American Journal of Plant Sciences
Vol.5 No.1(2014), Article ID:42301,8 pages DOI:10.4236/ajps.2014.51025
Influence of Additives on Enhanced in Vitro Shoot Multiplication of Stevia rebaudiana (Bert.)—An Important Anti Diabetic Medicinal Plant
Department of Biotechnology, Indian Institute of Horticultural Sciences, Bangaluru, India.
Email: *aswathiihr@gmail.com
Copyright © 2014 Thulasi Muneppa Sridhar, Chenna Reddy Aswath. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Thulasi Muneppa Sridhar, Chenna Reddy Aswath. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received October 10th, 2013; revised December 27th, 2013; accepted January 13th, 2014
KEYWORDS
Stevia rebaudiana; Nodal Explants; MS Medium; Growth Hormones; Additives; Shoot Proliferation
ABSTRACT
The present study was designed to develop an efficient protocol for micro propagation of S. rebaudiana from nodal explants and study the influence of additives on enhancement of shoot proliferation. A two-step protocol has been standardized in which, first step comprising growth hormones concentration is optimized and it was found that MS medium supplemented with 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA turned out to be the best treatment for shoot induction. In the second step, the best treatment for shoot induction was fortified with different growth additives for further shoot proliferation. Among the different types of additives used, casein hydrolysate at 0.05% (w/v) was found to be most effective, resulted with maximum of 15.0 shoots. 90% regeneration frequency and shoot length of 6.0 cm were recorded per explant. Thus, the procedure described is a quick and reliable method which could be applied for efficient large scale propagation, genetic transformation assays and secondary metabolite production of Stevia.
1. Introduction
Stevia rebaudiana (Bert.) is a herb from the asteracae family indigenous to higher elevations of northern Paraguay near Brazilian boarders [1], now being cultivated in many tropical and sub tropical countries. The leaves of Stevia rebaudiana mainly synthesize non-caloric thermo stable intense sweeteners, mainly steviosides that find applications in food and pharmaceutical industries. Purified steviosides imparts about 300 times sweetener than sucrose (0.4% solution) apart from being a calorie free biosweetener [2]. Another glycoside namely rebaudioside “A” which is about 400 times sweeter than sucrose is also present in Stevia leaves. Stevia rebaudiana leaves accumulate mixture of diterpene glycosides derived from the tetra cyclic diterpene steviol. These natural products taste intensely sweet and have similar biosynthetic origins to those of gibberellic acid [3].
About 30 million Indians are presently suffering from diabetes and it is estimated that by 2025 India’s contribution to the diabetic global population would be a whooping 80 million [4]. Therefore, the wave of “sugar free” has become a welcome trend. Stevia shows colorie free high potency sweetener does not contain calcium cyclamate, saccharin, and aspartame and causes no side effects [5]. The sweet compounds pass through the digestive process without chemically breaking down making Stevia safe for diabetes and obese people [6]. Stevia leaf tea offers excellent relief for an upset stomach. Like cucumber, a wet stevia leaf bag provides a cooling effect to eyes and helps to reduce weight and blood sugar management [7]. The Stevia powder also helps in rejuvenating the pancreatic gland [8]. Stevioside and rebaudioside induce insulin secretion [9] and the former acts as antitumour agent [10].
Propagation by seeds does not allow the production of homogeneous populations, resulting in great variability in important features like sweetening levels and composition, further vegetative propagation is not very successful because of low yield of cuttings from mother plants [11]. Hence, in vitro propagation is the sound technique for mass propagation of this plant because the species is becoming rare in natural habitat condition due to habit destruction and over exploitation.
Minor quantities of complex organic nutrients like amino acids, peptides, fatty acids, carbohydrates, vitamins on high proliferation and regeneration of plants in vitro, are well documented in many medicinal crops [12- 14].
Optimization of regeneration protocol supporting the action of growth additives as a supplement of growth regulators will be useful in the establishment of reliable regeneration protocols for various medicinal herbs of economic importance; however, no such attempts have been made in Stevia.
In this study, an attempt has been made to understand the role of different growth additives at various concentrations on enhancing the efficiency of shoot multiplication rate in Stevia rebaudiana.
2. Materials and Methods
2.1. Explant Preparation
Stevia rebaudiana plants were maintained in shade nets at Indian Institute of Horticultural Research, Bangalore, India. Surface sterilization of nodal explants was performed by washing the explants under running tap water. It was then washed with 1% (v/v) labolene solution and rinsed thoroughly with sterile water. The explants were disinfected by soaking in 0.2% (w/v) bavistin (Carbendazim 50% WP fungicide) for 5 min and washed five times with sterile distilled water. Surface decontamination was performed under aseptic conditions by immersing the explants in 0.1% (w/v) mercuric chloride for 2 min followed by rinsing 4 - 5 times with sterile distilled water to remove all the traces of mercuric chloride. Nodal explants were further trimmed at the cut ends and they were used as explants for in vitro regeneration.
2.2. Medium and Culture Conditions
Murashige and Skoog (MS) medium [15] used in this study was prepared by adding 3% (w/v) sucrose to MS basal salts and vitamins. Different concentrations of plant growth regulators (PGRs) were supplemented to the medium and the pH was adjusted to 5.7 before adding 0.7% (w/v) agar. MS medium devoid of growth regulators served as control. Agar fortified medium (10 mL) was dispensed into culture tubes and plugged with non absorbent cotton plugs. Culture bottles containing 50 mL medium were tightly closed with polypropylene caps. The tubes and bottles containing the media were autoclaved at 121˚C for 15 min. The cultures were incubated under 16 hrs photoperiod, irradiance of 40 μmol m−2·s−1 and temperature of at 24˚ ± 2˚.
Murashige and Skoog (MS medium composition)
• Major salts (macronutrients)
• Ammonium nitrate (NH4NO3) 1650 mg/l
• Calcium chloride (CaCl2·2H2O) 440 mg/l
• Magnesium sulphate (MgSO4·7H2O) 370 mg/l
• Potassium phosphate (KH2PO4) 170 mg/l
• Potassium nitrate (KNO3) 1900 mg/l
• Minor salts (micronutrients)
• Boric acid (H3BO3) 6.20 mg/l
• Cobalt chloride (CoCl2·6H2O) 0.025 mg/l
• Cupric sulphate (CuSO4·5H2O) 0.025 mg/l
• Ferrous sulphate (FeSO4·7H2O) 27.80 mg/l
• Manganese sulphate (MnSO4·4H2O) 22.30 mg/l
• Potassium iodide (KI) 0.83 mg/l
• Sodium molybdate (Na2MoO4·2H2O) 0.25 mg/l
• Zinc sulphate (ZnSO4·7H2O) 8.60 mg/l
• Na2EDTA·2H2O 37.20 mg/l
• Vitamins and organics
• Myo-Inositol 100 mg/l
• Niacin 0.5 mg/l
• Pyridoxine·HCl 0.5 mg/l
• Glycine 2.0 mg/l
2.3. Bud Break and Shoot Multiplication
For axillary shoot initiation and multiplication, nodal explants were inoculated on MS medium supplemented with different hormones such as 6-benzyl amino purine (BAP) (0.5 - 3.0 mg/l) and Kinetin (Kin) (0.1 - 1.0 mg/l) and in combination with auxins such as NAA and IAA (0.1 mg/l). Combination of both the cytokinins (BAP - 1.0 - 2.0 mg/land KIN - 0.50 mg/l) was also used for this study. Total number of shoots per explant and length of the shoots were recorded after 4 weeks of culture.
2.4. Fortification of Growth Additives to Culture Medium
To study the influence of various additives on shoot proliferation, explants were cultured on MS medium supplemented with 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA along with different growth additives such as casein hydrolysate (CH), malt extract (ME), yeast extract (YE) (0.025%, 0.050%, 0.075% & 0.1%, w/v) and coconut water (CW) (5%, 10%, 15%, 20%, v/v). Sub culturing was done two times in the same medium after a 4 weeks interval. The number and length of the shoots per explant were recorded after 4 weeks of culture.
2.5. Culture Conditions and Statistics
The cultures were incubated under 16 hrs photoperiod, irradiance of 40 μmol m−2·s−1 and temperature of 24˚C ± 2˚C with a relative humidity of 55% - 60%. Explants were sub cultured every 4 week, data were recorded after four weeks of multiple shoot induction, each treatment had five replicates of 10 explants each. All data are statistically analyzed by analysis of variance (ANOVA).
3. Results and Discussion
3.1. Influence of Plant Growth Regulators on Multiple Shoot Induction
Axillary shoot system is the best suited in vitro culture system for conservation purposes, since it eliminates the risk of somaclonal variation [16]. Bud break from nodal explant were observed in vitro one week after inoculation on MS basal medium supplemented with various plant growth regulators.
3.2. Influence of Cytokinins
Morphogenetic responses of nodal explants to various cytokinins alone (BAP and Kin) and in combination with auxins (NAA and IAA) are summarized and presented in Table 1. Nodal explants that were cultured on MS medium devoid of PGR’s (control) did not showed any regeneration response. However, the multiplication rate and shoot number were remarkably increased in cultures supplemented with PGR’s. The percentage response (represented in Graph 1), average number of shoots per explant as well as the mean length of shoots varied with the type of growth regulator used as well as its concentration and the results have been presented (Table 1).
Among the two cytokinins tested, BAP was found to be more efficient than Kin with respect to shoot initiation and subsequent multiplication. Medium supplemented with BAP alone (1.0 - 3.0 mg/L) recorded 2.0 - 2.80 number of shoots with an average shoot length of 1.2 - 2.4 cm. Among various concentrations of BAP, 2.0 mg/L BAP was proved to be most effective with 60% regeneration and a maximum of 2.80 number of shoots with 2.40 cm shoot length per culture. Similar findings were reported earlier in nodal explants of Orthosiphon stamineus cultured on MS medium containing BAP [17]. In Kinetin alone (0.1 - 1.0 mg/l) supplemented medium, an average of 1.2 - 2.0 shoots with 2.6 - 5.6 cm shoot length was obtained. The superiority of BAP over Kinetin on induction of multiple shoots has been reported in several
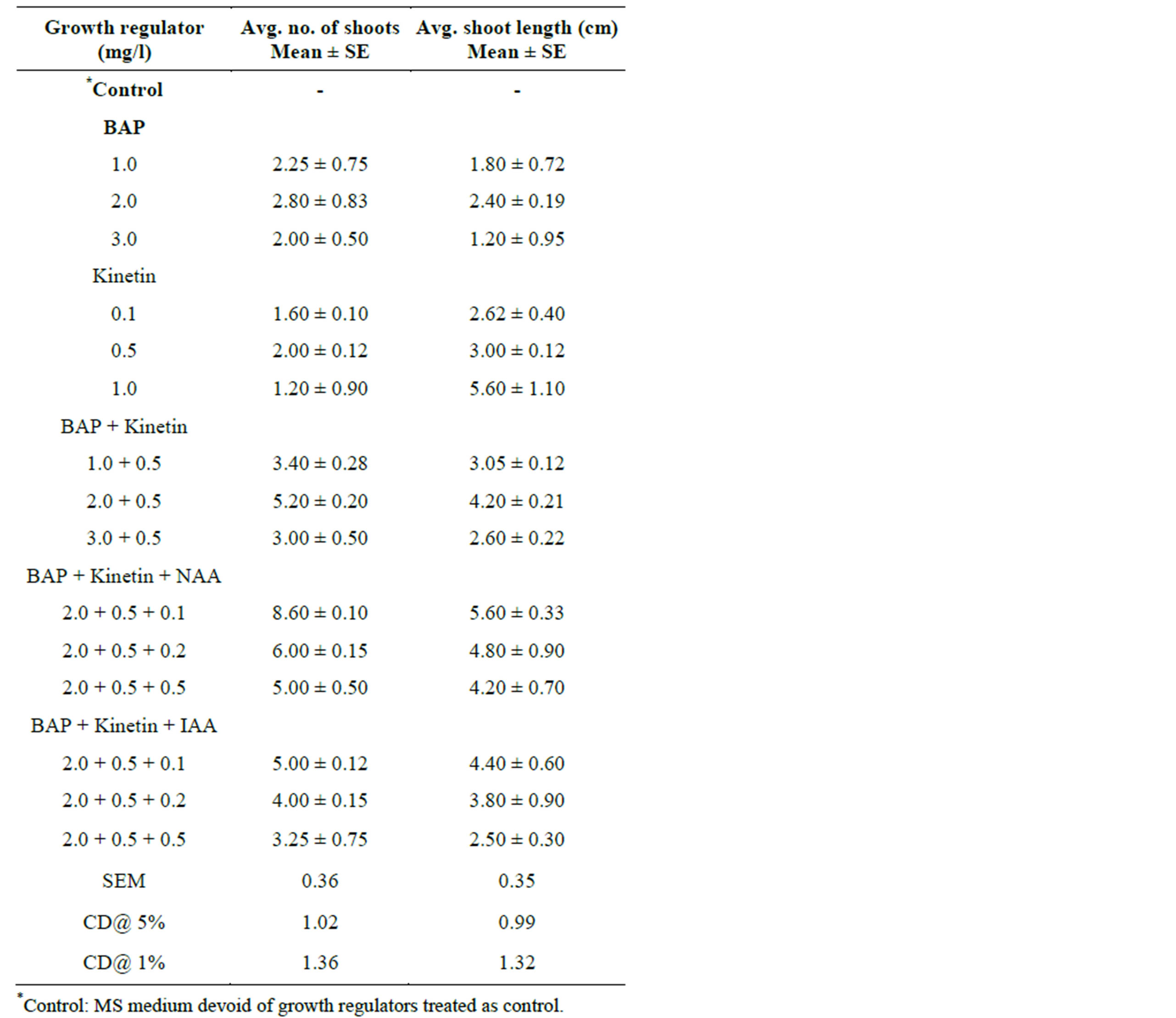
Table 1. Effect of BAP alone or in combination with Kinetin & NAA/ IAA on multiple shoot induction from nodal bud explants of S. rebaudiana in MS medium. Observations were taken after 4 weeks of culture.
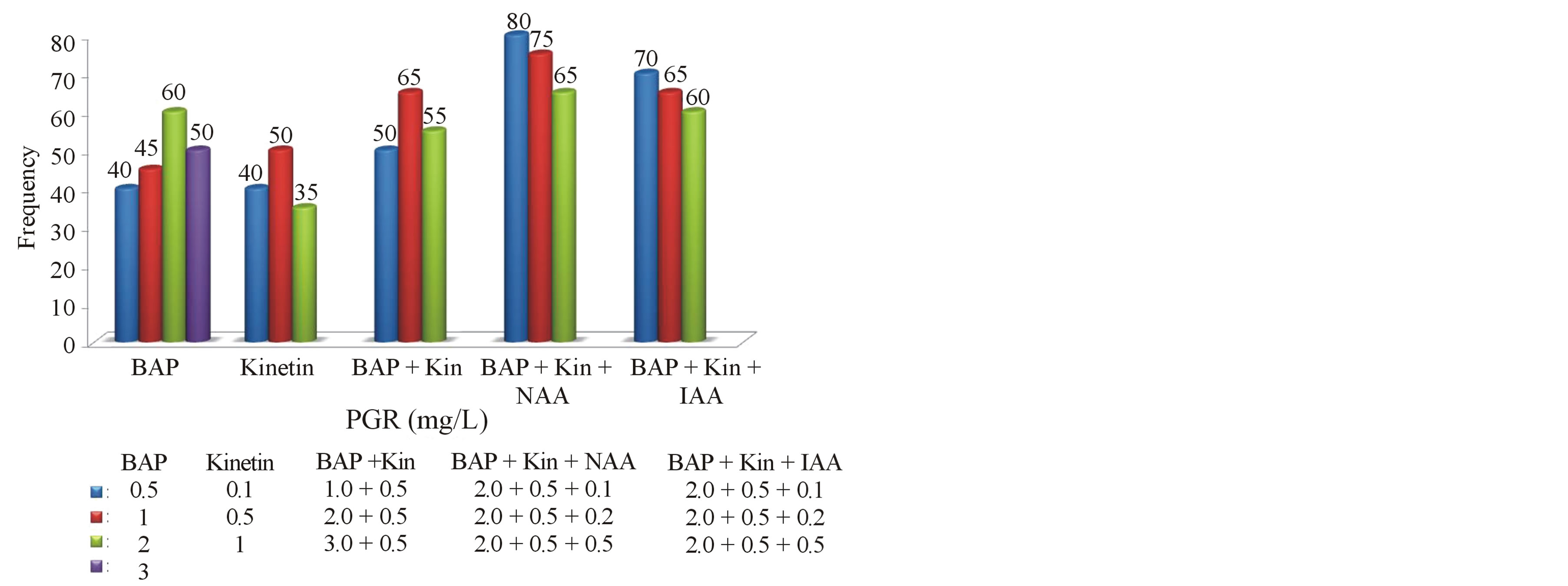
Graph 1. Frequency of regenerated shoots on MS medium supplemented with growth regulators at different concentrations.
medicinal species such as Salvia branchyodon [18], Pogostemon heyneanus [19] and Penthorum chinense [20]. However, the highest mean shoot length (5.6 cm) was recorded in Kinetin (1.0 mg/l) cultures. Earlier In vitro studies also reported the effect of kinetin on shoot length in Celastrus paniculatus [21]. Upon increasing the concentration of growth regulators, a reduction in the number of shoots per culture was observed. Similar trend was observed in Solanum nigrum using BAP at higher concentrations [22].
MS medium supplemented with a combination of (BAP 1.0 - 3.0 mg/l) and Kinetin (0.5 mg/l) in different concentrations was found to be effective in enhancing the shoot number and length per explant (Table 1). Maximum regeneration response of 65% was observed with BAP and Kin at 2.0 mg/l and 0.5 mg/l respectively, producing an average of 5.2 number of shoots and 4.2 cm shoot length per culture. The combined effect of BAP and Kinetin on efficient shoot induction has been well documented and proved in another asteracae member, Capsicum chinense [23] and Bambusa balcooa [24].
3.3. Influence of Two Cytokinins and Auxin Combination
It is well established that proper ratio of auxin and cytokinins is necessary for morphogenesis leading the formation of complete plantlets [25]. The efficiency of the optimal concentration of BAP and Kinetin (1.0 - 3.0 mg/l) with auxins (0.1 mg/l) was also tested for multiple shoot induction. Highly efficient regeneration frequency of 60% - 80% was observed in all the combinations tested. Increase in frequency and number of shoot formation was observed when BA and Kin used in combination with auxins (both NAA and IAA at 0.1 mg/l). Similar response was also observed in the propagation of Hemidismus [26]. High frequency (80%) and maximum number (8.6) of multiple shoots with shoot length of (5.0 cm) were induced on MS medium supplemented with 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA. When BAP and Kinetin were combined and used at their optimal concentrations with NAA, healthy shoots were produced. MS medium supplemented with BA and kinetin in combination with NAA found to be the best in shoot proliferation compared with same combination using NAA. The maximum regeneration frequency (70%), shoot number (5.0) and shoot length (4.4 cm) were observed in the combination of 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l IAA.
In the present study BAP and Kinetin in combination with NAA was found to be an effective combination for shoot regeneration and multiplication. Similar regeneration response was reported in Gymnema sylvastre [27] using BAP, Kinetin and NAA at their optimum concentrations.
3.4. Influence of Growth Additives on Multiple Shoot Induction
Synergistic effect of complex organic extracts such as casein hydrolysate, coconut water, malt extract and yeast extract was studied after determing the optimum cytokinin and auxin levels for shoot sprouting to improve the shoot multiplication rate in vitro. The proliferation effect of in vitro shoot buds on growth additives induced medium with different concentrations of cytokinins in combination with auxins showed variation in the regeneration percentage(represented in Graphs 2 and 3), no of shoots and shoot length and the results have been presented (Table 2 and Figure 1).
3.5. Casein Hydrolysate (CH)
Casein hydrolysate can be a source of calcium, phosphate, several micro elements, vitamins and most importantly, a
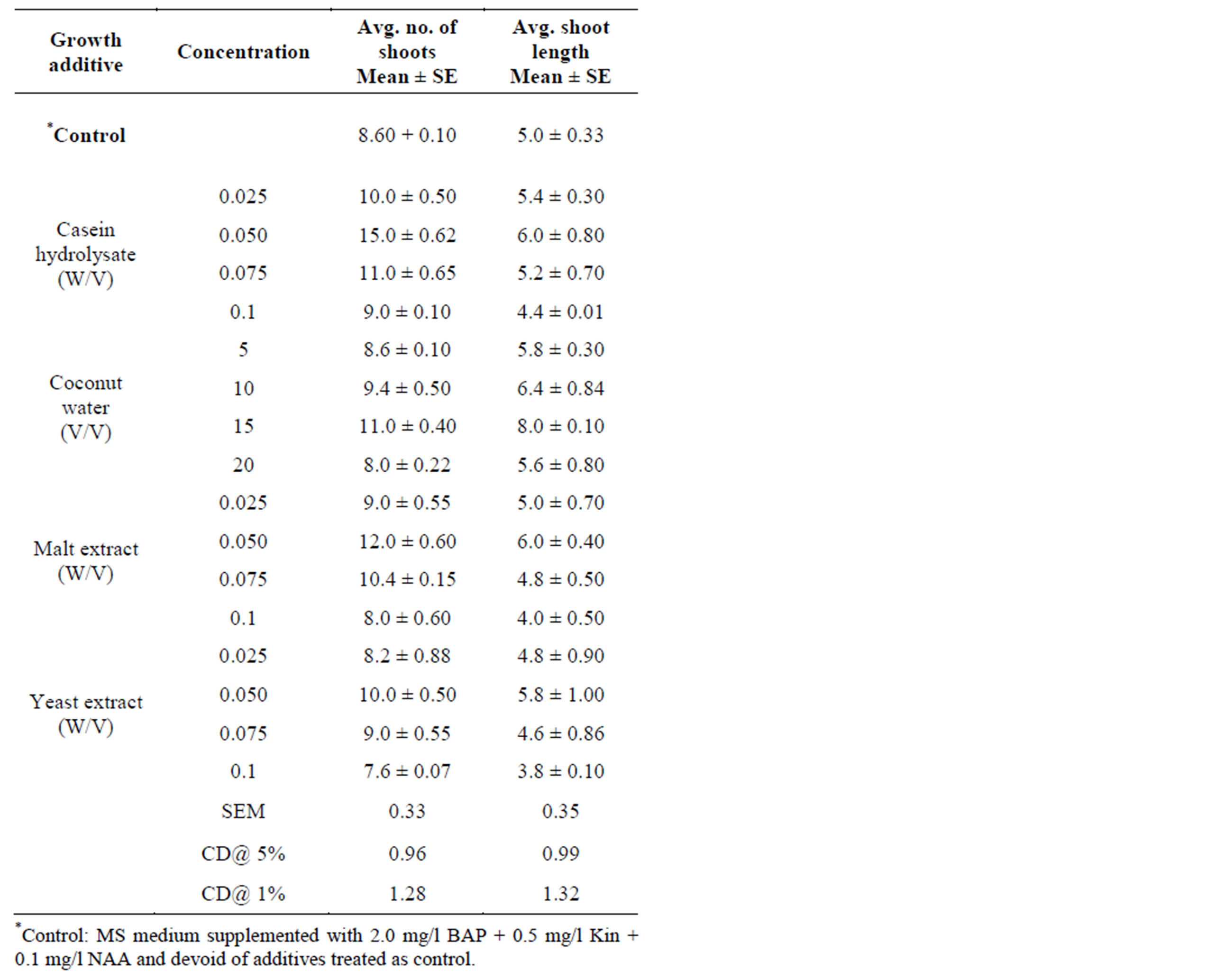
Table 2. Effect of different growth additives on multiple shoot regeneration from nodal bud explants of S. rebaudiana in MS medium supplemented with 2.0 mg/l BAP + 0.5 mg/l Kinetin + 0.1 mg/l NAA. Observations were taken after 4 weeks of culture.

Figure 1. Shoot initiation from nodal bud explants cultured on (A) MS + BAP (2.0 mg/l). Shoot multiplication from nodal bud explants cultured on (B) MS + BAP (2.0 mg/l) + Kin (0.5 mg/l) + NAA (0.1 mg/l) + 0.05% Malt extract. (C) MS + BAP (2.0 mg/l) + Kin (0.5 mg/l) + NAA (0.1 mg/l) + 10% Coconut extract. (D) MS + BAP (2.0 mg/l) + Kin (0.5 mg/l) + NAA (0.1 mg/l) + 0.05% Casein hydrolysate. Bar (A & C)—1 cm; Bar (B & D)—0.9 cm.
mixture of up to 18 amino acids [28]. Casein hydrolysate supplementation to the culture medium successfully induced shoot initiation within 4 - 5 days and subsequent multiplication from nodal bud explants. These results are in agreement with the previous report, where CH without growth regulators in the nutrient medium reduces the time period of shoot initiation up to 3 - 4 days as well as new shoot proliferation from nodal buds and also increased the percentage of explant response [29].
High frequency (90%) and maximum number (15) of shoots was obtained on MS medium supplemented with 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA when 0.05% CH was added. Similar results have also been found with CH in Anogeissus pendula, A. latifolia in vitro studies [30]. Induction of healthy shoot formation has
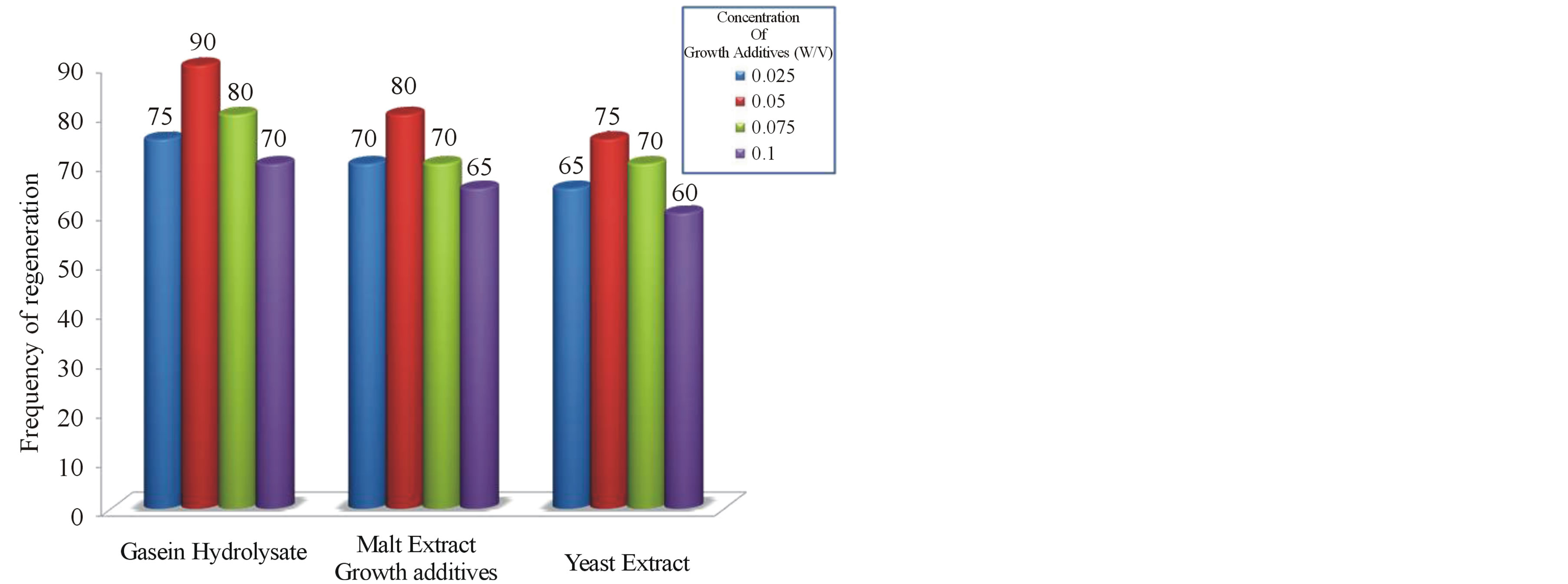
Graph 2. Frequency of regenerated shoots on MS + BAP (2.0 mg/l) + Kin (0.5 mg/l) + NAA (0.1 mg/l) supplemented with growth additives at different concentrations.
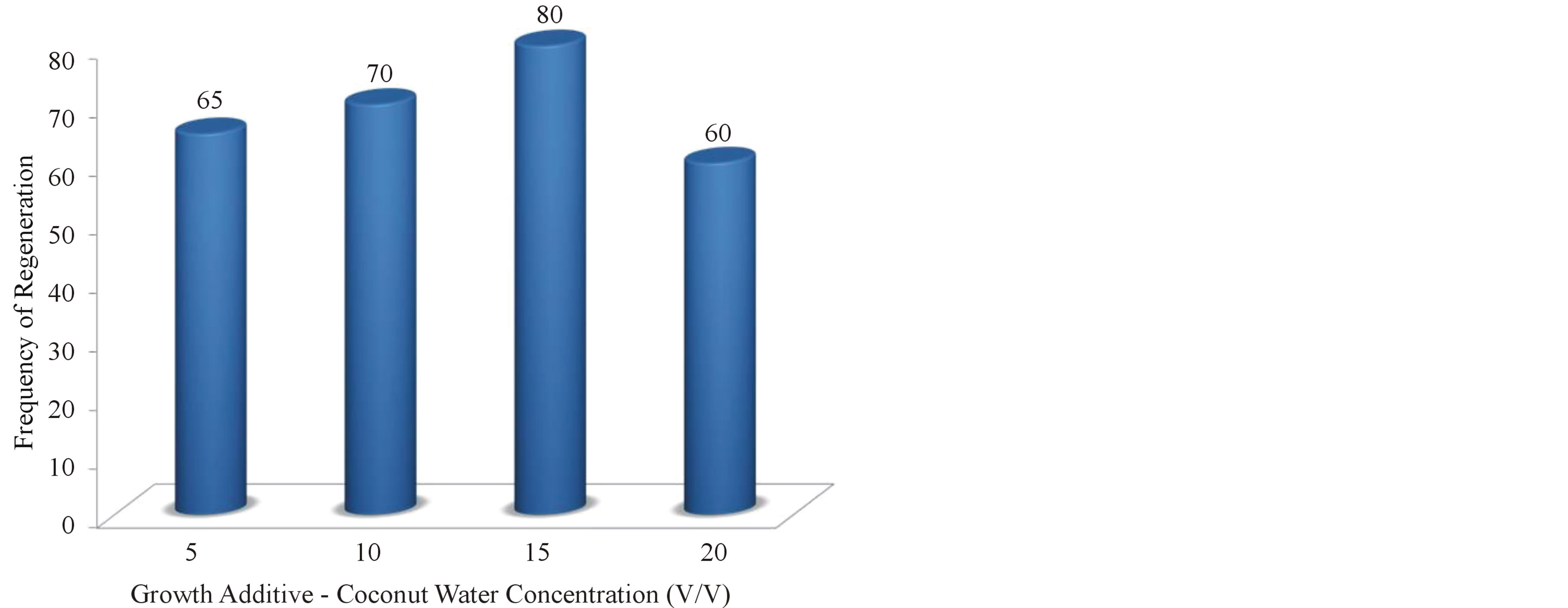
Graph 3. Frequency of regenerated shoots on MS + BAP (2.0 mg/l) + Kin (0.5 mg/l) + NAA (0.1 mg/l) supplemented with growth additive-Coconut Water at different concentrations.
been reported in Crataeva nurvala [31] using CH as medium supplement. Higher concentrations of casein hydrolysate (0.075% & 0.1%) resulted with no further increase in the number of shoots. The higher concentrations of CH (0.1%) in the growth medium did not support shoot growth as a consequence of which shoots remained compact and stunted. At higher doses (0.075% & 0.1% CH) basal callus was formed at the cut ends, there by which further diminishes the absorbance of nutrients resulted in stunted growth of the shoots (Figure 2). This is possibly due to maladjustment of in vitro cultured cells to the excessive organic nitrogen in the medium. Similar research findings have been reported in Asclepias regeneration studies, where CH in the growth medium produces appreciable amount of callus at the cut ends and readily turned brown and retarded the vigorous growth of shoots [32].
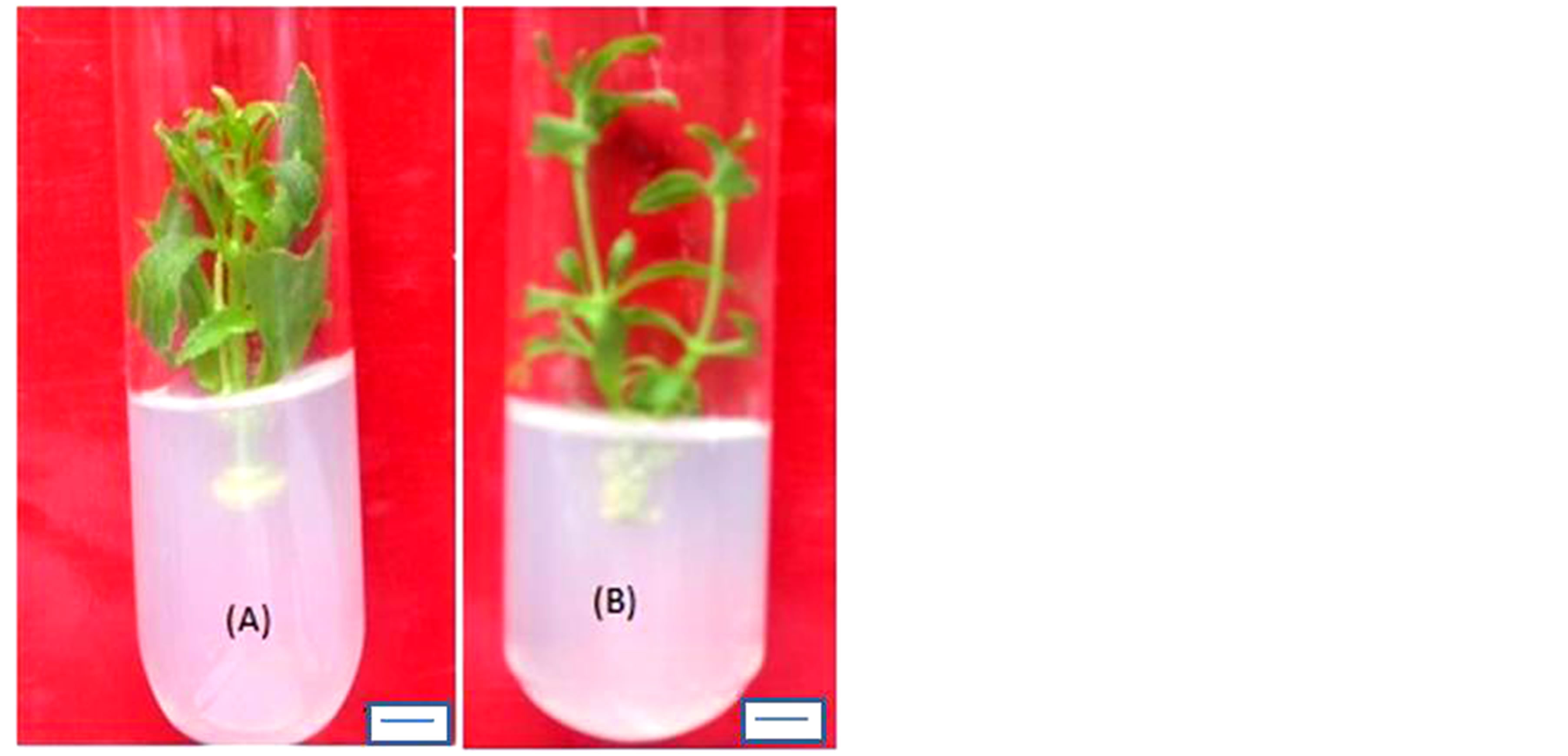
Figure 2. Shoot showing basal callus growth at cut portions from nodal explants cultured on (A) MS + BAP (2.0 mg/l) + NAA (0.1 mg/l) + 0.75% Casein hydrolysate. (B) and (C) MS + BAP (2.0 mg/l) + Kin (0.5 mg/l) + NAA (0.1 mg/l) + 0.75% Yeast extract. Bar (A)—1 cm; Bar (B)—0.9 cm.
3.6. Coconut Water (CW)
Coconut water is the colorless liquid endosperm of green coconuts (Cocos nucifera). However, coconut milk is the extract of white, solid endosperm of matured coconut after grinding and squeezing. Both of them are used in tissue culture media, but the coconut water is the more complex combination of compounds [33]. CW contains growth hormones and is liberally made use in tissue culture [12]. When added to the culture medium containing auxin, the liquid CW can induce plant cells to divide and grow rapidly this is clearly evident in the present research addition of CW into the media enhanced shoot growth and maximum shoot length (8.0 cm) was obtained on 15% CW supplemented medium. Similar research findings were obtained earlier with addition of CW enhanced the shoot growth and development of medicinal plants propagated in vitro [34]. Among the different concentrations tried, high frequency (80%) and maximum number (10.0) of shoots was obtained on MS medium supplemented with 2.0 mg/l BAP +0.5 mg/l Kin + 0.1 mg/l NAA when 15% CW was added. Whereas at higher concentrations (20%) callus formation at the cut ends was noted, there by which diminishes the shoot multiplication rate further.
3.7. Malt Extract (ME)
Malt extract, mainly a source of carbohydrates, was shown to initiate embryogenesis in nucellar explants [35]. Some plant hormones, such as auxins and gibberellins have been identified in malt extract [36]. In malt extract fortified medium shoot initiation and multiplication was successfully induced from axillary bud explants. Among the different concentrations of malt extract used, at 0.05% ME supplemented with 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA maximum number (12) of shoots with maximum shoot length (6.0 cm) was obtained. Similar trend was reported earlier, where ME was found to be necessary for improving the quality and quantity of shoot proliferation from nodal bud explants in Gymnema sylvestre [27]. In the present study shoot bud initiation and multiplication were higher at lower concentrations of ME (i.e. 0.025%, 0.05%), whereas at higher doses (i.e. 0.075%, 0.1% ME), the shoot number and shoot length was slightly decreased due to callus formation at the cut ended portions.
3.8. Yeast Extract (YE)
Addition of yeast extract to culture medium enhances the shoot proliferation rate in vitro. Among the different concentrations of yeast extract used, at 0.05% YE supplemented with 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA maximum number (10) of shoots with shoot length of (5.8 cm) was obtained. The promotive effect of YE on shoot proliferation was reported earlier in Lavandula latifolia in vitro studies [37]. In the present study similar to other growth additives such as CH, CW and ME, YE also favours the shoot multiplication, whereas at higher concentrations shoot multiplication rate was decreased due to basal callus formation at the cut ends of nodal explants.
Application of growth additives is adapted to the cultural needs [38] i.e. objectives of the experimental studies like micro propagation, regeneration, cytodifferentiation, androgenesis, biosynthesis of secondary metabolites and biotransformation of cells as well as the particular plant species taken. In the present study growth additives such as CH and ME have promontory effect on in vitro shoot multiplication rate compared to other growth additives. Where as CW has pronounced effect on axillary shoot length besides the number of shoots produced. YE have less beneficial effect on shoot proliferation and shoot length in vitro. Over all Casein hydrolysate has turned out to be the best growth additive for regeneration of Stevia rebaudiana. Hence, optimum medium composition for high frequency shoot multiplication where outlined.
4. Conclusion
The outlined procedure offers a potential system for improvement, conservation and mass propagation of S. rebaudiana from nodal bud explants. A two-step procedure has been standardized for maximum shoot proliferation, where in the first step growth hormones concentration has been standardized and it was found that 2.0 mg/l BAP + 0.5 mg/l Kin + 0.1 mg/l NAA , proven as the best treatment. In the second step, the best hormone treatment was fortified with growth additives for further shoot proliferation, among which 0.05% CH was found to be the best growth additive concentration for enhancing shoot multiplication. The efficient regeneration protocol developed in the presented study for enhanced shoot multiplication seems to be beneficial for producing Stevia cultures on large scale to meet the industrial needs. Apart from this the present study appears highly useful for future line of work involving metabolic engineering of Stevia genes leading to genetic improvement of this highly commercial medicinal herb.
Acknowledgements
The authors are thankful to Department of Biotechnology (DBT) for providing financial assistance. The authors are also thankful to the Director, IIHR for giving permission to carry out the proposed research.
REFERENCES
- D. D. Soejarto, C. M. Compadre, P. J. Medon, S. K. Kamath and A, D. Kinghorn, “Potential Sweetening Agents of Plant Origin, II. Field Search for Sweet Tasting Stevia species,” Economic Botany, Vol. 37, No. 1, 1983, pp. 71- 79. http://dx.doi.org/10.1007/BF02859308
- M. Bridel and R. Lavielle, “The Sweet Principle of Kaahe-e (Stevia rebaudiana),” Pharmaceutical Chemistry Journal, Vol. 14, 1931, pp. 99-154.
- A. S. Richman, M. Gizen, Al N. Starratt, Z. Y. Yang and J. E. Brandle, “Diterpene Synthesis in Stevia rebaudiana: Recruitment and Up-Regulation of Key Enzymes from the Gibberellin Biosynthetic Pathway,” The Plant Journal, Vol. 19, No. 4, 1999, pp. 411-421. http://dx.doi.org/10.1046/j.1365-313X.1999.00531.x
- D. Preethi, T. M. Sridhar and C. V. Naidu, “Effect of Bavistin and Silver Thiosulphate on in Vitro Plant Regeneration of Stevia rebaudiana,” Journal of Phytology, Vol. 3, 2011, pp. 74-77.
- M. D. Tofazzal Islam, “Stevia rebaudiana News: Is There Any Safe and Natural Alternative to Sugar? From This,” Financial Express, 2006, pp. 8-26.
- V. Yodyingyuad and S. Bunyawong, “Effects of Stevioside on Growth and Reproduction,” Human Reproduction, Vol. 6, 1991, pp. 158-165.
- D. Preethi, P. Shanmukanad, S. Hemadri Reddy, S. P. Jeevan Kumar, P. Josthna and C. V. Naidu, “In Vitro Plant Regeneration of Stevia rebaudiana,” Journal of Tropical Medicinal Plants, Vol. 9, No. 1, 2008, pp. 71- 76.
- P. B. Jeppesen, S. Gregersen and S. E. Rolfsen, “Antihyperglycemic and Blood Pressure-Reducing Effects of Stevioside in the Diabetic Goto-Kakizaki Rat,” Metabolism, Vol. 52, No. 3, 2003, pp. 372-378. http://dx.doi.org/10.1053/meta.2003.50058
- R. Abudula, P. B. Jeppesen, D. S. E. Rolfsen, J. Z. Xiao and K. Hermansen, “Rebaudioside A Potently Stimulates Insulin Secretion from Isolated Mouse Isolates: Studies on the Dose-Glucose and Calcium-Dependency,” Metabolism, Vol. 53, No.10, 2004, pp. 1378-1381. http://dx.doi.org/10.1016/j.metabol.2004.04.014
- K. Yasukawa, S. Kitanaka and S. Seo, “Inhibitory Effect of Stevioside on Tumor Promotion by 12-O-Tetradecanoylphorbol-13-Acetate in Two Stage Carcinogenesis in Mouse Skin,” Biological and Pharmaceutical Bulletin, Vol. 25, No. 11, 2002, pp. 1488-1490. http://dx.doi.org/10.1248/bpb.25.1488
- S. Nakamura and Y. Tamura, “Variation in the Main Glycosides of Stevia,” Japanese Journal of Tropical Agriculture, Vol. 29, 1985, pp. 109-116.
- S. Jayakumar and R. R. Lingam, “Influence of Additives on Enhanced In Vitro Shoot Multiplication of Orthosiphon aristatus (Blume) Miq,” Notulae Scientia Biologicae, Vol. 5,No. 3, 2013, pp. 338-345.
- S. Neelam and K. P. S. Chandel, “Effects of Ascorbic Acid on Axillary Shoot Induction in Tylophora indica (Burm. F.) Merr,” Plant Cell Tissue and Organ Culture, Vol. 29, No. 2, 1992, pp. 109-113. http://dx.doi.org/10.1007/BF00033615
- A. Vasudevan, N. Selvaraj, A. Ganapathi, S. Kasthurirengan, V. R. Anbbazhagan and M. Manickavasagam, “Glutamine: A Suitable Nitrogen Source for Enhanced Shoot Multiplication in Cucumis sativus,” Biologia Plantarum, Vol. 48, No. 1, 2004, pp. 125-128. http://dx.doi.org/10.1023/B:BIOP.0000024288.82679.50
- T. Murashige and F. A. Skoog, “Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiology Plant, Vol. 15, No. 3, 1962, pp. 473- 497. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
- P. Larkin and W. Scowcraft, “Somaclonal Variation—A Novel Source of Variability from Cell Culture for Plant Improvement,” Theoretical and Applied Genetics, Vol. 60, No. 4, 1981, pp. 197-214. http://dx.doi.org/10.1007/BF02342540
- L. W. Leng and C. Lai-Keng, “Plant Regeneration from Stem Nodal Segments of Orthosiphon stamineus Benth. A Medicinal Plant with Diuretic Activity,” In Vitro Cellular & Developmental Biology—Plant, Vol. 40, No. 1, 2004, pp. 115-118. http://dx.doi.org/10.1079/IVP2003500
- D. Misick, D. Grubisic and R. Konjevic, “Micro Propagation of Salvia brachyodon through Nodal Explants,” Biologia Plantarum, Vol. 50, No. 3, 2006, pp. 473-476. http://dx.doi.org/10.1007/s10535-006-0074-5
- M. E. Hembrom, K. P. Martin, S. K. Patchathundikandi and J. Madassery, “Rapid in Vitro Production of True-toType Plants of Pogostemon heyneaus through Dedifferentiated Axillary Buds,” In Vitro Cellular & Developmental Biology—Plant, Vol. 42, No. 3, 2006, pp. 283-286. http://dx.doi.org/10.1079/IVP2006757
- H. Cao, J. Yang, Z. S. Peng, C. Y. Kang, D. C. Chen, Z. C. Gong and X. Tan, “Micro Propagation of Penthorium chinense through Axillary Bud,” In Vitro Cellular & Developmental Biology—Plant, Vol. 12, 2007, pp. 33-38.
- M. S. Rao and S. D. Purohit, “In Vitro Shoot Bud Differentiation and Plantlet Regeneration in Celastrus paniculates Wild,” Biologia Plantarum, Vol. 50, No. 4, 2006, pp. 501-506. http://dx.doi.org/10.1007/s10535-006-0079-0
- T. M. Sridhar and C. V. Naidu, “High Frequency Plant Regeneration, in Vitro Flowering of Solanum nigrum (L.) —An Important Antiulcer Medicinal Plant,” Journal of Phytology, Vol. 3, No. 2, 2011, pp. 85-93.
- K. Sanatombi and G. J. Sharma, “In Vitro Propagation of Capsicum chinense Jacq,” Biologia Plantarum, Vol. 52, No. 3, 2008, pp. 517-520. http://dx.doi.org/10.1007/s10535-008-0100-x
- D. Negi and S. Saxena, “Micro Propagation of Bambusa balcooa Roxb. through Axillary Shoot Proliferation,” In Vitro Cellular & Developmental Biology—Plant, Vol. 47, No. 5, 2011, pp. 604-610. http://dx.doi.org/10.1007/s11627-011-9403-2
- E. F. George and P. D. Sherrington, “Plant Propagation by Tissue Culture,” Handbook and Dictionary of Commercial Laboratories, Exgenetics Ltd., England, 1984.
- J. Patnaik and B. K. Debata, “Micro Propagation of Hemidesmus indicus (L.) R. Br. through Axillary Bud Culture,” Plant Cell Reports, Vol. 15, No. 6, 1996, pp. 427- 430. http://dx.doi.org/10.1007/BF00232069
- N. Komalavalli and M. V. Rao, “In Vitro Micro Propagation of Gymnema sylvestre—A Multipurpose Medicinal Plant,” Plant Cell, Tissue and Organ Culture, Vol. 61, No. 2, 2000, pp. 239-245. http://dx.doi.org/10.1023/A:1006421228598
- E. F. George and G. J. De Klerk, “The Components of Plant Tissue Culture Media I: Macro and Micro Nutrients,” Plant Propagation by Tissue Culture, 3rd Edition, Springer-Verlag, Dordretch, Vol. 1, 1984, pp. 65-113.
- F. Parabia, B. Gami, L. L. Kothari, J. S. S. Mohan and M. H. Parabia, “Effect of Plant Growth Regulators on in Vitro Morphogenesis of Leptadenia reticulata Retz. from Nodal Explant,” Current Science, Vol. 92, 2007, pp. 1290- 1293.
- S. Saxena and V. Dhawan, “Large-Scale Production of Anogeissus pendula and Anogeissus latifolia by Micro Propagation,” In Vitro Cellular & Developmental Biology—Plant, Vol. 37, No. 5, 2001, pp. 586-591. http://dx.doi.org/10.1007/s11627-001-0103-1
- N. Walia, A. Kour and S. B. Babbar, “An Efficient, in Vitro Cyclic Production of Shoots from Adult Trees of Crataeva nurvala Buch. Ham,” Plant Cell Reports, Vol. 26, No. 3, 2007, pp. 277-284. http://dx.doi.org/10.1007/s00299-006-0239-x
- S. H. Reddy, M. Chakravarthi, K. N. Chandrashekara and C. V. Naidu, “Influence of Bavistin and Silver Thiosulphate on in Vitro Regeneration of Asclepias curassavica (L.) Using Nodal Explants,” American Journal of Plant Sciences, Vol. 3, 2012, pp. 941-946. http://dx.doi.org/10.4236/ajps.2012.37111
- Z. Molnar, E. Virag and V. Ordog, “Natural Substances in Tissue Culture Media of Higher Plants,” Acta Biologica Szegediensis, Vol. 55, No. 1, 2011, pp. 123-127.
- W. Tefera and S. Wannakrairoj, “Micro Propagation of Karwan,” Science Asia, Vol. 30, 2004, pp. 9-15. http://dx.doi.org/10.2306/scienceasia1513-1874.2004.30.009
- T. S. Rangan, “Clonal Propagation, Cell Culture and Somatic Cell Genetics of Plants, Laboratory Procedures and Their Applications,” Academic Pres, Inc., Orland, Vol. 1, 1984, pp. 68-73.
- L. Dix and J. Van Staden, “Auxin and Gibberellin Like Substances in Coconut Milk and Malt Extract,” Plant Cell, Tissue and Organ Culture, Vol. 1, 1982, pp. 239-245. http://dx.doi.org/10.1007/BF02318920
- M. C. S. Gras and M. C. Calvo, “Micro Propagation of Lavandula latifolia through Nodal Bud Culture of Mature Plants,” Plant Cell, Tissue and Organ Culture, Vol. 45, No. 3, 1996, pp. 259-261. http://dx.doi.org/10.1007/BF00043639
- K. Vinod, G. Parvatam and A. R. Gokare, “AgNO3-A Potential Regulator of Ethylene Activity and Plant Growth Modulator,” Electronic Journal of Biotechnology, Vol. 12, No. 2, 2009, pp. 1-15.
Abbreviations
BAP—6 Benzyl Amino Purine;
Kin—Ki-netin;
NAA—Naphthalene Acetic Acid;
IAA—Indole Acetic Acid;
CH—Casein Hydrolysate;
CW—Coconut Water;
YE—Yeast Extract;
ME—Malt Extract.
NOTES
*Corresponding author.

