and Iachello, F. (1983) Group theory of the Morse oscillator. Chemical Physics Letters, 99(1), 27-30. [32] Levine, R.D. (1983) Representation of one-dimensio  S. R. Karumuri et al. / Journal of Biophysical Chemistry 1 (2010) 119-133 Copyright © 2010 SciRes. http://www.scirp.org/ journal/JBPC/Openly accessible at 133 quen- J.R., Stein, P. d infrared isotope shift. Journal o of nonfundamental Raman lines. ) Resonance Raman spectros- loroiron(III) octaethyl- and tetraphenyl- motion in a morse potential by a quadratic Hamiltonian. Chemical Physics Letters, 95(2), 87-90. [33] Child, M.S. and Halonen, L.O. (1984) Overtone fre cies and intensities in the local mode picture. Advances in Chemical Physics, 57, 1-58. [34] Li, X.-Y., Czernuszewicz, R.S., Kincaid, and Spiro, T.G., (1990) Consistent porphyrin force field. 2. Nickel octaethylporphyrin skeletal and substituent mode assignments from nitrogen-15, meso-d4, and me- thylene-d16 Raman anf por Physical Chemistry, 94(1), 47-61. [35] Kitagawa, T., Abe, M. and Ogoshi, H. (1978) Resonance Raman spectra of octaethylporphyrinato-Ni(II) and meso- deuterated and 15N substituted derivatives. I. Observa- tion and assignments Journal of Chemical Physics, 69(10), 4516-4525. [36] Czernuszewicz, R.S., Macor, K.A., Li, X.-Y., Kincaid, J.R. and Spiro, T.G. (1989 copy reveals a1u vs. a2u character and pseudo-Jahn- Teller distortion in radical cations of nickel(II), cop- per(II), and ch phyrins. Journal of the American Chemical Society, 111(11), 3860-3869. |

