Food and Nutrition Sciences
Vol.4 No.8A(2013), Article ID:35315,7 pages DOI:10.4236/fns.2013.48A025
Development of a Rapid and Simple Non-Derivatization Method to Determine Constituents and Antioxidative Capacity of Camellia Oils by HPTLC
![]()
1State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, China; 2School of Pharmacy, Jiangxi Science and Technology Normal University, Nanchang, China.
Email: #zhaojing.cpu@163.com
Copyright © 2013 Guang-Ping Lv et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 10th, 2013; revised May 10th, 2013; accepted May 17th, 2013
Keywords: Antioxidant; Camellia oleifera Abel; Camellia Oil; HPTLC; Non-Derivatization
ABSTRACT
Camellia oil is an edible vegetable oil with high value of nutrition and health protection function such as antioxidant and adjusting blood fat. In this study, a simple, rapid and effective HPTLC method was developed for analyzing the composition and antioxidant constituents of camellia oil. The HPTLC was performed on G60 plate with n-hexane-diethyl ether-acetic acid (6:4:0.1, v/v/v) as mobile phase combined with two coloration methods (ethanol containing 10% phosphomolybdic acid, ethanol containing 0.03% DPPH) and scanning densitometry technique. The unsaturated fatty glyceride, free fatty acids, sterols and lipids including triolein, oleic acid, ergosterin, β-sitosterol, tocopherol and phospholipids in camellia oils were determined and performed densitometrically at λs1 = 620 nm and λs2 = 517 nm. The results show that the main components of different samples of camellia oil are similar, however the contents are diverse. The antioxidative test shows that camellia oil has obvious antioxidant capability as olive oil, especially the pressed virgin oil. Therefore, this non-derivatization HPTLC method can be used for composition and antioxidative capacity determination of camellia oils.
1. Introduction
Food provides not only essential nutrients required for life, but also bioactive compounds useful to maintain good health and disease prevention. Camellia oil is an edible vegetable oil that can be obtained from the mature seeds of Camellia oleifera Abel which mainly distributed in southern China, such as Guangxi, Hunan, and Jiangxi provinces [1]. It is an important woody oil crop [2] and also grown as an ornamental plant in Western countries. Camellia oil can be used both for medicine and food dual purposes such as directly given in the form of injection [3] or used as dissolvent of liquid soluble drug or ointment base [4], especially it listed as medicinal oil in the Chinese pharmacopoeia [5]. Simultaneously, camellia oil is high quality natural cooking oil, because of its high quality and healthful properties. The monounsaturated fatty acid contains the best natural vegetable oil, which is hailed as olive oil of eastern world [4,6-10]. Besides, it also has been used in cosmetics [11-13].
Camellia oil was traditionally applied as a health caring medicine to prevent coronary heart disease, hypertension, cerebrovascular disease [14], arteriosclerosis, stomachache [7] as well as burning injury in China [15]. Recent evidence concerning an association between bioactive phytochemicals in the diet and health has imparted impetus to the utilization of camellia oil for most healthy edible oil, keeping its bioactive phytochemicals intact. Camellia oil is predominantly composed of glyceride of unsaturated fatty acid such as oleic acid (75% - 80%) and linoleic acid, while the saturated fats are presented in low amounts [13]. Camellia oil also contains many other natural antioxidants with various biological activities, such as phytosterols, tocopherol, free fatty acids (FFAs) [16]. The camellia oil is helpful in protecting liver against carbon tetrachloride toxicity [17]. It has been observed to suppress cholesterol content in the body and provide resistance to oxidative stress [18]. Antioxidants are essential for human health. Dietary antioxidants play an important role in controlling oxidative stress [19]. Robust epidemiological evidence suggests the crucial role of diets in prevention of chronic degenerative diseases [20]. Supplementation of natural antioxidants through a balanced diet containing enough antioxidants could be most effective in protecting against various oxidative stressors [21].
Up to date, gas chromatography (GC) and gas chromatography with mass spectrometry (GC/MS) are widely considered as conventional procedures for the determination of FA and phytosterols in oils [22-28]. Yuan et al. using near infrared transmittance spectroscopy (NITS) to detect FA composition of the edible oils [1]. The high performance liquid chromatography with mass spectrometry (HPLC-MS) method was applied for the determination of triacylglycerols composition of camellia oil [29]. These techniques are accurate, but also have the disadvantages of expensive, time-consuming and derivatization that make the sample preparation procedure inconvenience in popularization. In contrast, the high performance thin layer chromatography (HPTLC) method has several advantages including the lower cost, less rigorous sample preparation, the ability to analyze multiple samples simultaneously, and the ease of visualization [30]. In this work, the optimized HPTLC method enables the main chemical components of camellia oil separated on the thin layer plate even without derivatization processing. The HPTLC combining with scan technique will transform the spots of different components into chromatographic peak signals, appraise the retention factor (Rf) and peak areas of samples which can quickly analyze the main components of camellia oil. Simultaneously, the antioxidation ability of camellia oil was evaluated for the first time, and indicated the main antioxidation components in camellia oil, this method also can be used to analyze other edible vegetable oils directly.
2. Materials and Methods
2.1. Materials
Acetic acid, n-hexane, diethyl ether, petroleum ether (60˚C - 90˚C), acetone, ethyl acetate, toluene-methanol and chloroform were purchased from UNI-CHEM d.o.o. (Belgrade, Serbia and Montenegro). All chemicals reagents used were analytical grade. Triolein (98%, HPLC), oleic acid (99%, HPLC), beta-sitosterol (98%, HPLC), ergosterol (98%, HPLC), tocopherol (98%, HPLC) and 2, 2-diphenly-1-picrylhydrazyl (DPPH) were purchased from Sigma Company (Sigma, USA). Refined camellia oils were collected from Ji’an (SM01 and SM02), Nanchang (SM03), Yongfeng (SM04) and Ganzhou (SM05) of Jiangxi Province; Refined camellia oil (SM06) was collected from Fangchenggang of Guangxi Province; Refined camellia oil (SM07) was collected from Yichun of Jiangxi Province; Pressed virgin oil (SM08) was collected from Jiangxi Province; Virgin oil (SM09) was made in the Institute of Chinese Medical Sciences, University of Macau, Macao, China; Refined olive oil (SM10) was collected from Italy. All refined oils are commercially available. Voucher specimens of these samples were deposited at the Institute of Chinese Medical Sciences, University of Macau, Macao SAR, China.
2.2. Preparation of Oil Samples and Standard Solutions
The seeds of Camellia oleifera Abel were powdered by a laboratory grinder after discarding the shells and dried to constant weight at 65˚C. The prepared sample (5 g) was macerated with 50 mL solvent (petroleum ether, 60˚C - 90˚C) at 80˚C for 6 h by using Soxhlet apparatus. Finally, the solvent was dried under nitrogen protection. The residual is the crude oil samples (extracted), marked as SM09.
A stock solution containing 0.25 mg/mL of phospholipid, tocopherol, ergosterin and β-sitosterol, 0.5 mg/mL of oleic acid and 1 mg/mL of triolein was prepared in methanol, vortex for 2 min. The solution was stored at 4˚C freezer for use.
The oil sample (25 μL, accurately weighed) was dissolved in 1 mL of n-hexane and vortex for 2 min; the sample (2.5%) was collected for analysis.
2.3. HPTLC Methods
Chromatography was performed on silica G60 TLC plate (20 cm × 10 cm, Merck, Darmstadt, Germany), and a HPTLC system (Desaga GmbH, Germany) including AS30 HPTLC Applicator, CD 60 HPTLC densitometer with Pro Quant Windows software. The plate was heated at 105˚C for 30 min before use. The plate was allowed to cool to room temperature before spotting the samples. Mixed standards (3 μL) and samples (3 μL) of camellia oil were spotted on the plate as bands 4 mm wide, 18 mm apart and 10 mm from the bottom edge. The plate was developed to a distance of 95 mm with n-hexane-diethyl ether-acetic acid (6:4:0.1, v/v/v) in a Desaga 20 cm × 10 cm glass flat-bottom chamber after equilibration with mobile phase vapor for 30 min. The developed plate was colorized with ethanol containing 10% phosphomolybdic acid solution and heated at 95˚C on a YOKO-XR plate heater (Wuhan YOKO technology Ltd., China) for 5 min to make spots colored clearly. The plate was then scaned at λs1 (scan wavelength) = 620 nm in positive signal mode by use of the densitometer.
The chromatography for evaluating antioxidant capability of camellia oil, was performed on silica G60 TLC plate and a HPTLC system, the procedure was the same as mentioned above, the developed plate was colorized with ethanol containing 0.03% DPPH solution and heated at 40˚C on a YOKO-XR plate heater (Wuhan YOKO technology Ltd., China) for 30 min and at room temperature for 12 h to make spots colored clearly. The treated plate scanned at λs2 (scan wavelength) = 517 nm in negative signal mode by use of the densitometer.
2.4. Validation of the Method
The precision was expressed as instrumental precision, identical and different plate precision. Instrumental precision was performed by scanning the same spot of the investigated compound of SM08 sample (3 μL) six times, and variations were expressed by the relative standard deviations (RSD). Identical plate precision was determined by analyzing six spots of the SM08 sample (3 μL) on one plate, while different plate precision was tested by determining one spot of the SM08 sample (3 μL) per plate on six TLC plates. The RSDs of the investigated compounds were calculated.
The repeatability was evaluated by preparing and analyzing six solutions of the SM08 sample (25 μL each). One spot of each solution was analyzed on the same plate, and RSDs of the investigated compounds were calculated.
The stability of six investigated compounds were also determined by injecting SM08 sample at 0, 4, 8, 12, 16, 20 and 24 h, and scanning each band respectively. The RSDs of the investigated compounds were calculated.
3. Result and Discussion
3.1. Optimization of Method
Mobile phases and developing mode were optimized to obtain good separation. Petroleum ether-acetone (4:1 or 3:1, v/v), n-hexane-ethyl acetate (5:1 or 4:1, v/v), toluene-methanol-acetic acid (90:4:4, v/v/v), chloroform-acetone-acetic acid (85:15:1, v/v/v), n-hexane-acetone-acetic acid (90:10:1 or 80:20:1, v/v/v), were tested and demonstrated not available for simultaneous separation of lowand high-polarity compounds in camellia oil in one run. The optimum development reagents are: n-hexanediethyl ether-acetic acid (6:4:0.1, v/v/v). As the results, five main investigated compounds and the components in ten oil samples were well separated in once development (Figure 1).
The saturation time (10 min, 20 min, 30 min, 40 min) and the spray reagents (5% vanillin-H2SO4, iodine vapor, ethanol containing 10% phosphomolybdic acid) were also optimized. The results show that saturating the plate for 30 min and using the ethanol containing 10% phosphomolybdic acid as spray reagents show good separation for HPTLC analysis.
3.2. Method Validation
Instrumental precision (RSD, %) was less than 1.47% (n = 6). For identical plates, and the different plates precision were also determined. The overall RSDs of identical and different plate were less than 2.09% (n = 6) and 3.01% (n = 6), respectively. The repeatability for all the analysis was less than 3.71%. The stability results prove it was stable within 24 h, which RSDs was less than
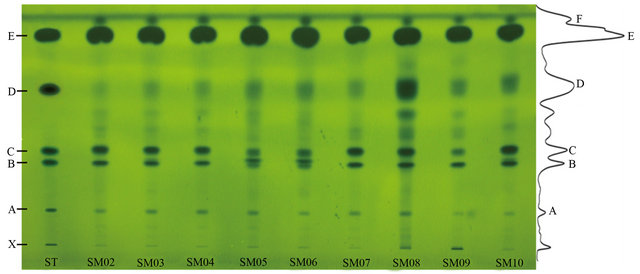
Figure 1. HPTLC of mixed standards and oil samples developed on silica G60 TLC plate (20 cm × 10 cm) and colored with ethanol containing 10% phosphomolybdic acid, viewed at white light. ST, mix standards; X, phospholipid; A, tocopherol; B, ergosterin; C, β-sitosterol; D, oleic acid; E, triolein. SM02 to SM07 are refined camellia oil; SM08 is pressed virgin camellia oil; SM09 is extracted virgin camellia oil; SM10 is refined olive oil.
2.0%. The established method showed good stability, precision and repetitiveness.
3.3. Determination of Main Components in Oil Samples
The proposed method was applied to the determination of oil samples. The representative chromatograms of the standard solutions and the oil samples were shown in Figure 1. The results show that, the components could be simultaneously separated in one run. The main components of camellia oil and olive oil samples are similar: tocopherol with Rf values of 0.23, ergosterin with Rf values of 0.41, β-sitosterol with Rf values of 0.46, oleic acid with Rf values of 0.67, triolein with Rf values of 0.88 and one unknown compound with Rf values of 0.94, respectively. However, the results show that pressed virgin oil is different from others, which contains more free fatty acid. Therefore, TLC could rapidly and easily analysis free fatty acid in vegetable oil and discriminate the pressed virgin oil [25,31].
Maximum detection wavelengths which are set in scan detector are important for the detection sensitivity. Spots of tocopherol, ergosterin, β-sitosterol, oleic acid and triolein were scanned from 400 to 900 nm so as to record their spectrum and to obtain their wavelengths of maximum absorption. Densitograms were recorded at the wavelength of maximum absorption (620 nm) of primary components. The detection wavelengths of the investigated compounds were selected based on their absorption spectra.
The contents of components in ten oil samples were determined using TLC scanning (TLCs). Their TLCs profiles were shown in Figure 2. Generally, the components are showing high similarities of ten oil samples in tocopherol, ergosterin, β-sitosterol, triolein. However, there are two obvious discrimination: 1) Virgin oil samples (SM08, SM09) have high content of polarity compounds which relatively more ingredients were retained in the origin shown in Figure 1. From the band (Rf~0) which corresponding with standard, it may be the phospholipids for this kind of components was reported as common component in virgin camellia oil [32]. 2) Pressed virgin oil (SM08) has more FFAs which was shown in Figure 1 bond D. FFAs in edible oils are undesirable, it may results in lower flavour quality and stability of the oil, moreover high levels of FFAs will result in rancidity of the oil and usually removed from crude oil by refining in industrial production [33].
3.4. Antioxidative Capacity of Oil Samples
The quality of edible oil is determined by various factors such as flavour, free fatty acids (FFAs) content, oxidation, etc. The free radical scavenging activity of the oil samples was done using DPPH. The TLC chromatogram of camellia oil and olive oil samples was colored with ethanol containing 0.03% DPPH solution. Figure 3 shows the results, which revealed sterols and unsaturated fatty acid esters in camellia oil have high antioxidant capacity [18].
The contents of antioxidative components in ten oil samples were determined using TLC scanning (TLCs). For the DPPH have maximum absorption at 517 nm. The plate colored with ethanol containing 0.03% DPPH, scanned at λs2 (scan wavelength) = 517 nm in negative signal mode by use of the densitometer.
The densitogram results for oil samples in Figure 4 provide a visible method of the antioxidative capacity between virgin oil and refined oil. It also proves that pressed virgin oils can significantly reduce the superoxide radicals (Figure 4), because it has more FFAs content. Camellia virgin oil with more antioxidative components than other refined oil. The vegetable oil sold in markets has lost parts of antioxidative components during the refining procedures in order to increase its stability and flavour. The camellia oil sold in markets has similar components and antioxidative capability comparing with imported olive oil, which has high price. Therefore, camellia oil has potentiality to be used as excellent healthy edible oil.
4. Conclusion
In this study, a simple and rapid method to simultaneous determination of unsaturated fatty glyceride, free fatty acids, sterols and other components of camellia oil was set up. This research breaks through the traditional derivatization analysis method and exhibits a powerful potential for the analysis of FFAs, phytosterols, etc. from edible oils and evaluates the antioxidative capacity of
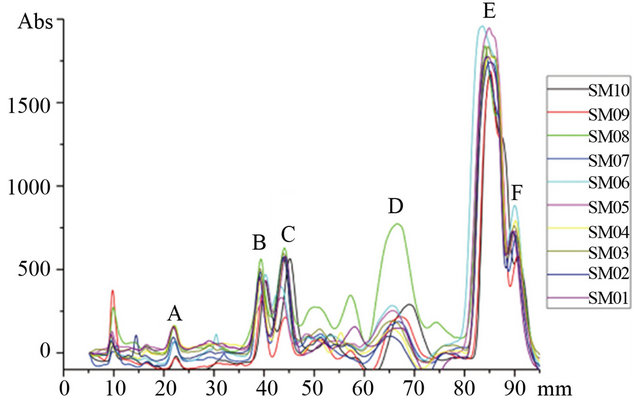
Figure 2. TLC densitograms (at 620 nm) of ten oil samples. SM01 to SM07 are refined camellia oil; SM08 is pressed camellia oil; SM09 is extra camellia oil; SM10 is refined olive oil.
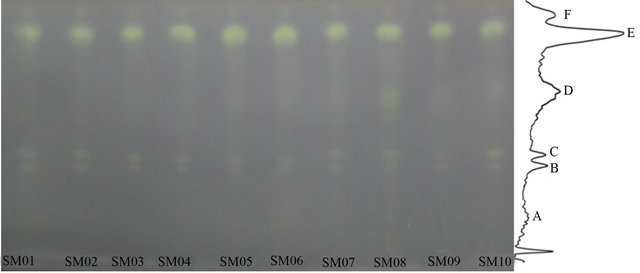
Figure 3. Chromatograms of oil samples’ antioxidative capability. Oil samples developed on silica G60 TLC plate (20 cm × 10 cm) and colored with ethanol containing 0.03% DPPH, viewed at white light. SM01 to SM07 are refined camellia oil; SM08 is pressed virgin camellia oil; SM09 is extra virgin camellia oil; SM10 is refined olive oil.

Figure 4. Typical TLC densitograms of ten oil samples 1), refined camellia oil 2), pressed virgin oil 3), extracted virgin camellia oil 4). SM01 to SM07 are refined camellia oil; SM08 is pressed camellia oil; SM09 is extracted camellia oil; SM10 is refined olive oil.
vegetable oil directly. The developed non-derivatization TLC method can be used as an economical alternative method for routine quality control of vegetable oil.
5. Acknowledgements
The research was partially supported by grants from the Science and Technology Development Fund of Macao (028/2007/A2) to S.P. Li, and the University of Macau (MYRG085) to J. Zhao.
REFERENCES
- J. Yuan, C. Wang, H. Chen, H. Zhou and J. Ye, “Prediction of Fatty Acid Composition in Camellia oleifera Oil by Near Infrared Transmittance Spectroscopy (NITS),” Food Chemistry, Vol. 138, No. 2-3, 2013, pp. 1657-1662. doi:10.1016/j.foodchem.2012.11.096
- X. Zhong, B. Zhang and J. Zhu, “The Comprehensive Utilization of Camellia oleifera Seed,” Science and Technology of Cereals, Oils and Foods, Vol. 15, No. 2, 2007, pp. 34-36.
- Y. Zeng, W. Hu and H. Xia, “Study on Refining Technology of Tea Seed Oil for Injection,” China Oils and Fats, Vol. 28, No. 3, 2003, pp. 28-31.
- M. Lai and L. Yang, “Research Progress of Pharmacological Action of Tea Oil and the Clinic Application,” Journal of External Therapy of Traditional Chinese Medicine, Vol. 16, No. 3, 2007, pp. 6-7.
- C. P. Commission, “Chinese Pharmacopoeia,” China Medical Science Press, Beijing, 2010.
- L. Ma and Y. Chen, “Analyzed Camellia Oil of Function Characteristics,” Chinese Agricultural Science Bulletin, Vol. 25, No. 8, 2009, pp. 82-84.
- X. Wu, Y. Huang and Z. Xie, “Health Functions and Prospective of Camellia Oil,” Food Science and Technology, No. 8, 2005, pp. 94-96.
- S. Liao, D. Ji and H. Tong, “Study on Fatty Acid Composition and Nutrition Health Protection Function of the Oiltea Camellia Seed Oil,” Journal of Cereals & Oils, No. 6, 2005, pp. 7-9.
- A. Wang, D. Yuan, K. Sun, Y. Pan and J. Guan, “Effects of Camellia Oil on Permeation of Non-Steroidal Anti-Inflammatory Drugs,” Journal of Shenyang Pharmaceutical University, Vol. 23, No. 10, 2006, pp. 621-624.
- F. Mao, H. Wang, F. Liu, Y. Lin and H. Ni, “Extraction and Free Radical Scavenging Effects of Camellia oleifera Seed Oil,” Journal of Northwest Forestry University, Vol. 24, No. 5, 2009, pp. 125-128.
- C. Xiong, P. Chen, G. Liu, X. Nie, Z. Huang, L. He and X. Wen, “Tea Seed Oil Refining and Its Application in Cosmetics,” Applied Chemical Industry, Vol. 40, No. 2, 2011, pp. 235-238.
- Y. Huang, X. Zheng, H. Huang, F. You and H. Zhong, “Analysis of Spectral Properties of Cosmetic Camellia Oil,” Fujian Analysis & Testing, Vol. 17, No. 1, 2008, pp. 7-9.
- C. Chanya and P. Amorn, “Use of Tea (Camellia oleifera Abel.) Seeds in Human Health,” In: V. R. Preedy, R. R. Watson and V. B. Patel, Eds., Nuts and Seeds in Health and Disease Preventionl, Academic Press, London, Burlington, San Diego, 2011, pp. 1115-1122.
- X. Deng, G. Xie and S. Huang, “Preparation of Healthy Tea Oil and Its Function of Adjusting Blood Fat,” China Oils and Fats, Vol. 27, No. 5, 2002, pp. 96-98.
- X. Chen and R. Huang, “Therapeutic Effects of Tea Oil and Cod-Liver Oil on Alkali Corneal Burn,” Practical Pharmacy and Clinical Remedies, Vol. 10, No. 4, 2007, pp. 214-215.
- Y. Bai, D. Song, F. Zhang, X. Xiao and Q. Wang, “Comparison of Nutritional Value between Camellia oleifera and Olive Oils,” China Oils and Fats, Vol. 33, No. 3, 2008, pp. 39-41.
- C. P. Lee, P. H. Shih, C.-L. Hsu and G.-C. Yen, “Hepatoprotection of Tea Seed Oil (Camellia oleifera Abel.) against CCl4-Induced Oxidative Damage in Rats,” Food and Chemical Toxicology, Vol. 45, No. 6, 2007, pp. 888- 895. doi:10.1016/j.fct.2006.11.007
- C. P. Lee and G. C. Yen, “Antioxidant Activity and Bioactive Compounds of Tea Seed (Camellia oleifera Abel.) Oil,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 13, 2006, pp. 779-784. doi:10.1021/jf052325a
- E. Niki, “Free Radicals in the 1900’s: from in Vitro to in Vivo,” Free Radical Research, Vol. 33, No. 6, 2000, pp. 693-704. doi:10.1080/10715760000301221
- Van’t Veer Pieter, M. C. Jansen, M. Klerk and F. J. Kok, “Fruits and Vegetables in the Prevention of Cancer and Cardiovascular Disease,” Public Health Nutrition, Vol. 3, No. 1, 2000, pp. 103-110.
- G. Cao, E. Sofic and R. L. Prior, “Antioxidant Capacity of Tea and Common Vegetables,” Journal of Agricultural and Food Chemistry, Vol. 44, No. 11, 1996, pp. 3426- 3431.
- S. L. Estévez and R. Helleur, “Fatty Acid Profiling of Lipid Classes by Silica Rod TLC-Thermally Assisted Hydrolysis and Methylation-GC/MS,” Journal of Analytical and Applied Pyrolysis, Vol. 74, No. 1-2, 2005, pp. 3-10. doi:10.1016/j.jaap.2004.11.017
- W. Vetter, M. Schröder and K. Lehnert, “Differentiation of Refined and Virgin Edible Oils by Means of the Transand cis-Phytol Isomer Distribution,” Journal of Agricultural and Food Chemistry, Vol. 60, No. 24, 2012, pp. 6103-6107. doi:10.1021/jf301373k
- D. Gomathi, G. Ravikumar, M. Kalaiselvi, B. Vidya and C. Uma, “HPTLC Fingerprinting Analysis of Evolvulus Alsinoides (L.) L,” Journal of Acute Medicine, Vol. 2, No. 3, 2012, pp. 77-82. doi:10.1016/j.jacme.2012.08.004
- J. Giacometti, “Determination of Aliphatic Alcohols, Squalene, α-Tocopherol and Sterols in Olive Oils: Direct Method Involving Gas Chromatography of the Unsaponifiable Fraction Following Silylation,” Analyst, Vol. 126, No. 4, 2001, pp. 472-475. doi:10.1039/b007090o
- J. Ma, H. Ye, Y. Rui, G. Chen and N. Zhang, “Fatty Acid Composition of Camellia oleifera Oil,” Journal für Verbraucherschutz und Lebensmittelsicherheit, Vol. 6, No. 1, 2011, pp. 9-12. doi:10.1007/s00003-010-0581-3
- T. Řezanka and K. Sigler, “Odd-Numbered Very-LongChain Fatty Acids from the Microbial, Animal and Plant Kingdoms,” Progress in Lipid Research, Vol. 48, No. 3-4, 2009, pp. 206-238. doi:10.1016/j.plipres.2009.03.003
- C. Ruiz-Samblás, F. Marini, L. Cuadros-Rodríguez and A. González-Casado, “Quantification of Blending of Olive Oils and Edible Vegetable Oils by Triacylglycerol Fingerprint Gas Chromatography and Chemometric Tools,” Journal of Chromatography B, Vol. 910, 2012, pp. 71-77. doi:10.1016/j.jchromb.2012.01.026
- A. Zeb, “Triacylglycerols Composition, Oxidation and Oxidation Compounds in Camellia Oil Using Liquid Chromatography—Mass Spectrometry,” Chemistry and Physics of Lipids, Vol. 165, No. 5, 2012, pp. 608-614. doi:10.1016/j.chemphyslip.2012.03.004
- Z. Liu, “The Methodological Study in Construction of TLC Fingerprints of Chinese Medicine,” MA. Sc. Thesis, Sichuan University, Chengdu, 2005.
- F. Tang, X. Li, W. Wu and J. Liu, “Study on Methylesterification Conditions of Fatty Acid in Camellia Oil,” Cereals & Oils, No. 8, 2010, pp. 36-39.
- L. Zhang and N. Chen, “Study on Refining Technology of Camellia oil,” Anhui Agricultural Science Bulletin, Vol. 13, No. 15, 2007, pp. 125-127.
- X. Shi and Y. Tu, “A Simple Refining Method of Camellia Oil,” Journal of Tea, Vol. 33, No. 3, 2007, pp. 158-161.
NOTES
*Authors have contributed equally to this work.
#Corresponding author.

