American Journal of Plant Sciences
Vol.5 No.15(2014), Article
ID:48074,9
pages
DOI:10.4236/ajps.2014.515257
Thermo-Protective Role of 28-Homobrassinolide in Brassica juncea Plants
Sirhindi Geetika1, Kaur Harpreet1, Bhardwaj Renu2, Kaur Nirmal Spal1, Sharma Poonam1
1Department of Botany, Punjabi University, Patiala, India
2Department of Botanical and Environmental Sciences, GNDU, Amritsar, India
Email: geetikasir123@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 9 April 2014; revised 25 June 2014; accepted 16 July 2014
ABSTRACT
The present work was undertaken to study the effects of 28-homobrassinolide on growth, stress markers, antioxidative enzyme [superoxide dismutase (EC 1.15.1.1), guaiacol peroxidase (EC 1.11.1.7), catalase (EC 1.11.1.6)] activities and protein content in 10 days old seedlings of Brassica juncea L. treated with different degrees (4˚C, 44˚C) of temperature. 28-homobrassinolide at 10−9 M concentration lowered temperature stress. Different degrees of temperature treatment alone decreased the enzyme activities and protein concentration of seedlings. However, pre-sowing treatments of 28-homobrassinolide lowered the temperature stress and enhanced the contents of MDA and proline, activities of enzymes and protein concentration of seedlings.
Keywords:Antioxidative Enzymes, 28-Homobrassinolide, Brassinosteroids, Temperature Stress, Lipid Peroxidation, Guaiacol Peroxidase, Catalase

1. Introduction
Temperature is one of the most important environmental stresses that adversely affect plant growth and development thereby limiting plant productivity. It causes heat injury and chilling stress, which result in degradation and collapsing of proteins, lipids and bring changes in metabolism. These changes cause breakdown of cell products and membranes etc. Temperature stress accelerates generation of reactive oxygen species (ROS) that have the capacity to initiate lipid peroxidation and degrade proteins, lipids and nucleic acids [1] . Plants respond to temperature stress by changes in the levels of antioxidants and antioxidative enzymes [2] [3] . Several hormones are implicated in modulating the plant responses to oxidative stress, including ethylene [4] , abscisic acid [5] , salicylic acid (SA) [6] and brassinosteroids (BRs) [7] .
Brassinosteroids are a class of plant polyhydroxy steroids that are ubiquitously distributed in the plant kingdom. These compounds, when applied to plants, improve their quality and yield. They have been further explored for stress-protective properties in plants against a number of stresses like chilling [8] , salt [9] , heat [10] and heavy metals [11] [12] . However, it is unclear whether BRs are involved in the modulation of plant responses to oxidative stresses. The influence of BRs on the response of the antioxidative enzymes of plants under stress conditions has been studied recently [7] [13] . The available data show that the changes induced in the activity of antioxidative enzymes by BRs differ with plant species and with stress conditions [9] [14] [15] .
Brassica juncea L. is an important oilseed crop known for its oil content, edible and medicinal uses. The seed is a warming stimulant with antibiotic effects. The chemical constituents of B. juncea include glucosinolates, ascorbate, foliate, myrosinase, and sterols (brassicasterol, sitosterol and brassinosteroids). The present study was undertaken to observe the growth, contents of stress markers and antioxidants activities under the influence of 28-homobrassinolide (28-homoBR).
2. Materials and Methods
2.1. Chemicals
28-homoBL was obtained from Sigma-Aldrich Chemicals Pvt Ltd., INDIA. Chemicals and reagents used for various biochemical analyses were purchased from Merck. The experiments were performed under controlled conditions in plant growth chamber.
2.2. Collection of Seeds and Experimental Setup
Seeds of Brassica juncea CV-201 (certified) were procured from Department of Plant Breeding, Punjab Agriculture University, Ludhiana, India. Healthy seeds were manually selected and treated with 5% hypochlorite (v/v) for 5 minutes and then washed for 30 minutes in free flowing tap water followed by 4 - 5 times washing with deionised water. Seed priming was done to surface sterilized seeds with different concentrations of 28- homoBL (1 μM, 1 nM, and 1 pM) and DW as control, for 6 hours. 28-homoBL treated and untreated seeds were sowed in petriplates for seven days and then 7-day-old seedlings were exposed to 44˚C and 4˚C for 5 h daily for three days. After 44˚C and 4˚C temperature shock treatment, seedlings were transferred to normal lab conditions of plant growth chamber, observing 24˚C temperatures, 16/8 hours dark and light period and light intensity fall uniformly on each petriplate at 200 PAR while humidity was set at 80%. Present study was conducted to elucidate the effect of 28-homoBL on antioxidant system to mitigate toxic effect of temperature stress during seedling growth.
2.3. Treatment of 28-HomoBL and Temperature
Our experiment consisted of 12 treatments with 3 replication of each treatment. The treatment included 28-homoBL (1 μM, 1 nM, 1 pM) and temperature (24˚C, 4˚C, 44˚C) and combination of 28-homoBL and Temp (1 μM + 4˚C, 1 nM + 4˚C and 1 pM + 4˚C, 1 μM + 44˚C, 1 nM + 44˚C and 1 pM + 44˚C). The growth parameters in terms of root and shoot length were examined after 10 days after sowing (DAS).
2.4. Estimation of MDA and Proline Content
MDA and proline content was determined using colorimetric method. The level of MDA was measured by Thiobarbituric acid reaction method [15] . Proline estimation was done following method of Bates et al. [16] . The plant material was homogenized with 3% sulpho-salicylic acid. The homogenate was filtered and glacial ace-tic acid and acid ninhydrin was added to the supernatant. After shaking for 1 minute, the reaction mixture was incubated at 100˚C for 1 h. Reaction was stopped by adding toluene and absorbance was taken at 520 nm using spectrophotometer.
2.5. Preparation of Enzyme Extract
One gram of shoot tissue of Brassica juncea was homogenized in 3 ml of pre-chilled phosphate buffer, (pH 7.2) in chilled pestle and mortar. The homogenates were centrifuged at 15,000 rpm for 15 minute at 4˚C and supernatant collected and used for enzyme activities of SOD and POD along with total proteins.
2.6. Superoxide Dismutase Activity (EC 1.15.1.1)
The assay of superoxide dismutase was carried out based on the reduction of nitro blue tetrazolium (NBT) [17] . To 0.5 ml of enzyme extract, 1.8 μl of 50 mM of Sodium Carbonate buffer (pH 10), 750 μl of 96 μM NBT and 150 μl Triton X-100 were added. The reaction was initiated by adding 0.4 ml of 1 mM hydroxylamine hydrochloride. Absorbance was taken at 540 nm using spectrophotometer mentioned elsewhere, and activity of SOD was taken as an increase in absorbance for 2 min at 25˚C. The control was simultaneously run without enzyme extract. Units of SOD were expressed as amount of enzyme required for inhibiting the reduction of NBT by 50%. The specific activity was expressed in terms of Units∙mg−1 of protein.
2.7. Activity of Guaiacol Peroxidase (EC 1.11.1.7)
Guaiacol peroxidase was assayed by mixing 50 μl of Guaiacol, 30 μl of H2O2 and 3 ml of potassium phosphate buffer and enzyme extract. Blank was prepared by adding all the reagents except enzyme extract [18] .
2.8. Activity of Catalase (EC 1.11.1.6)
CAT activity was measured according to Aebi [19] by taking 3 ml reaction mixture containing 1.5 ml of 100 mM phosphate buffer (pH 7.0), 0.05 ml of 75 mM H2O2 and 0.05 ml enzyme extract. The reaction was started by addition of H2O2 and CAT activity was measured as decrease in absorbance at 240 nm for 1 min.
2.9. Determination of Total Proteins
Total proteins were estimated by the method of Lowry et al. [20] . One ml of enzyme extract was kept in 1 ml of ice cold 20% TCA for 18 hours. Homogenate was centrifuged and pellet was dissolved in 0.1 N NaOH for protein estimation. The absorbance was measured at 750 nM.
3. Statistical Analysis
All analysis was done on a completely randomized de-sign. All data obtained was subjected to unpaired t-test. Each data was the mean of three replicates (n = 3) except for shoot and root length where n = 5 and comparisons of p-values < 0.05 were considered significant and different from control.
4. Results and Discussions
4.1. Effect of 28-homoBL and Temperature on Growth
28-homoBL treated seeds resulted in increased percent shoot length (Table 1) of Brassica juncea as compared to control. Maximum (39%) increase in shoot length was observed in seedlings treated with 10−9 M 28-homoBL concentrations. Seedlings grown in presence of 4˚C and 44˚C showed (13% and 27%) decrease respectively. Interestingly seeds grown in presence of 4˚C temperature after treatment with 10−9 M concentrations of 28-homoBL showed (16%) increase in shoot length rate as compared to control distilled water seeds.
Seedling growth in terms of root length showed synergistic mechanism of negative effect on growth particularly on root length. Root length affected negatively in all concentrations of 28-homoBL except 10−9 M where (1%) increase was found. Overall 28-homoBL showed stimulatory effect on shoot length and inhibitory effect on root length in presence or absence of temperature.
In case of fresh and dry weights, maximum decrease was found in seedlings treated with 44˚C temperature (14%) and (18%) respectively but when supplementation of different concentrations of 28-homoBL is given maximum increase (25%) fresh weight and (14%) dry weight was found in seedlings treated with 4˚C temperature stress.
4.2. Estimation of MDA and Proline Content
There are number of factors, plants showed at morphological and biochemical level which can be taken as stress
Table 1 . Influence of pretreatment of 28-homoBL on shoot, root length and fresh, dry weights under temperature stress.
Values are the mean of three replicates measurements. *Indicates significant difference compared to the control at p < 0.05.
indicators under inadequate environmental conditions. Lipid peroxidation (MDA) increases in presence of temperature (Figure 1(A)) stress indicated that extreme temperatures (4˚C, 44˚C) were lethal and responsible for disintegration of plasma membrane and thus caused decrease in growth of cell. In present study 20% and 23% of lipid peroxidation increase was observed in presence of 4˚C and 44˚C temperature. 28-homoBL decreases lipid peroxidation that was harmful to the cells [21] . 1 nM 28-homoBL treatment resulted in about 7% decrease in MDA content but in other 28-homoBL (1 μM and 1 pM) treatments 6% to 5% deccrease in MDA content was noticed. Enhancement of MDA content subjected to 1˚C and −10˚C temperatures was noticed in Avena nuda, a cold tolerant plant species [22] . It was noticed that instead of low temperature, high temperature also damages cellular membranes due to lipid peroxidation. The results showed that MDA level was significantly increased with rising temperature. There are reports that stresses disrupts membrane permeability is by peroxidation of the lipid membrane [23] . Membrane injury under High temperature is related to increased production of highly toxic reactive oxygen species [24] . Lipid peroxidation measured as the amount of produced MDA when polyunsaturated fatty acids in the membrane undergo oxidation by the accumulation of free oxygen radicals. Lipid peroxidation is ascribed to oxidative damage and is often used as an indicator of increased damage [25] -[27] . 28-homoBL application protects membranes from damage by various stress factors by reducing MDA content [28] . From present study it was observed that this protection of cell membrane by 28-homoBL is very much dose-dependent along with absence or presence of any stress factor such as temperature in present case.
Proline is an amino acid which starts accumulating in higher amount in plants under inadequate environmental conditions which can be taken as stress marker. Presence of extreme temperatures such as 4˚C and 44˚C (Figure 1(B)) in seedlings growth environment enhanced proline content up to 34% and 13% as compared to control (untreated distilled water). On the other hand the promoting effect of temperature on proline content stimulation was higher in presence of 28-homoBL which increased with increase in 28-homoBL concentration. These results showed that in contrast to 28-homoBL, the temperature stimulatory effect on proline content was sustainable and that for the neutralization of its effect higher concentration of 28-homoBL was needed. 28-homoBL application can promote the biosynthesis of proline under heat stresses [29] . 1 nM 28-homoBL was most effective in proline accumulation in which about 126% of more proline accumulated as compared to untreated control distilled water seedlings. These results are in accordance with Rizhsky et al. [30] and Amirjani [31] .
4.3. Superoxide Dismutase (SOD) Peroxidase (POD) and Catalase Activities
The presence of stress in the cell leads to the formation of ROS, which causes further severe oxidative damage
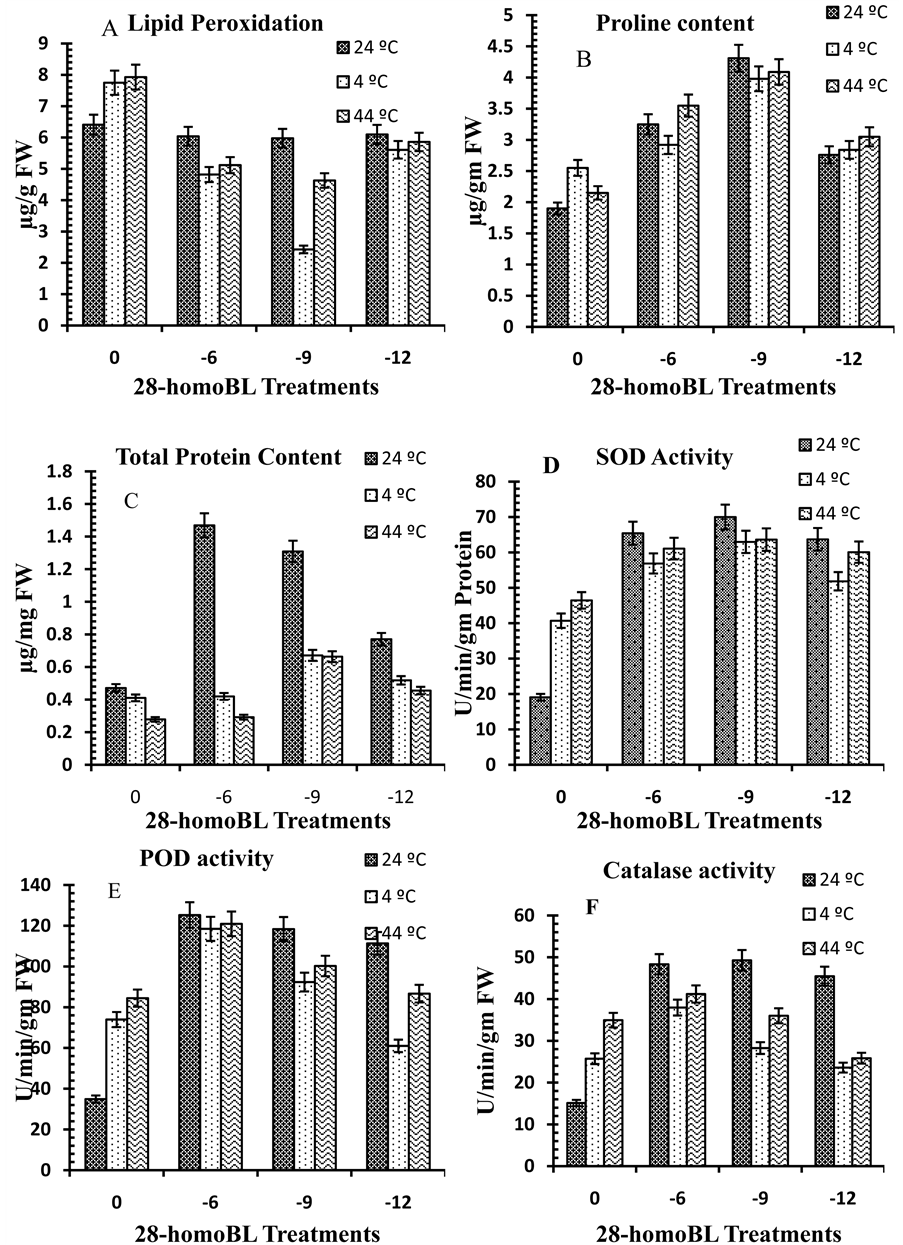
Figure 1. Levels of biochemical and antioxidant enzymes of Brassica juncea L. in presence and absence of 28-homoBL treatments and stress (A) Lipid peroxidaion (B) Proline content (C) Total Proteins (D) SOD activity (E) POD activity (F) CAT activity (values represent average of triplicates and expressed as mean ± SD).
to different cell organelles and biomolecules. To scavenge ROS, plants possess a well-organized antioxidative defense system comprising enzymatic and nonenzymatic antioxidants. The cooperative function of these antioxidants plays an important role in scavenging ROS and maintaining the physiological redox status of organisms [32] . The expressions of the antioxidant enzyme-related genes, i.e., SOD, POD and CAT were up-regulated by extreme temperature treatments in comparison with the control. Up-regulation in the expression of genes, and hence the increased activities of the antioxidant enzymes suggest that temperature adaptation improved the antioxidant capacity, which may effectively lessen ROS injury during extreme temperature stresses.
In this study, a significant increase of SOD activity in seedlings was observed at extreme temperature (4˚C and 44˚C) and this increase was further enhanced in 28-homoBL treated seedlings (Figure 1(C)) of Brassica juncea L. and its involvement in plant tolerance to oxidative stress caused by abiotic stress. This may be attributed to the increased production of superoxide, resulting in the activation of existing enzyme pools or increased expression of genes encoding SOD [33] . The present study first time revealed that exogenous 28-homoBL application increases SOD activity to many folds which help the plant in upgrading its antioxidant capacity to scavenge more free radicles. Under temperature alone SOD activity increased to 2 folds as compared to control distilled water seedlings but his increase in SOD was 2 - 4 times more in seedlings but this increase in SOD was 2 - 4 times more in seedling shaving 28-homoBL treatment before exposing to extreme temperatures. Increased SOD activity caused by extreme temperatures has been previously observed in several plant species, and is routinely considered to be an adjustment response to stress [34] .
Extreme temperatures decreased the CAT activity in Brassica seedlings and addition of BRs increased its activity (Figure 1(D)). CAT is an important oxidizing enzyme that helps in the removal of H2O2 and helps in detoxifying harmful metabolic products; its activity appears to be positively correlated with an increase in growth. A decrease in CAT activity, extreme temperatures can be attributed to inhibition of the CAT synthesis and other oxidase proteins [35] . A similar increase in CAT activity of sorghum seedlings under water stress caused by the application of brassinosteroids was reported previously [36] . In contrast to CAT activity, temperature stress increased the POD activity in Brassica seedlings (Figure 1(E)). An increase in POD activity is a common response to oxidative and abiotic stresses. In plants, POD protects cells against harmful concentrations of hydroperoxides and helps in a variety of cellular functions [37] . Increased total peroxidase activities in response to salinity were reported by [38] . A similar increase in POD was also observed after the application of Ni to the leaves of Silene italic [39] . The high POD activity in Brassica seedlings treated with extreme temperatures observed in the present study may indicate an initiation of disruption in the biochemical processes. However, we observed that 28-homobrassinolides applied to temperature stressed Brassica seedlings reduced the POD activity. Similarly, a reduction in POD activity in putrescine-alleviated salt stress in spinach leaves was reported [40] .
4.4. Protein Content
The present finding revealed that exogenous application of 28-homoBL provokes accumulation of protein content (Figure 1(F)) as high as 177% more in 1 nM 28-homoBL treated seedlings as compared to untreated control seedlings. 28-homoBL seed pre-sowing treatments enhanced protein content in 10 days old B. juncea seedlings under temperature stress conditions as compared to control. Earlier reports indicated that 24-epibrassinolide-treated seedlings of B. napus showed maximum resistance to lethal heat treatments compared to control seedlings and this was found to correlate with higher levels of heat-shock proteins and corresponding mRNA during heat stress [8] [9] [41] . In this study 28-homoBL application provokes alterations in protein content in very dose-dependent and specificity manner in polypeptide profiles.
5. Conclusion
The present study shows that, although temperature is an essential factor for normal plant growth and physiological processes, extreme on both sides of mercury is toxic and may result in growth inhibition and altered metabolic processes. The observations of the present study clearly indicate temperature stress-protective properties of 28-homoBL in Brassica juncea plants. Stress ameliorative properties of BRs are clearly demonstrated by better growth, accumulation of proline content and antioxidative enzymes in seedlings to which different degrees of temperatures and concentration of 28-homobrassinolides are applied. It points to the possibility of 28-homoBL regulated stress-protection in plants but extensive studies are still needed on various aspects related to stress.
Acknowledgements
Authors are highly acknowledged to the Head, Department of Botany, Punjabi University, Patiala for providing experimental facilities and UGC-New Delhi for financial assistance in major research project.
References
- Halliwell, B. and Gutteridge, J.M.C. (1999) Free Radicals in Biology of Medicine. Oxford University Press, London.
- Noctor, G. and Foyer, C.H. (1998) Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annual Review Plant Physiology and Plant Molecular Biology, 49, 249-279. http://dx.doi.org/10.1146/annurev.arplant.49.1.249
- Pandey, V., Dixit, V. and Shyam, R. (2005) Antioxidative Responses in Relation to Growth of Mustard (Brassica juncea cv. Pusa Jaikisan) Plants Exposed to Hexavalent Chromium. Chemosphere, 61, 40-47. http://dx.doi.org/10.1016/j.chemosphere.2005.03.026
- Vahala, J., Ruonala, R., Keinänen, M., Tuominen, H. and Kangasjärvi, J. (2003) Ethylene Insensitivity Modulates Ozone-Induced Cell Death in Birch. Plant Physiology, 132, 185-195. http://dx.doi.org/10.1104/pp.102.018887
- Kovtun, Y., Chiu, W.L. and Tena, S.J. (2000) Functional Analysis of Oxidative Stress—Mitogen Activated Protein Kinase Cascade in Plants. Proceeding of National Academy of Sciences, USA. 97, 294-295. http://dx.doi.org/10.1073/pnas.97.6.2940
- Metwally, A., Finkemeier, I., Georgi, M. and Dietz, K.J. (2003) Salicylic Acid Alleviates the Cadmium Toxicity in Barley Seedlings. Plant Physiology, 132, 272-281. http://dx.doi.org/10.1104/pp.102.018457
- Cao, S.Q., Xu, Q.T., Cao, Y.J., Qian, K., An, K., Zhu, Y., Hu, B.Z., Zhao, H.F. and Kuai, B.K. (2005) Loss of Function Mutation in DET2 Gene Lead to an Enhanced Resistance to Oxidative Stress in Arabidopsis. Plant Physiology, 123, 57-66. http://dx.doi.org/10.1111/j.1399-3054.2004.00432.x
- Dhaubhadel, S., Chaudhary, S., Dobinson, K.F. and Krishna, P. (1999) Treatment with 24-Epibrassinolide (Brassinosteroid) Increases the Basic Thermotolerance of Brassica napus Tomato Seedlings. Plant Molecular Biology, 40, 333-342. http://dx.doi.org/10.1023/A:1006283015582
- Ozdemir, F., Bor, M., Demiral, T. and Turkan, I. (2004) Effects of 24-Epibrassinolide on Seed Germination, Seedling Growth, Lipid Peroxidation, Proline Content and Antioxidative System of Rice (Oryza sativa L.) under Salinity Stress. Plant Growth Regulation, 42, 203-211. http://dx.doi.org/10.1023/B:GROW.0000026509.25995.13
- Dhaubhadel, S., Browning, K.S., Gallie, D.R. and Krishna, P. (2002) Brassinosteroid Functions to Protect the Translational Machinery and Heat Shock Protein Synthesis Following Thermal Stress. Plant Journal, 29, 681-691. http://dx.doi.org/10.1046/j.1365-313X.2002.01257.x
- Bajguz, A. (2000) Blockage of Heavy Metal Accumulation in Chlorella vulgaris Cells by 24-Epibrassinolide. Plant Physiology Biochemistry, 38, 797-801. http://dx.doi.org/10.1016/S0981-9428(00)01185-2
- Janeczko, A., Koscielniak, J., Pilipowicz, M., Lukaszewsa, G.S. and Skoczowspi, A. (2005) Protection of Winter Rape Photosystem 2 by 24-Epibrassinolide under Cadmium Stress. Photosynthetica, 4, 293-298. http://dx.doi.org/10.1007/s11099-005-0048-4
- Hayat, S., Ali, B., Hasan, S. and Ahmad, A. (2007) Effect of 28-Homobrassinolide on Salinity Induced Changes in Brassica juncea. Turkish Journal of Biology, 31, 141-146.
- Almeida, J.M., Fidalgo, F., Confraria, A., Santos, A., Pires, H. and Santos, I. (2005) Effect of Hydrogen Peroxide on Catalase Gene Experation, Isoform Activities and Levels in Leaves of Potato Sprayed with Homobrassinolide and Ultrastructure Changes in Mesophyll Cells. Functional Plant Biology, 32, 707-720. http://dx.doi.org/10.1071/FP04235
- Hayat, S., Ali, B., Hassan, S.A. and Ahmad, A. (2007) Brassinosteroids Enhanced Antioxidants under Cadmium Stress in Brassica juncea. Environmental and Experimental Botany, 60, 33-41. http://dx.doi.org/10.1016/j.envexpbot.2006.06.002
- Heath, R.L. and Packer, L. (1968) Photoperoxidation in Isolated Chloroplast. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Archives of Biochemistry and Biophysics, 125, 189-198. http://dx.doi.org/10.1016/0003-9861(68)90654-1
- Lowry, H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Estimation with Folin-Phenol Reagent. Journal of Biological Chemistry, 193, 265.
- Kono, Y. (1978) Generation of Superoxide Radical during Autoxidation of Hydroxylamine and an Assay for Superoxide Dismutase. Archives of Biochemistry and Biophysics, 186, 189-195. http://dx.doi.org/10.1016/0003-9861(78)90479-4
- Putter, J. (1974) Peroxidase. In: Bergmeyer, H.U., Ed., Methods of Enzymatic Analysis. Verlag Chemie, Weinhan, 685-690.
- Bates, L.S., Waldren, R.P. and Teare, I.D. (1973) Rapid Determination of Free Proline for Water Stress Studies. Plant and Soil, 39, 205-208. http://dx.doi.org/10.1007/BF00018060
- Arora, N., Bhardwaj, R., Sharma, P. and Arora, H.K. (2008) Effects of 28-Homobrassinolide on Growth, Lipid Peroxidation and Antioxidative Enzyme Activities in Seedlings of Zea mays L. under Salinity Stress. Acta Physiology Plant, 30, 833-839. http://dx.doi.org/10.1007/s11738-008-0188-9
- Liu, W., Yu, K., He, T., Li, F., Zhang, D. and Liu, J. (2013) The Low Temperature Induced Physiological Responses of Avena nuda L., a Cold-Tolerant Plant Species. The Scientific World Journal, 2013, Article ID: 658793. http://dx.doi.org/10.1155/2013/658793
- Mandhania, S., Madan, S. and Sawhney, V. (2006) Antioxidant Defense Mechanism under Salt Stress in Wheat Seedlings. Plant Biology, 227, 227-231. http://dx.doi.org/10.1007/s10535-006-0011-7
- Hernández, J.A., Jiménez, A., Mullineaux, P. and Sevilia, F. (2000) Tolerance of pea (Pisum sativum L.) to Long Term Salt Stress is Associated with Induction of Antioxidant Defences. Plant, Cell and Environment, 23, 853-862. http://dx.doi.org/10.1046/j.1365-3040.2000.00602.x
- Jimenez, A., Creissen, G., Kular, B., Firmin, J., Robinson, S., Verhoeyen, M. and Mullineaux, P. (2002) Changes in Oxidative Processes and Components of the Antioxidant System during Tomato Fruit Ripening. Planta, 214, 751-758. http://dx.doi.org/10.1007/s004250100667
- Bor, M., Ozdemir, F. and Turkan, I. (2003) The Effect of Salt Stress on Lipid Peroxidation and Antioxidants in Leaves of Sugar Beet Beta vulgaris L. and Wild Beet Beta maritima L. Plant Science, 164, 77-84. http://dx.doi.org/10.1016/S0168-9452(02)00338-2
- Meloni, D.A., Oliva, M.A., Martinez, C.A. and Cambraia, J. (2003) Photosynthesis and Activity of Superoxide Dismutase, Peroxidase and Glutathione Reductase in Cotton under Salt Stress. Environmental Experimental Botany, 49, 69-76. http://dx.doi.org/10.1016/S0098-8472(02)00058-8
- Arora, N., Bhardwaj, R., Sharma, P. and Arora, H.K. (2008) Effects of 28-Homobrassinolide on Growth, Lipid Peroxidation and Antioxidative Enzyme Activities in Seedlings of Zea mays L. under Salinity Stress. Physiologiae Plantarum, 30, 833-839.http://dx.doi.org/10.1007/s11738-008-0188-9
- Kumar, S., Gupta, D. and Nayyar, H. (2012) Comparative Response of Maize and Rice Genotypes to Heat Stress: Status of Oxidative Stress and Antioxidants. Acta Physiologiae Plantarum, 34, 75-86. http://dx.doi.org/10.1007/s11738-011-0806-9
- Rizhsky, L., Liang, H., Shuman, J., Shulaev, V., Davletova, S. and Mittler, R. (2004) When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiology, 134, 1683-1696. http://dx.doi.org/10.1104/pp.103.033431
- Amirjani, M. (2012) Estimation of Wheat Responses to “High” Heat Stress. American-Eurasian Journal of Sustainable Agriculture, 6, 222-233.
- Cho, U.H. and Seo, N.H. (2005) Oxidative Stress in Arabidopsis thaliana Exposed to Cadmium Is Due to Hydrogen Peroxide Accumulation. Plant Science, 168, 113-120. http://dx.doi.org/10.1016/j.plantsci.2004.07.021
- Mishra, S., Srivastava, S., Tripathi, R.D., Govindarajan, R., Kuriakose, S.V. and Prasad, M.N.V. (2006) Phytochelatin Synthesis and Response of Antioxidants during Cadmium Stress in Bacopa monnieri L. Plant Physiology and Biochemistry, 44, 25-37. http://dx.doi.org/10.1016/j.plaphy.2006.01.007
- Bavita, A., Shashi, B. and Navtej, S.B. (2012) Nitric Oxide Alleviates Oxidative Damage Induced by High Temperature Stress in Wheat. Indian Journal of Experimental Biology, 50, 372-378.
- Das, P.K., Kar, M. and Mishra, D. (1978) Nickel Nutrition of Plants: I. Effect of Nickel on Some Oxidase Activities during Rice (Oryza sativa L.) on Seed Germination. Zeitschrift für Pflanzenphysiologie, 90, 225-233. http://dx.doi.org/10.1016/S0044-328X(78)80235-9
- Vardhini, B.V. and Rao, S.S.R. (2003) Amelioration of Osmotic Stress by Brassinosteroids on Seed Germination and Seedling Growth of Three Varieties of Sorghum. Plant Growth Regulation, 41, 25-31. http://dx.doi.org/10.1023/A:1027303518467
- Beauchamp, C. and Fridovich, I. (1971) Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Analytical Biochemistry, 44, 276-287. http://dx.doi.org/10.1016/0003-2697(71)90370-8
- Sancho, M.A., Milrod de Forchetti, S., Pliego, F., Valpuesta, V. and Queseda, M.A. (1996) Peroxidase Activity and Isoenzymes in the Culture Medium of NaCl Adapted Tomato Suspension Cells. Plant Cell Tissue and Organ Culture, 44, 161-167. http://dx.doi.org/10.1007/BF00048195
- Gabbrielli, R., Pandolfini, T. and Vergnano, O. (1987) Peroxidase Involvement in Tolerance Mechanisms. Giorn Botany Italy, 21, 200-201.
- Ozturk, L. and Demir, Y. (2003) Effects of Putrescine and Ethephon on Some Oxidative Stress Enzyme Activities and Proline Content in Salt Stressed Spinach Leaves. Plant Growth Regulation, 40, 89-95. http://dx.doi.org/10.1023/A:1023078819935
- Kagale, S., Divi, U.K., Kronchko, J.E., Keller, W.A. and Krishna, P. (2007) Brassinosteroid Conifers Tolerance in Arabidopsis thaliana and Brassica napus to a Range of Abiotic Stresses. Planta, 225, 353-364. http://dx.doi.org/10.1007/s00425-006-0361-6


