International Journal of Clinical Medicine
Vol.2 No.5(2011), Article ID:8532,11 pages DOI:10.4236/ijcm.2011.25111
The Clinical Application of Ambulatory Blood Pressure Monitoring in Pediatrics
![]()
Children’s Hospital of Pittsburgh of UPMC, Department of Pediatrics, School of Medicine, University of Pittsburgh, Pittsburgh, USA.
Email: yosuke.miyashita@chp.edu
Received August 15th, 2011; revised September 25th, 2011; accepted October 7th, 2011.
Keywords: Pediatric Hypertension, Ambulatory Blood Pressure Monitoring, White Coat Hypertension, Masked Hypertension
ABSTRACT
Management of hypertension (HTN) largely relies on proper and accurate measurement of blood pressure (BP). Even following the criteria for HTN diagnosis defined in the Fourth report on high BP in children and adolescents, inaccurate diagnosis and misdiagnosis can occur with white coat effect and masked HTN. The use of Ambulatory Blood Pressure Monitoring (ABPM) has been increasing in pediatrics in the last 20 years. The main use of ABPM is to differentiate between sustained HTN and white coat HTN in patients who have elevated casual BP measurements and to detect masked HTN in high risk patients. ABPM is most useful in patients with casual BP within 20% of the 95th percentile for age, gender, and height. This report will highlight the use of ABPM in the evaluation of elevated BP and management of HTN in pediatrics. The discussion includes a review of various non-invasive BP measuring techniques, a description of ABPM and ABPM-unique data and diagnoses, updated ABPM clinical data more specific to pediatrics, its use in HTN clinical trials, and future outlook and direction of ABPM in pediatrics.
1. Introduction
The diagnosis and proper management of hypertension (HTN) relies on accurate blood pressure (BP) measurements, traditionally performed by manual or automated devices in clinical settings, known as casual BP. However, repeated studies have shown that this procedure is often inaccurate due to inadequate or poorly maintained equipment and observer bias [1], and disregard of circadian rhythm of BP [2]. An alternative method such as home self-BP measurement is also problematic as they are vulnerable to observer error in reporting [3] and device inaccuracies [4]. Regardless of the measuring method, elevated BP measurements tend to fall on subsequent measurements as a result of accommodation effect and regression to the mean. To address this issue, the Fourth report on high BP in children and adolescents strictly defines diagnosis of HTN as the average systolic blood pressure (SBP) and/or diastolic blood pressure (DBP) ≥ 95th percentile for gender, age, and height on at least 3 separate occasions [5].
Even with this definition, there are additional diagnoses known as white coat hypertension (WCH) and masked HTN, discussed more in detail in later section, that complicate the proper diagnosis and management of BP. Since the 1990s, the use of ambulatory blood pressure monitoring (ABPM) has been on the increase in pediatrics [2,6], and it appears to be a useful tool to overcome much of the potential difficulties of BP measurements discussed above. This review will first compare various traditional non-invasive BP measurement techniques in pediatrics. Then ABPM is discussed extensively including description of the study, ABPM-unique data and diagnoses, ABPM related clinical data concentrating more in pediatrics, and potential benefits of ABPM in HTN clinical trials. Finally, needs and future opportunities for further ABPM research will be discussed.
2. Blood Pressure Measurement Techniques
Traditional non-invasive blood pressure measurement options include auscultation using mercury or aneroid sphygmomanometer and oscillometric devices, either clinic or home monitors. Table 1 summarizes the strengths and weaknesses of each method. Oscillometric devices offer convenience and minimization of observer bias, but they measure mean arterial BP then calculate SBP and DBP values instead of direct measurement of these values [7]. The algorithms used to calculate SBP and DBP vary depending on manufacturer and devices, and they
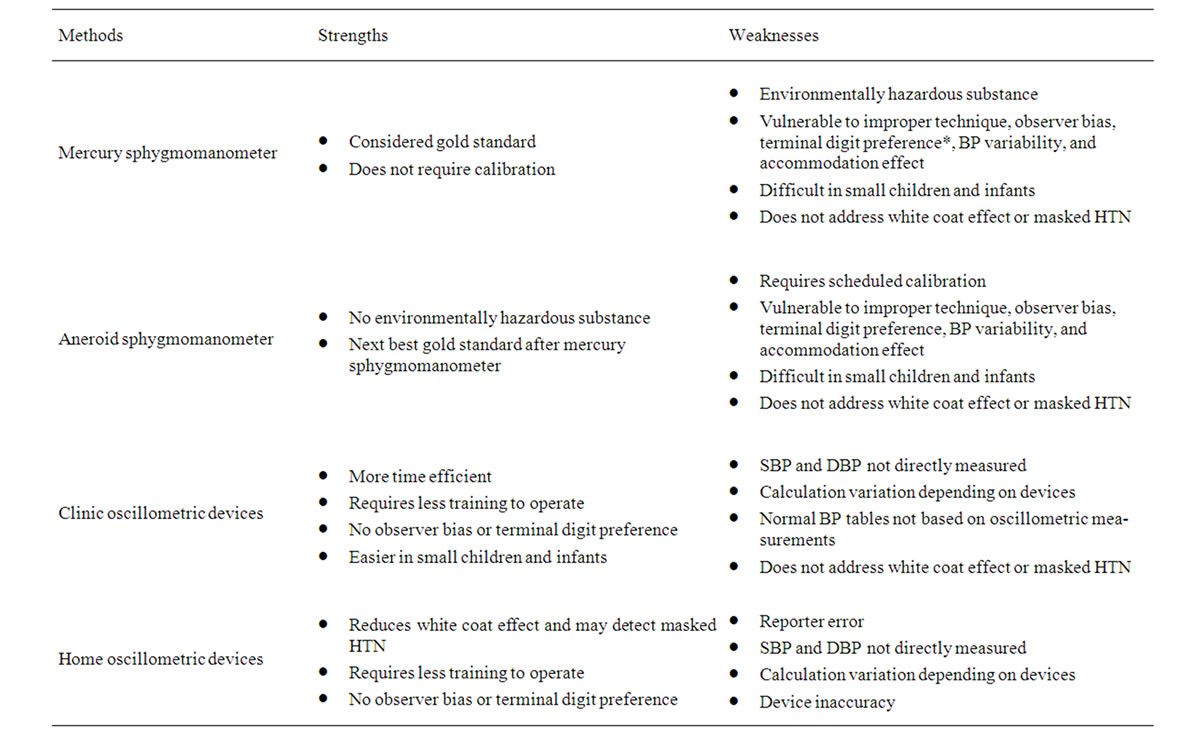
Table 1. Traditional blood pressure measurement techniques BP: blood pressure, SBP: systolic blood pressure, DBP: diastolic blood pressure, HTN: hypertension. *Terminal digit preference: a type of observer bias where observer has tendency to round readings to a certain number (usually zero) leading to overestimation or underestimation of actual BP.
have been reported to be always not consistent with auscultative BP values [8]. In addition, the pediatric normative BP tables are based on auscultatory measurements, and therefore, the preferred method of measurement is auscultation [5]. Thus, the Fourth report recommends repeated BP measurement by auscultation for any oscillometric BP measurement that exceed the 90th percentile for age, gender, and height [5]. Interested readers are encouraged to review the extensive discussion on proper environment, equipment, body position, and technique for accurate BP measurement in the Fourth report [5].
As noted in Table 1, despite proper techniques that should be taken with every auscultative BP measurement, it is still vulnerable to misclassification due to white coat effect and masking of HTN. ABPM is a newer tool that is aimed to reduce this misclassification and to make proper diagnosis of HTN.
3. Ambulatory Blood Pressure Monitoring
3.1. Description of the Equipment and the Study
ABPM equipment usually consists of a cuff, a monitor, and a rubber tubing that connects the two parts. The cuff is usually worn around the non-dominant arm of patients for 24 hours continuously, and the monitor is usually attached to their waistline, usually on belts. For ABPM to be practical in pediatric usage, the monitor should be small in size and light in weight. There are now available monitors ranging from 168 to 457 g [9]. There are a number of ABPM manufactures but almost all the published reports of pediatric ABPM used devices from Spacelabs Healthcare (Issaquah, WA, USA). Typically, ABPM records BP every 15 to 30 minutes during awake hours and every 20 to 60 minutes during sleep hours, and the data are analyzed in relation to a timed activity diary recorded by the family [9]. Most authorities agree that at least one valid BP recording per hour is required for an interpretable study [9]. Readings can take place during usual activity of children and adolescents including going to school, doing homework, eating, and non-vigorous activity like walking and sleeping. Activities not permitted while on ABPM include vigorous exercise, contact sports, showering, bathing, and swimming. The raw recorded BPs are then interpreted by a software that calculates various mean BP values and ABPM-unique data called nocturnal dip and BP load, discussed further in the next section. Currently, pediatric normative ABPM data is defined by German healthy school children initially published by Soergel et al. [10] and later revised using LMS-transformed data ([L] degree of skewness, [M] ageand gender-specific estimates of the distribution median, [S] coefficient of variation) by Wühl et al. [11]. Currently, routine use of ABPM is recommended for children aged 5 years and older [9], and the normative tables are available for ages 5 to 16 year olds [10,11]. A sample ABPM interpretation report, which includes 95th percentile threshold for patient’s age and gender, various mean BP values, BP load, nocturnal dip status, and physician’s interpretation, is shown as Figure 1.
3.2. ABPM-Unique Data
Contrary to casual BP measurement, ABPM records and calculates more comprehensive BP data including various mean BP values, nocturnal dip, and BP load as summarized in Table 2. The mean BP, both systolic and diastolic, can be calculated for the entire 24 hour period as well as awake and sleep periods. These values can then be compared to the published ABPM normative data which also include 24 hour, awake, and sleep BP values.
Nocturnal dipping refers to the physiological decline
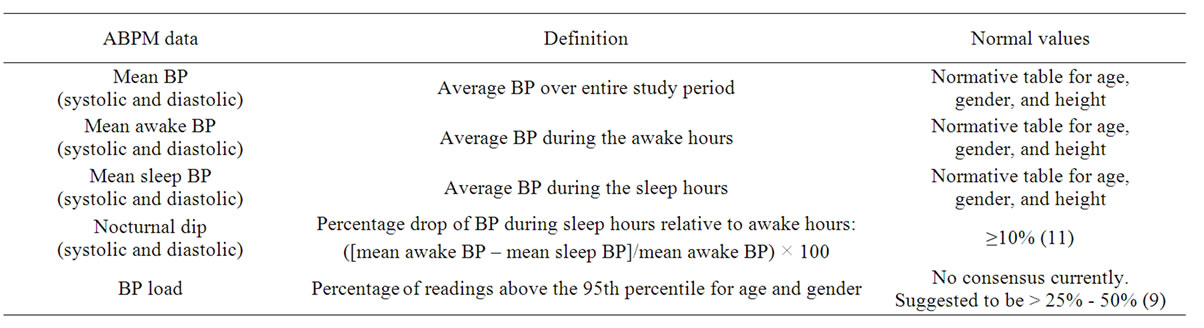
Table 2. ABPM unique data.
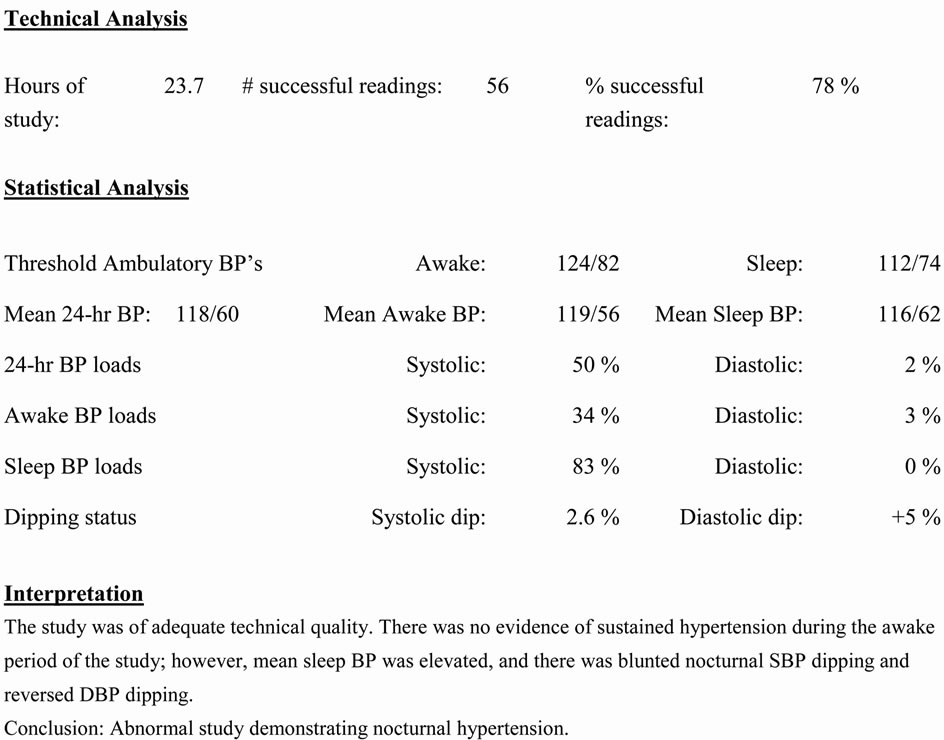
Figure 1. A sample of ambulatory blood pressure monitoring interpretation report.
in BP observed at night due to circadian rhythm. Normal dipping is currently defined as ≥10% decline in mean SBP and DBP from day to night [12], as blunted nocturnal dipping by itself may have clinical significance, as discussed in later section. There may be racial differences in nocturnal dipping [13], but presently, there is no race-specific definition established. BP load is defined as percentage of ABPM measurements greater than the 95th percentile for age, gender, and height [14]. At this point, there is no consensus cut-off percentage that defines abnormal BP load in pediatrics with suggested percentage ranging from 25% to 50% [9,14-16], and greater than 40% - 50% appears to be associated with target organ damage [15,17].
3.3. ABPM-Unique Diagnosis
With ABPM, additional BP related diagnoses such as WCH and masked HTN can be made with more certainty. WCH is defined as BP levels ≥ 95th percentile for age, gender, and height in physician’s office or clinic but are normal (<90th percentile) outside of clinical setting. This is believed to be due to transient, stress-induced elevation of BP when children are in medical setting. The prevalence of pediatric WCH have been reported to range from 10% - 60% depending on methodology used for casual BP measurement, diagnostic thresholds for casual and ambulatory BP, and study population (healthy population versus referred patients for elevated BP) [18]. The likelihood of WCH appears to decrease as casual BP measurements increase. Sorof et al. reported that prevalence of WCH decreased from 87% to 15% when cut-off value was increased from 95th percentile for age, gender, and height to 20% above the 95th percentile [19]. As discussed later, the degree of casual BP elevation may help to select which patients are appropriate to have ABPM for evaluation of WCH.
Masked HTN is a converse of WCH as defined as normal casual BP (<90th percentile for age, gender, height) but elevated ambulatory levels. The prevalence of masked HTN in pediatrics is also unclear with reported prevalence ranging from 4.2% to 20% [20-24] depending on study methods including study population and technique to detect masked HTN: ABPM versus home BP measurements. Masked HTN also includes patients with nocturnal HTN such as the sample ABPM interpretation in Figure 1. ABPM has the capability to pick up nocturnal HTN where both casual BP measurement and home BP measurement likely will miss this diagnosis.
3.4. ABPM versus Home BP Monitoring
Currently, there is no consensus on which technique (ABPM versus home BP monitoring) would be the most efficient and cost-effective way to diagnose WCH and masked HTN in pediatrics. According to the American Heart Association consensus statement, ABPM is a superior technique and is considered the gold standard for evaluation of both WCH and masked HTN [9]. However, proponents of home BP monitoring ague that there is not enough data to support one method being superior over the other [25]. Two studies have compared diagnosis of HTN comparing casual BP, home BP, and ABPM. In a sub-study of the ESCAPE (Effect of Strict blood pressure Control and ACE inhibition on Progression of chronic renal failure in pEdiatric patients) trial with children ages 3 to 19 years old with mild to moderate renal failure, home BP monitoring was superior to casual BP in detecting HTN, but even the maximum diagnostic sensitivity by both methods compared to ABPM was 81% [26]. This means that one out of four children diagnosed as hypertensive by ABPM could be missed by combination of home BP and casual BP [26]. In the second study, in children ages 6 to 18 years old referred for suspected HTN, home BP monitoring had sensitivity, specificity and positive and negative predictive values of 55%, 92%, 74% and 82%, respectively for diagnosis of sustained HTN, 89%, 92%, 70% and 98%, respectively for diagnosis of WCH, and 36%, 96%, 50% and 93%, respectively for diagnosis of masked HTN when compared to ABPM diagnosis [21].
3.5. Adult ABPM Data
ABPM has been in use longer in adults, and because of more frequent cardiovascular events, normative data is more evidence based on patient outcome. Normal 24-hour ABPM cut-off for adults has been defined as <130/80 [27,28]. In hypertensive European adult population, higher ambulatory SBP or DBP predicted cardiovascular events even after adjustment for classic risk factors including casual BP [29]. In general rural Japanese adult population, ABPM showed stronger predictive power for stroke risk than casual BP measurement [30].
Studies have also shown that ABPM predicts target organ damages better than casual BP. PIUMA (Progetto Ipertensione Umbria Monitoraggio Ambulatoriale) study, an Italian adult registry of morbidity and mortality in subjects with primary HTN, showed that ABPM stratified cardiovascular risks including left ventricular hypertrophy (LVH) independent of casual BP [31,32]. LVH has been established as an independent risk factor for cardiovascular disease in adults [33,34]. Similarly, ABPM results and carotid intima-media thickness, a known risk factor for stroke [35], appears to correlate better than casual BP. In a cross-sectional analysis of 214 healthy adult volunteers, 4 automated ambulatory blood pressure taken every 2.5 hours correlated positively with carotid intima-media thickness and carotid plaque even when adjusted for demographic covariates, automated casual BP, and manual casual BP [36].
The long-term clinical outcome data in adults with masked HTN and WCH are available. As expected, adults with masked HTN, diagnosed by ABPM, in multiple studies have shown higher rates of cardiovascular mortality and events compared to normotensive subjects [37]. The available data on WCH appears to be inconclusive both in target organ damage and patient outcome. Traditionally, WCH was viewed as a benign condition as number of studies illustrated similar rates of cardiovascular events between WCH subjects and normotensive subjects [38]. However, in a larger, international collaborative study, the incidence of stroke tended to increase after 6 years of follow-up, raising a question of whether WCH as a benign condition [39]. The data is also inconclusive for target organ damage, as some studies showed an association between WCH and intermediate target organ damage, by echocardiogram and carotid ultrasound, while others showed lack of association [38]. Table 3 summarizes the relationship between various BP diagnoses and their cardiovascular risks.
Finally, given the relative high prevalence of WCH, performing ABPM appears to be cost-effective in adults. A study showed that in new patients being evaluated for elevated BP, ABPM being incorporated in diagnostic process potentially saved 3% to 14% for cost of care and 10% to 23% reduction in treatment days taking in account for estimates of testing costs, treatment costs, and prevalence of WCH [40].
3.6. Pediatric Data
Early pediatric studies showed validity and reproducibility of ABPM. Lurbe et al. found valid measurements in 76.4 ± 15.6% of total measurements in unselected children between ages 3 to 18 years, and 84.1% of all monitoring had successful measurement rate of >80%, and 64% had successful measurement rate ≥ 90% [41]. Kahn et al. found similar results with 83% of the ABPM studies considered successful [42].
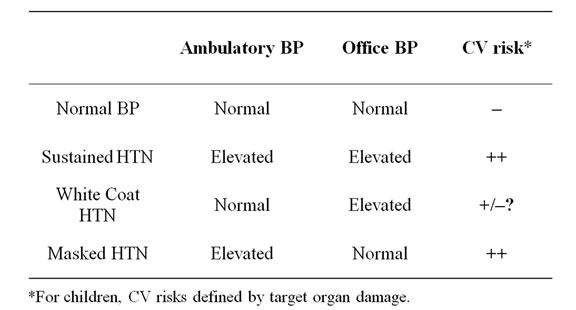
Table 3. Definition of blood pressure diagnosis with ABPM and their cardiovascular (CV) risks.
In contrast to adults, there is no pediatric data available to date that correlates patient outcome with ABPM data largely due to very low incidence of cardiovascular events. However, there have been an increasing number of studies that show an association between abnormal ABPM results with target organ abnormalities, including the heart, the carotid intima-media thickness, the kidneys, and perhaps a unique concern to pediatrics, central nervous system development.
Among these organs, the most studied is the heart, specifically the correlation between BP and LVH. Multiple pediatric studies have found a correlation between elevated casual BP measurements with LVH [43-46], and these results have been duplicated by ABPM studies demonstrating correlation between HTN and LVH [17,47-49]. To expand on this point, ABPM-unique data, such as 24 hour systolic BP index (defined as percentage over the 95th percentile for age, gender, and height) and night time mean SBP have been found to correlate with left ventricular mass index [17,50]. Recent pediatric studies also suggest an association between abnormal ABPM with increased carotid intima-media thickness [49,51,52]. Because obesity is also associated with increased carotid intima-media thickness, a study by Lande et al. may be most intriguing since this study had a control group with matched age, gender, and body mass index [52]. There have also been recent reports to suggest that hypertensive target organ damage may include abnormalities in central nervous system development. Children and adolescents aged 10 to 18 years old who were proven to have sustained HTN by ABPM demonstrated lower parental ratings of executive function compared to normotensive controls [53]. These parental ratings improved 12 months later with antihypertensive therapy, confirmed with lower ABPM measurements [54]. Further, odds of having learning disabilities were about 4 times higher for hypertensive subjects (proven either by ABPM or 3 casual BP measurements) compared to normotensive subjects adjusted for age, gender, obesity, and low socioeconomic status, regardless of whether they were receiving Attention Deficit Hyperactivity Disorder therapy [55].
ABPM can also be a useful tool to “unmask” masked HTN in high risk patients and to provide a marker of progression in diabetic nephropathy. A cross-sectional study in children after renal transplantation showed masked HTN in 24%, uncontrolled HTN (defined as daytime ABPM HTN undetected by casual BP while on antihypertensive therapy) in 21%, and non-nocturnal dipping (defined as <10%) in 71% of patients [56]. A cross-sectional multicenter trial of chronic peritoneal dialysis children found day time SBP load to be an independent predictor of LVH in a population where prevalence of LVH was found to be high at 70.2% [57]. ABPM abnormalities also have been shown to correlate with progression of diabetic nephropathy in both type I and type II diabetes mellitus (DM). Persistent microalbuminuria occurs during the subclinical stage of diabetic nephropathy, before the onset of overt proteinuria in DM. In a study among adolescents and young adults with type I DM, non-nocturnal dipping ABPM pattern was observed in 80% of proteinuric subjects, 58% of micro-albuminuric subjects, 18% in normoalbuminuric subjcts, and 10% in non-diabetic controls [58]. The same study group also showed that nocturnal increase in SBP preceded the development of micro-albuminuria in adolescents and young adults with type I DM initially without microalbuminuria and HTN, and the risk of microalbuminuria increased when nocturnal dipping fell to <10% [59]. In type II DM, the data appears to be similar. In adolescents with recent diagnosis of type II DM, casual BP was not significantly different in patients with microalbuminuria versus without micro-albuminuria, while average daytime SBP and average daytime SBP load detected by ABPM were significantly higher in patients with microalbuminuria compared to patients without microalbuminuria and non-diabetic control subjects [60].
Similar to adults, the prognostic significance of WCH in pediatrics is unclear. Recent reports have mixed conclusions on whether WCH is associated with target organ damages, in particular, left ventricular mass index and carotid intima-media thickness [22,61,62]. Among children referred for suspected hypertension who underwent ABPM, WCH subjects had left ventricular mass index between those of confirmed hypertensives and normotensives, although the difference between WCH subjects and normotensives did not reach statistical signifycance [22]. No significant differences were found in the carotid intima-media thickness between the three groups [22]. A Hungarian school-based adolescent study showed higher intima-media thickness in both WCH and hypertensive subjects compared to normotensive controls while there was no difference between left ventricular mass index between WCH subjects and controls [61]. Finally, a study among children referred for suspected hypertension showed left ventricular mass index greater than 95th percentile for age in 33% of WCH males and 36% of WCH females [62]. Based on these results, some of the pediatric HTN experts feel that WCH is a prehypertension condition, thus warranting continued follow-up of BPs.
As expected, the relationship between BP and target organ damage is much clearer in masked HTN, both in otherwise healthy population and in high risk populations such as patients with chronic kidney disease. The BP associated target organ damages appear to be indistinguishable from those of sustained HTN, which parallel the adult data. A study among 592 youth ages 6 to 18 years old, subjects with masked HTN had higher left ventricular mass index and a higher rates of left ventricular mass index above the 95th percentile for age and gender compared to normotensive controls [20]. Among 85 patients referred for elevated BP, left ventricular mass indexes were significantly larger in ABPM confirmed hypertensives and masked hypertensives compared to confirmed normotensives [22]. The evidence for target organ damage from masked HTN are even more pronounced in high risk patients. A cross-sectional analysis of Chronic Kidney Disease in Children (CKiD) cohort showed that children 9 through 15 years of age with chronic kidney disease stage 2 through 4 (estimated glomerular filtration rate 15 to 89 ml/min/1.73 m2) had 38% prevalence of HTN and 18% confirmed HTN by ABPM [63]. Further, 17% of this cohort had LVH, and a multivariable analysis showed that both masked and confirmed HTN were the strongest independent predictors of LVH with both odds ratios just over 4 [63]. Thus the relationship between various BP related diagnoses and cardiovascular risks, as indicted in Table 3, appear to stay intact both in otherwise healthy hypertensive and high risk children and adolescents.
Finally, once proper diagnosis of HTN is achieved and therapy is started, ABPM can be used to assess the treatment efficacy more completely. One of the early pediatric ABPM study by Flynn is an example of this use. Out of seven patients, four patients were determined to have poorly controlled HTN by ABPM, not detected by casual BP measurements, prompting a change in their therapy or further diagnostic testing [6].
In summary, there is increasing number of published pediatric ABPM reports. Early studies showed validity and reproducibility of ABPM in children and adolescents. More recent reports have shown correlation between abnormal ABPM studies with various hypertensive target organ damages including the heart, the carotid intimamedia thickness, and the central nervous system development. ABPM also appears to be useful in detecting masked HTN in high risk population including dialysis and renal transplant patients, and in predicting development of microalbuminuria, an early sign of diabetic nephropathy. Lastly, similar to adults, the relationship between WCH and target organ damage is unclear at present time, and target organ damages in masked HTN patients appear to be indistinguishable from those of sustained HTN, both in otherwise healthy and in high risk populations.
3.7. Current Clinical Indications in Pediatrics
The main use of ABPM is to differentiate between sustained HTN and WCH in patients who have elevated casual BP measurements and to detect masked HTN in high risk patients. ABPM is most useful in patients with casual BP within 20% of the 95th percentile for age, gender, and height since patients with casual BP greater than 20% above the 95th percentile are more likely to have sustained HTN and not WCH [64]. Table 4 summarizes the current clinical indications for use of ABPM. ABPM is also useful in follow-up evaluations in determining effectiveness of antihypertensive therapy, evaluation of resistant HTN, and assessment of dipping status in high risk patients such as DM.
3.8. Clinical Trial Uses in Pediatrics
As illustrated in the previous section, pediatric ABPM clinical research data have been increasing in the recent years. In clinical trials, potential benefits of ABPM include confirmation of HTN diagnosis prior to enrollment and exclusion, elimination of observer bias, reduction of measurement variability between trial centers, reduction of placebo effect, and perhaps most importantly, reducetion of sample size. As another sub-study of ESCAPE trial, Gimpel et al. showed that standard deviation of ABPM responses were up to 39% smaller than those of casual BP measured responses. Using power analysis, they showed that depending on the magnitude of the aimed BP reduction, sample size can be reduced by 57% - 75% with the use of ABPM [65]. Many pediatric studies are often limited by difficulty in enrolling large number of patients, and not only ABPM will help to overcome this limitation, but it will also reduce the number of patients who would receive placebo medication in blinded placebo-controlled drug trials.
4. Current Needs and Future Directions
Even with increasing pediatric ABPM data, there is a sufficient need for further clinical research in many areas. First and foremost is the necessity for normative data based on larger population that include nonwhite populations [9]. Second is a further analysis to correlate ABPM results with well-defined or intermediate end points for sustained hypertensive patients and those with WCH, which should help to define more evidence-based normal values. Related to this analysis, there is a need to evaluate efficacy of therapy with ABPM and reversal of target organ damage [9]. Third is to develop standardized protocols appropriate for validation of monitors specific for pediatric patients [9]. Fourth is pediatric-specific cost effective analysis. A number of adult studies have suggested significant cost savings with use of ABPM, but there has only been one notable pediatric cost effective analysis of ABPM use in the initial BP evaluation. In this study, Swartz et al. projected saving of $2.4 million per 1000 patients with use of ABPM [66]. The largest cost difference between evaluations based on casual BP measurement versus ABPM in this analysis was the echocardiogram obtained in patients determined to have sustained HTN solely by casual BP. However, if WCH also becomes an indication for target organ damage investigation, as suggested by some of the studies discussed above, further revised cost analysis is needed. Fifth is the use of ABPM in pediatric non-renal transplant patients to detect masked HTN in this high risk group. There is a number of pediatric ABPM reports in renal transplant patients [56,67,68], but data in cardiac [69-71], liver [72], small bowel, lung, and hematopoietic cell transplantations are scarce. Finally, there is a concern that undergoing ABPM itself can produce abnormal nocturnal dipping. An adult study with veterans with chronic kidney disease showed that among the patients with most amount of sleep, wearing ABPM monitor was associated with less time spent in bed at night, less sleeping hours during those hours, reduced sleep efficiency, and more sedentary during waking hours around ABPM monitoring. Further analysis showed that waking after sleep onset more than the median time was associated with greater odds for non-dipping (OR = 10.5, p = 0.008) [73]. Thus, a pediatric study with similar design would be intriguing.
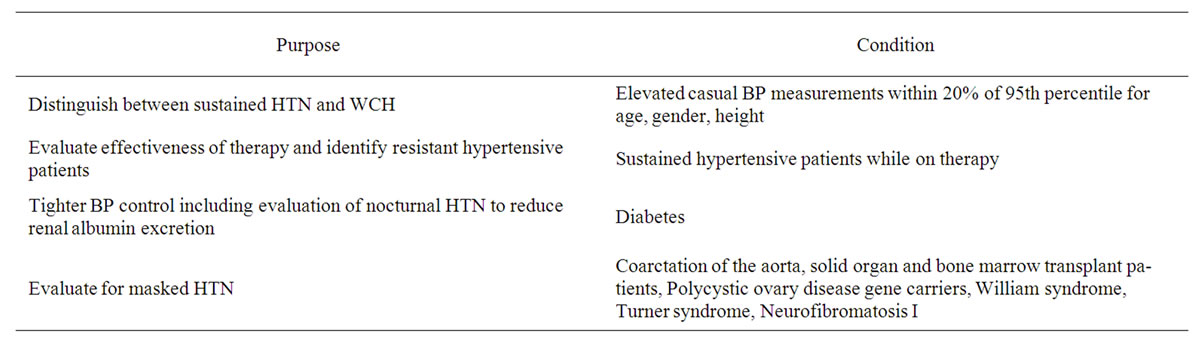
Table 4. Clinical uses of ABPM in pediatrics.
Lastly, the roles of casual BP, home BP, and ABPM will need to be better defined in evaluation of elevated BP. While American Heart Association ABPM consensus statement suggests superiority of ABPM over home BP measurements [9], others suggest that home BP measurements and ABPM can be interchangeable [21,25,74]. Thus, further studies are needed in pediatric patients to clarify who, when, and in which clinical settings, ABPM versus home BP measurements, are appropriate.
5. Conclusions
Since the initial report on blood pressure in children and adolescent was released in 1977, the understanding of BP evaluation and management in pediatrics has evolved extensively, including proper diagnosis of sustained HTN versus WCH and detection of masked HTN. Parallel to adults, recent pediatric ABPM studies are starting to uncover the clinical significance of HTN, especially with target organ damage both in otherwise healthy hypertensive patients and high risk patients. ABPM also has many future needs, which also means ample opportunities for many future clinical trials. These trials will hopefully help to further clarify the proper indication and role of ABPM in elevated BP evaluation and HTN management in children and adolescents.
6. Key Points
1) Inaccurate diagnosis of HTN occur due to WCH and masked HTN even with proper BP measurement techniques and following the Fourth report HTN diagnosis criteria.
2) ABPM is a tool to aid in proper BP evaluation for children and adolescents aged 5 years and older, especially to differentiate between sustained HTN and WCH in patients who have elevated casual BP measurements and to detect masked HTN in high risk patients. ABPM is most useful in patients with casual BP within 20% of the 95th percentile for age, gender, and height.
3) Unlike adults, pediatric ABPM data have not been correlated with patient outcome data. However, pediatric ABPM data have shown abnormal ABPM to correlate with hypertensive target organ damage including LVH, increased carotid intima-media thickeness, and abnormal central nervous system development. ABPM also appears to be useful in detecting masked HTN in high risk population and in predicting development of diabetic nephropathy.
4) ABPM is a promising tool in pediatric BP clinical trials including antihypertensive drug trials, and there are many future clinical research needs and opportunities.
REFERENCES
- M. J. Stewart and P. L. Padfield, “Measurement of Blood Pressure in the Technological Age,” British Medical Bulletin, Vol. 50, No. 2, 1994, pp. 420-442.
- R. J. Portman and R. J. Yetman, “Clinical Uses of Ambulatory Blood Pressure Monitoring,” Pediatric Nephrology, Vol. 8, No. 3, 1994, pp. 367-376. doi:10.1007/BF00866367
- T. Mengden, M. R. M. Hernandez, B. Beltran, E. Alvarez, K. Kraft and H. Vetter, “Reliability of Reporting SelfMeasured Blood Pressure Values by Hypertensive Patients,” American Journal of Hypertension, Vol. 11, No. 12, 1998, pp. 1413-1417. doi:10.1016/S0895-7061(98)00241-6
- T. G. Wells, W. A. Neaville, J. R. Arnold and C. W. Belsha, “Evaluation of Home Blood Pressure Monitors in Children and Adolescents,” American Journal of the Medical Sciences, Vol. 315, No. 2, 1998, pp. 110-117. doi:10.1097/00000441-199802000-00007
- American Academy of Pediatrics, “The 4th Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents,” Pediatrics, Vol. 114, No. 2, 2004, pp. 555-576. doi:10.1542/peds.114.2.S2.555
- J. T. Flynn, “Impact of Ambulatory Blood Pressure Monitoring on the Management of Hypertension in Children,” Blood Pressure Monitor, Vol. 5, No. 4, 2000, pp. 211-216. doi:10.1097/00126097-200008000-00003
- L. Butani and B. Z. Morgenstern, “Are Pitfalls of Oxcillometric Blood Pressure Measurements Preventable in Children?” Pediatric Nephrology, Vol. 18, No. 4, 2003, pp. 313-318.
- M. K. Park, S. W. Menard and C. Yuan, “Comparison of Auscultatory and Oscillometric Blood Pressures,” Archives of Pediatrics & Adolescent Medicine, Vol. 155, No. 1, 2001, pp. 50-53.
- E. Urbina, B. Alpert, J. Flynn, L. Hayman, G. A. Harsh- -field, M. Jacobson, et al., “Ambulatory Blood Pressure Monitoring in Children and Adolescents: Recommendations for Standard Assessment: A Scientific Statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young and the Council for High Blood Pressure Research,” Hypertension, Vol. 52, No. 3, 2008, pp. 433-451. doi:10.1161/HYPERTENSIONAHA.108.190329
- M. Soergel, M. Kirschstein, C. Busch, T. Danne, J. Gellermann, R. Holl, et al., “Oscillometric Twenty-Four-Hour Ambulatory Blood Pressure Values in Healthy Children and Adolescents: A Multicenter Trial Including 1141 Subjects,” Journal of Pediatrics, Vol. 130, No. 2, 1997, pp. 178-184. doi:10.1016/S0022-3476(97)70340-8
- E. Wuhl, K. Witte, M. Soergel, O. Mehls and F. Schaefer, “Distribution of 24-h Ambulatory Blood Pressure in Children: Normalized Reference Values and Role of Body Dimensions,” Journal of Hypertension, Vol. 20, No. 10, 2002, pp. 1995-2007. doi:10.1097/00004872-200210000-00019
- D. K. Wilson, D. A. Sica and S. B. Miller, “Ambulatory Blood Pressure Nondipping Status in Salt-Sensitive and Salt-Resistant Black Adolescents,” American Journal of Hypertension, Vol. 12, No. 2, 1999, pp. 159-165. doi:10.1016/S0895-7061(98)00234-9
- G. A. Harshfield, P. Barbeau, P. A. Richey and B. S. Alpert, “Racial Differences in the Influence of Body Size on Ambulatory Blood Pressure in Youths,” Blood Pressure Monitor, Vol. 5, No. 2, 2000, pp. 59-63.
- S. Koshy, C. Macarthur, S. Luthra, M. Gajaria and D. Geary, “Ambulatory Blood Pressure Monitoring: Mean Blood Pressure and Blood Pressure Load,” Pediatric Nephrology, Vol. 20, No. 10, 2005, pp. 1484-1486. doi:10.1007/s00467-005-2014-6
- R. Lubrano, E. Travasso, C. Raggi, G. Guido, R. Masciangelo and M. Elli, “Blood Pressure Load, Proteinuria and Renal Function in Pre-Hypertensive Children,” Pediatric Nephrology, Vol. 24, No. 4, 2009, pp. 823-831. doi:10.1007/s00467-008-1077-6
- E. Lurbe, J. M. Sorof and S. R. Daniels, “Clinical and Research Aspects of Ambulatory Blood Pressure Monitoring in Children,” Journal of Pediatrics, Vol. 144, No. 1, 2004, pp. 7-16. doi:10.1016/j.jpeds.2003.09.050
- J. M. Sorof, G. Cardwell, K. Franco and R. J. Portman, “Ambulatory Blood Pressure and Left Ventricular Mass Index in Hypertensive Children,” Hypertension, Vol. 39, No. 4, 2002, pp. 903-908. doi:10.1161/01.HYP.0000013266.40320.3B
- G. S. Stergiou, N. J. Yiannes, V. C. Rarra and C. V. Alamara, “White-Coat Hypertension and Masked Hypertension in Children,” Blood Pressure Monitor, Vol. 10, No. 6, 2005, pp. 297-300. doi:10.1097/00126097-200512000-00002
- J. M. Sorof, T. Poffenbarger, K. Franco and R. Portman, “Evaluation of White Coat Hypertension in Children: Importance of the Definitions of Normal Ambulatory Blood Pressure and the Severity of Casual Hypertension,” American Journal of Hypertension, Vol. 14, No. 9, 2001, pp. 855-860. doi:10.1016/S0895-7061(01)02180-X
- E. Lurbe, I. Torro, V. Alvarez, T. Nawrot, R. Paya, J. Redon, et al., “Prevalence, Persistence, and Clinical Significance of Masked Hypertension in Youth,” Hypertension, Vol. 45, No. 4, 2005, pp. 493-548. doi:10.1161/01.HYP.0000160320.39303.ab
- G. S. Stergiou, V. C. Rarra and N. G. Yiannes, “Prevalence and Predictors of Masked Hypertension Detected by Home Blood Pressure Monitoring in Children and Adolescents: The Arsakeion School Study,” American Journal of Hypertension, Vol. 22, No. 5, 2009, pp. 520-524. doi:10.1038/ajh.2009.34
- S. Stabouli, V. Kotsis, S. Toumanidis, C. Papamichael, A. Constantopoulos and N. Zakopoulos, “White-Coat and Masked Hypertension in Children: Association with TargetOrgan Damage,” Pediatric Nephrology, Vol. 20, No. 8, 2005, pp. 1151-1155. doi:10.1007/s00467-005-1979-5
- S. Matsuoka and M. Awazu, “Masked Hypertension in Children and Young Adults,” Pediatric Nephrology, Vol. 19, No. 6, 2004, pp. 651-654. doi:10.1007/s00467-004-1459-3
- K. L. McNiece, M. Gupta-Malhotra, J. Samuels, C. Bell, K. Garcia, T. Poffenbarger, et al., “Left Ventricular Hypertrophy in Hypertensive Adolescents: Analysis of Risk by 2004 National High Blood Pressure Education Program Working Group Staging Criteria,” Hypertension, Vol. 50, No. 2, 2007, pp. 392-395. doi:10.1161/HYPERTENSIONAHA.107.092197
- G. Stergiou, A. Vazeou and C. Stefanidis, “American Heart Association’s Statement That ‘In Children Ambulatory Blood Pressure is Superior to Home’ Not Proven,” Hypertension, Vol. 52, No. 6, 2008, p. e145. doi:10.1161/HYPERTENSIONAHA.108.122309
- E. Wuhl, C. Hadtstein, O. Mehls and F. Schaefer, “Home, Clinic, and Ambulatory Blood Pressure Monitoring in Children with Chronic Renal Failure,” Pediatric Research, Vol. 55, No. 3, 2004, pp. 492-497. doi:10.1203/01.PDR.0000106863.90996.76
- J. A. Staessen, L. Beilin, G. Parati, B. Waeber and W. White, “Task Force IV: Clinical Use of Ambulatory Blood Pressure Monitoring. Participants of the 1999 Consensus Conference on Ambulatory Blood Pressure Monitoring,” Blood Pressure Monitor, Vol. 4, No. 6, 1999, pp. 319-331. doi:10.1097/00126097-199900460-00005
- W. B. White, “Ambulatory Blood-Pressure Monitoring in Clinical Practice,” New England Journal of Medicine, Vol. 348, No. 24, 2003, pp. 2377-2378. doi:10.1056/NEJMp030057
- D. L. Clement, M. L. De Buyzere, D. A. De Bacquer, P. W. de Leeuw, D. A. Duprez, R. H. Fagard, et al., “Prognostic Value of Ambulatory Blood-Pressure Recordings in Patients with Treated Hypertension,” New England Journal of Medicine, Vol. 348, No. 24, 2003, pp. 2407-2415. doi:10.1056/NEJMoa022273
- T. Ohkubo, A. Hozawa, K. Nagai, M. Kikuya, I. Tsuji, S. Ito, et al., “Prediction of Stroke by Ambulatory Blood Pressure Monitoring versus Screening Blood Pressure Measurements in a General Population: The Ohasama Study,” Journal of Hypertension, Vol. 18, No. 7, 2000, pp. 847-854. doi:10.1097/00004872-200018070-00005
- P. Verdecchia, C. Porcellati, G. Schillaci, C. Borgioni, A. Ciucci, M. Battistelli, et al., “Ambulatory Blood Pressure. An Independent Predictor of Prognosis in Essential Hypertension,” Hypertension, Vol. 24, No. 6, 1994, pp. 793- 801.
- M. J. Koren, R. B. Devereux, P. N. Casale, D. D. Savage and J. H. Laragh, “Relation of Left Ventricular Mass and Geometry to Morbidity and Mortality in Uncomplicated Essential Hypertension,” Annals of Internal Medicine, Vol. 114, No. 5, 1991, pp. 345-352.
- G. de Simone, R. B. Devereux, S. R. Daniels, M. J. Koren, R. A. Meyer and J. H. Laragh, “Effect of Growth on Variability of Left Ventricular Mass: Assessment of Allometric Signals in Adults and Children and Their Capacity to Predict Cardiovascular Risk,” Journal of the American College of Cardiology, Vol. 25, No. 5, 1995, pp. 1056-1062. doi:10.1016/0735-1097(94)00540-7
- D. Levy, R. J. Garrison, D. D. Savage, W. B. Kannel, W. P. Castelli, “Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study,” New England Journal of Medicine, Vol. 322, No. 22, 1990, pp. 1561-1566. doi:10.1056/NEJM199005313222203
- D. H. O’Leary, J. F. Polak, R. A. Kronmal, T. A. Manolio, G. L. Burke and S. K. Wolfson Jr., “Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. Cardiovascular Health Study Collaborative Research Group,” New England Journal of Medicine, Vol. 340, No. 1, 1999, pp. 14-22. doi:10.1056/NEJM199901073400103
- T. W. Kamarck, D. E. Polk, K. Sutton-Tyrrell, M. F. Muldoon, “The Incremental Value of Ambulatory Blood Pressure Persists after Controlling for Methodological Confounds: Associations with Carotid Atherosclerosis in a Healthy Sample,” Journal of Hypertension, Vol. 20, No. 8, 2002, pp. 1535-1541. doi:10.1097/00004872-200208000-00016
- P. Verdecchia, F. Angeli, R. Gattobigio, C. Borgioni, C. Castellani, M. Sardone, et al., “The Clinical Significance of White-Coat and Masked Hypertension,” Blood Pressure Monitor, Vol. 12, No. 6, 2007, pp. 387-389. doi:10.1097/MBP.0b013e32824958e5
- F. Angeli, P. Verdecchia, R. Gattobigio, M. Sardone and G. Reboldi, “White-Coat Hypertension in Adults,” Blood Pressure Monitor, Vol. 10, No. 6, 2005, pp. 301-305. doi:10.1097/00126097-200512000-00003
- P. Verdecchia, G. P. Reboldi, F. Angeli, G. Schillaci, J. E. Schwartz, T. G. Pickering, et al., “Shortand Long-Term Incidence of Stroke in White-Coat Hypertension,” Hypertension, Vol. 45, No. 2, 2005, pp. 203-208. doi:10.1161/01.HYP.0000151623.49780.89
- L. R. Krakoff, “Cost-Effectiveness of Ambulatory Blood Pressure: A Reanalysis,” Hypertension, Vol. 47, No. 1, 2006, pp. 29-34. doi:10.1161/01.HYP.0000197195.84725.66
- E. Lurbe, B. Cremades, C. Rodriguez, M. I. Torro, V. Alvarez and J. Redon, “Factors Related to Quality of Ambulatory Blood Pressure Monitoring in a Pediatric Population,” American Journal of Hypertension, Vol. 12, No. 9, 1999, pp. 929-933. doi:10.1016/S0895-7061(99)00076-X
- I. A. Khan, M. Gajaria, D. Stephens and J. W. Balfe, “Ambulatory Blood Pressure Monitoring in Children: A Large Center’s Experience,” Pediatric Nephrology, Vol. 14, No. 8-9, 2000, pp. 802-805. doi:10.1007/s004679900291
- J. M. Sorof, A. V. Alexandrov, G. Cardwell and R. J. Portman, “Carotid Artery Intimal-Medial Thickness and Left Ventricular Hypertrophy in Children with Elevated Blood Pressure,” Pediatrics, Vol. 111, No. 1, 2003, pp. 61-66. doi:10.1542/peds.111.1.61
- S. R. Daniels, J. M. Loggie, P. Khoury and T. R. Kimball, “Left Ventricular Geometry and Severe Left Ventricular Hypertrophy in Children and Adolescents with Essential Hypertension,” Circulation, Vol. 97, No. 19, 1998, pp. 1907-1911.
- C. Hanevold, J. Waller, S. Daniels, R. Portman and J. Sorof, “The Effects of Obesity, Gender, and Ethnic Group on Left Ventricular Hypertrophy and Geometry in Hypertensive Children: A Collaborative Study of the International Pediatric Hypertension Association,” Pediatrics, Vol. 113, No. 2, 2004, pp. 328-333. doi:10.1542/peds.113.2.328
- J. M. Sorof, J. Turner, D. S. Martin, K. Garcia, Z. Garami, A. V. Alexandrov, et al., “Cardiovascular Risk Factors and Sequelae in Hypertensive Children Identified by Referral versus School-Based Screening,” Hypertension, Vol. 43, No. 2, 2004, pp. 214-218. doi:10.1161/01.HYP.0000114696.96318.4e
- V. Kotsis, S. Stabouli, V. Pitiriga, C. Papamichael, S. Toumanidis and N. Zakopoulos, “Impact of Gender on 24-h Ambulatory Blood Pressure and Target Organ Damage,” Journal of Human Hypertension, Vol. 20, No. 9, 2006, pp. 658-665. doi:10.1038/sj.jhh.1002047
- V. Kotsis, S. Stabouli, V. Pitiriga, S. Toumanidis, C. Papamichael and N. Zakopoulos, “Ambulatory Blood Pressure Monitoring and Target Organ Damage: Effects of Age and Sex,” Blood Pressure Monitor, Vol. 11, No. 1, 2006, pp. 9-15. doi:10.1097/01.mbp.0000189785.59994.20
- M. Litwin, A. Niemirska, J. Sladowska, J. Antoniewicz, J. Daszkowska, A. Wierzbicka, et al., “Left Ventricular Hypertrophy and Arterial Wall Thickening in Children with Essential Hypertension,” Pediatric Nephrology, Vol. 21, No. 6, 2006, pp. 811-819. doi:10.1007/s00467-006-0068-8
- C. W. Belsha, T. G. Wells, K. L. McNiece, P. M. Seib, J. K. Plummer and P. L. Berry, “Influence of Diurnal Blood Pressure Variations on Target Organ Abnormalities in Adolescents with Mild Essential Hypertension,” American Journal of Hypertension, Vol. 11, No. 4, 1998, pp. 410-417. doi:10.1016/S0895-7061(98)00014-4
- S. Stabouli, V. Kotsis, C. Papamichael, A. Constantopoulos and N. Zakopoulos, “Adolescent Obesity is Associated with High Ambulatory Blood Pressure and Increased Carotid Intimal-Medial Thickness,” Journal of Pediatrics, Vol. 147, No. 5, 2005, pp. 651-656. doi:10.1016/j.jpeds.2005.06.008
- M. B. Lande, N. L. Carson, J. Roy and C. C. Meagher, “Effects of Childhood Primary Hypertension on Carotid Intima Media Thickness: A Matched Controlled Study,” Hypertension, Vol. 48, No. 1, 2006, pp. 40-44. doi:10.1161/01.HYP.0000227029.10536.e8
- M. B. Lande, H. Adams, B. Falkner, S. R. Waldstein, G. J. Schwartz, P. G. Szilagyi, et al., “Parental Assessments of Internalizing and Externalizing Behavior and Executive Function in Children with Primary Hypertension,” Journal of Pediatrics, Vol. 154, No. 2, 2009, pp. 207-212. doi:10.1016/j.jpeds.2008.08.017
- M. B. Lande, H. Adams, B. Falkner, S. R. Waldstein, G. J. Schwartz, P. G. Szilagyi, et al., “Parental Assessment of Executive Function and Internalizing and Externalizing Behavior in Primary Hypertension after Anti-Hypertensive Therapy,” Journal of Pediatrics, Vol. 157, No. 1, 2010, pp. 114-119. doi:10.1016/j.jpeds.2009.12.053
- H. R. Adams, P. G. Szilagyi, L. Gebhardt and M. B. Lande, “Learning and Attention Problems among Children with Pediatric Primary Hypertension,” Pediatrics, Vol. 126, No. 6, 2010, pp. e1425-e1429. doi:10.1542/peds.2010-1899
- D. Paripovic, M. Kostic, B. Spasojevic, D. Kruscic and A. Pecoantic, “Masked Hypertension and Hidden Uncontrolled Hypertension after Renal Transplantation,” Pediatric Nephrology, Vol. 25, No. 9, 2010, pp. 1719-1724. doi:10.1007/s00467-010-1552-8
- Z. Bircan, A. Duzova, N. Cakar, A. K. Bayazit, A. Elhan, E. Tutar, et al., “Predictors of Left Ventricular Hypertrophy in Children on Chronic Peritoneal Dialysis,” Pediatric Nephrology, Vol. 25, No. 7, 2010, pp. 1311-1318. doi:10.1007/s00467-010-1481-6
- E. Lurbe, J. Redon, J. M. Pascual, J. Tacons and V. Alvarez, “The Spectrum of Circadian Blood Pressure Changes in Type I Diabetic Patients,” Journal of Hypertension, Vol. 19, No. 8, 2001, pp. 1421-1428. doi:10.1097/00004872-200108000-00010
- E. Lurbe, J. Redon, A. Kesani, J. M. Pascual, J. Tacons, V. Alvarez, et al., “Increase in Nocturnal Blood Pressure and Progression to Microalbuminuria in Type 1 Diabetes,” New England Journal of Medicine, Vol. 347, No. 11, 2002, pp. 797-805. doi:10.1056/NEJMoa013410
- [61] L. M. Ettinger, K. Freeman, J. R. DiMartino-Nardi and J. T. Flynn, “Microalbuminuria and Abnormal Ambulatory Blood Pressure in Adolescents with Type 2 Diabetes Mellitus,” Journal of Pediatrics, Vol. 147, No. 1, 2005, pp. 67-73. doi:10.1016/j.jpeds.2005.02.003
- [62] D. Pall, M. Juhasz, S. Lengyel, C. Molnar, G. Paragh, B. Fulesdi, et al., “Assessment of Target-Organ Damage in Adolescent White-Coat and Sustained Hypertensives,” Journal of Hypertension, Vol. 28, No. 10, 2010, pp. 2139- 2144.
- [63] R. E. Kavey, D. A. Kveselis, N. Atallah and F. C. Smith, “White Coat Hypertension in Childhood: Evidence for End-Organ Effect,” Journal of Pediatrics, Vol. 150, No. 5, 2007, pp. 491-497. doi:10.1016/j.jpeds.2007.01.033
- [64] M. Mitsnefes, J. Flynn, S. Cohn, J. Samuels, T. Blydt-Hansen, J. Saland, et al., “Masked Hypertension Associates with Left Ventricular Hypertrophy in Children with CKD,” Journal of the American Society of Nephrology, Vol. 21, No. 1, 2010, pp. 137-144. doi:10.1681/ASN.2009060609
- [65] J. W. Graves and M. M. Althaf, “Utility of Ambulatory Blood Pressure Monitoring in Children and Adolescents,” Pediatric Nephrology, Vol. 21, No. 11, 2006, pp. 1640-1652. doi:10.1007/s00467-006-0175-6
- [66] C. Gimpel, E. Wuhl, K. Arbeiter, D. Drozdz, A. Trivelli, M. Charbit, et al., “Superior Consistency of Ambulatory Blood Pressure Monitoring in Children: Implications for Clinical Trials,” Journal of Hypertension, Vol. 27, No. 8, 2009, pp. 1568-1574. doi:10.1097/HJH.0b013e32832cb2a8
- [67] S. J. Swartz, P. R. Srivaths, B. Croix and D. I. Feig, “CostEffecttiveness of Ambulatory Blood Pressure Monitoring in the Initial Evaluation of Hypertension in Children,” Pediatrics, Vol. 122, No. 6, 2008, pp. 1177-1181. doi:10.1542/peds.2007-3432
- [68] C. B. Sethna, M. B. Leonard, P. R. Gallagher and K. E. Meyers, “Serum Adiponectin Levels and Ambulatory Blood Pressure Monitoring in Pediatric Renal Transplant Recipients,” Transplantation, Vol. 88, No. 8, 2009, pp. 1030-1037. doi:10.1097/TP.0b013e3181b9e1ec
- [69] J. R. Ferraris, “ABPM vs Office Blood Pressure to Define Blood Pressure Control in Treated Hypertensive Paediatric Renal Transplant Recipients,” Pediatric Transplantation, Vol. 11, No. 1, 2007, pp. 24-30. doi:10.1111/j.1399-3046.2006.00595.x
- [70] J. J. O’Sullivan, G. Derrick and J. Gray, “Blood Pressure after Cardiac Transplantation in Childhood,” Journal of Heart and Lung Transplantation, Vol. 24, No. 7, 2005, pp. 891-895. doi:10.1016/j.healun.2004.05.025
- [71] S. L. Roche, J. Kaufmann, A. I. Dipchand and P. F. Kantor, “Hypertension after Pediatric Heart Transplantation Is Primarily Associated with Immunosuppressive Regimen,” Journal of Heart and Lung Transplantation, Vol. 27, No. 5, 2008, pp. 501-507. doi:10.1016/j.healun.2008.01.018
- [72] S. L. Roche, J. J. O’Sullivan and P. F. Kantor, “Hypertension after Pediatric Cardiac Transplantation: Detection, Etiology, Implications and Management,” Pediatr Transplant, Vol. 14, No. 2, 2010, pp. 159-168. doi:10.1111/j.1399-3046.2009.01205.x
- [73] M. E. Del Compare, D. D’Agostino, J. R. Ferraris, G. Boldrini, G. Waisman and R. T. Krmar, “Twenty-FourHour Ambulatory Blood Pressure Profiles in Liver Transplant Recipients,” Pediatr Transplant, Vol. 8, No. 5, 2004, pp. 496-501. doi:10.1111/j.1399-3046.2004.00192.x
- [74] R. Agarwal and R. P. Light, “The Effect of Measuring Ambulatory Blood Pressure on Nighttime Sleep and Daytime Activity-Implications for Dipping,” Journal of the American Society of Nephrology, Vol. 5, No. 2, 2010, pp. 281- 285. doi:10.2215/CJN.07011009

