 American Journal of Plant Sciences, 2012, 3, 1646-1653 http://dx.doi.org/10.4236/ajps.2012.311200 Published Online November 2012 (http://www.SciRP.org/journal/ajps) Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo Eros Vasil Kharshiing Department of Botany, St. Edmund’s College, Shillong, India. Email: eros.kharshiing@gmail.com Received August 9th, 2012; revised September 12th, 2012; accepted October 15th, 2012 ABSTRACT Bioactive compounds derived from plant natural compounds have proven to be valuable sources of metabolites which can seldom be obtained from other sources. Plants belonging to the genus Zanthoxylum have been valued across various cultures for their curative properties. Zanthoxylum armatum DC., belonging to the family Rutaceae is extensively used in traditional practices in North-Eastern India and neighbouring regions including South-East Asia. However, the poten- tial cytogenetic effects of Zanthoxylum armatum under in vivo conditions, and their causative mechanisms have not yet been studied in detail. The current study was undertaken to evaluate the cytotoxic and genotoxic potential of aqueous extracts of fruits of Z. armatum under in vivo conditions using the Allium test. Physiological and cellular data indicate that the extracts induce clumped chromosomes at metaphase stage of cell division coupled with mitotic arrest. Electron microscopy data reveal membrane damage of cellular organelles, chromatin condensation and chromatin marginalisa- tion in cell of roots incubated in the extracts. The extracts also induce concentration dependent protein precipitation and genomic DNA degradation. Keywords: Clumped Chromosomes; Chromatin Condensation and Marginalisation; TEM; Toxicity; Zanthoxylum armatum 1. Introduction Plant derived natural compounds such as quinine, mor- phine, digitoxin, artemisinin and various others have made important contributions to modern civilisation. Advances in natural products research have helped in developing leads towards the identification and isolation of com- pounds having significant biological activity [1]. Bioac- tive secondary metabolites which are produced by plants for their own defense have proven to be valuable sources of new drugs some of which cannot be obtained from other sources [2-4]. Research in plants therefore repre- sents an invaluable source for discovering new sub- stances, considering that most plants can contain a large number of secondary metabolites. From approximately more than 300,000 plant species reported, only a small percentage has been the subject of phytochemical and biological activity studies [5]. Plants belonging to the genus Zanthoxylum have been reported to harbour medicinal properties which justifies their use in traditional medicines worldwide [6,7]. Many species have been used in different parts of the world especially in Asia, Africa and America to treat a number of diseases in humans and animals [8-11]. Extracts of Z. nitidum exhibit antibacterial activity in vitro, as well as antioxidative properties [12,13]. Aqueous extracts of Z. macrophylla roots have been reported to show anti-sick- ling effects in human erythrocytes [14]. Extracts of Z. piperitum show scavenging activities towards active oxygen species [15]. Crude extracts of Z. acanthopodium and Z. alainthoides are reported to possess antioxidative properties [16,17]. Z. armatum contains numerous che- mical constituents reported to exhibit several biological activities [18,19]. The genus Zan thoxylum is also re- ported to harbour several metabolites that exhibit anti- proliferative activity [20,21]. While the putative curative properties of medicinal plants are widely reported, there are also reports of the in vitro toxicity effects of plant extracts utilised in traditional medicines [22-24]. In vitro assays indicate that extracts of such plants induce sig- nificant damage on cellular and nuclear material. Spiri- donov et al., [25] reported acute in vitro cytotoxicity of Potentilla erecta extracts, which is used for alleviating symptoms of disease in cancer patients. The extracts of aerial parts of Rumex acetosa used in traditional Korean and Japanese medicine is also reported to show toxicity Copyright © 2012 SciRes. AJPS  Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo 1647 in vitro [26]. Similar reports are also available for Plan- tago sp used in traditional Spanish medicine [27]. Plants extracts used in herbal remedies are also reported to show cytotoxic potential when used either singly or as concoctions with extracts of other plants [22]. Zanthoxylum armatum DC. (syn. Z. alatum Roxb.) belonging to the family Rutaceae is extensively used in traditional practices of the Khasi tribe in North-Eastern India and in neighbouring regions including South-East Asia. According to reports of ethnobotanical properties of the Zanthoxylum genus, the most commonly used ex- traction methods use mainly water as a solvent. Herbal formulations prepared from various parts of the plant are reported to be anti-helminthic and hypoglycaemic, and are used as treatment of cholera, tonic for fever, remedy for skin diseases, diseases of the mouth and teeth and others [28,29]. However based on currently available data, the potential cytogenetic effects of Z. armatum un- der in vivo conditions, and their causative mechanisms have not yet been studied in detail. The current study was undertaken to evaluate the cytotoxic and genotoxic po- tential of aqueous extracts of Z. armatum DC in an in vivo experimental system and gain some insight into the mechanism(s) by which these extracts exert their toxic potential. 2. Materials and Methods 2.1. Plant Material and Growth Conditions The plant specimen used in this study was collected from Shillong, Meghalaya and identified with the help of Dr. P. B. Gurung, Herbaria curator, Department of Botany, North Eastern Hill University, Meghalaya. A voucher specimen was deposited in the herbaria of the Depart- ment of Botany, St. Edmund’s College and assigned a voucher number SEC/BOT/EK/101 dated 01/07/2010. For the Allium test, onion bulbs of approximately 45 cm in diameter were incubated in double distilled water in cylindrical opaque plastic vials (25 mm × 50 mm) for 48 hr with water being changed every 24 hr. After 48 hr the water was substituted with the appropriate solutions and incubated for another 24 hr after which roots of equal length were harvested for analyses. The light con- ditions for growth were maintained at 14 hr light and 10 hrs dark cycles. Light (200 µmol·m−2·s−1) was supplied via cool white fluorescent tubes. Temperature was main- tained at 25˚C ± 1˚C respectively throughout the period of incubation. For each experiment a minimum of 5 bulbs were used. 2.2. Preparation of Aqueous Extract Fruits of locally growing Zanthoxylum armatum DC., were collected, washed and air dried in the shade until constant weight was obtained. The dried fruits were then ground to a coarse powder in a grinder at short bursts of 30 sec each. 5 gm of powdered material was soaked in 100 mL double distilled water and incubated in the dark for 48 hr at 25˚C ± 1˚C with frequent agitation. After 48 hr the extract was filtered through Whatmann filter paper No 1 and immediately used. Drying of the aqueous ex- tract in a rotary vacuum evaporator at 40˚C resulted in sticky brownish slurry, which could not be used further. Therefore, the fresh aqueous extracts, hereon referred to as Aqueous Extract of Dried Fruits (AEDF), were quan- tified as total phenolic content [30] and used directly for further experiments. 2.3. Determination of Total Phenolic Content Total phenolic content of AEDF was estimated according to Makkar et al., [30] with slight modifications. Fifty micro litre (50 µl) of AEDF extract for each sample was taken in a test tube and the volume was made to 1.0 ml with double distilled water. Then, 0.5 ml of 1 N Folin Ciocalteu reagent was added and mixed thoroughly after which 2.5 ml of 0.7 M Na2CO3·H2O solution was added. The resulting solution was mixed thoroughly and incu- bated for 40 minutes at 25˚C ± 1˚C. Absorbance was measured spectrophotometrically at 725 nm and total phenolic content was estimated as tannic acid equivalent and expressed as mg/ml. Tannic acid was used for gener- ating the standard curve. 2.4. Root Growth Measurement and Mitotic Squash Preparations Root lengths were measured at 0 hr, 24 hr, 48 hr and 72 hr after incubation. The initial incubation in double dis- tilled water was considered as 0 (zero) hr for all experi- ments. Five of the longest roots per bulb were considered. All measurements are represented as the mean ± S.D. of 5 roots per bulb with a minimum of 5 bulbs per experi- ment. Root squash preparations were prepared for analyses of mitotic cells. At 72 hr of incubation, 5 - 10 of the longest roots from each bulb were harvested at a distance of 5 mm from the root tip. The roots were immediately boiled in 2% (w/v) aceto-orcein. From each root a seg- ment of 1 mm from the root tip was dissected out. This was used for squash preparation for analyses of mitotic cells. For each analysis a minimum of 1000 cells were examined per root tip with a minimum of 5 roots per bulb. Images of cells were taken with a microscope (Weswox TRHL-66A) fitted with a digital camera (Sony, Japan). 2.5. Transmission Electron Microscope Studies After incubation for 24 hr in AEDF, the roots were har- Copyright © 2012 SciRes. AJPS  Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo 1648 vested for TEM studies. Root tips of approximately 3 mm in length, measured from the tip of roots incubated in AEDF and the corresponding regions of the control roots were fixed in Karnovsky’s fixative for 1.5 hr. The fixed roots were then washed thrice by immersion in 0.1 M cacodylate buffer for 15 min. The fixed roots were further processed and examined using 100 CXII JEOL transmission electron microscope at the Sophisticated Analytical Instrumentation Facility, North-Eastern Hill University, Shillong as described earlier by Choudhury and Sharan [31]. A minimum of 3 roots each from 5 dif- ferent bulbs were used. Bulbs grown only in double dis- tilled water were taken as negative controls. 2.6. Protein Precipitation Studies Bovine serum albumin (Fraction V) diluted in double distilled water to a final concentration of 1 mg/mL was taken in different tubes. Increasing amounts of AEDF quantified as total phenolic content were added into each tube, mixed thoroughly and centrifuged at 2000 g for 10 minutes at room temperature. From each tube equal volumes of solution were taken for total proten quantifi- cation and the remaining solutions were incubated for 24 hr at 25˚C ± 1˚C in the dark. After incubation, the tubes were centrifuged at 2000 g for 10 minutes at room tem- perature and from each tube equal volumes of solution were taken for total protein quantification. Total protein concentration was determined by the Bradford assay [32]. 2.7. Genotoxicity Studies For in vivo studies, total genomic DNA was extracted from roots of Allium bulbs incubated in increasing con- centrations of AEDF for 24 hrs as described earlier. Ge- nomic DNA extraction was modified from Križman et al., [33]. Briefly, fresh root tissue was homogenised with CTAB buffer (20 mM EDTA, 100 mM Tris-HCl [pH 8.0], 1.4 M NaCl, 2% [w/v] N-Cetyl-N,N,N, Trimethyl- ammonium bromide [CTAB] and 1% [w/v] Polyviny- polypyrrolidone) in a ratio of 0.2 gm:1.5 ml (tissue: buffer) alongwith β-Mercaptoethanol (50 µl/ml buffer) and 0.5% (w/v) activated charcoal and incubated at 55˚C for 30 min with constant agitation followed with cen- trifugation at 12,500 g for 10 min at room temperature. The resulting supernatant was mixed with equal volumes of chloroform:isoamyl alcohol (24:1) solution and cen- trifuged at 12,500 g for 10 min at room temperature. The supernatant was treated with 5 µl RNaseA (1 mg/ml) for 30 min and the DNA was precipitated with cold isopro- panol, washed twice with 70% cold ethanol and dis- solved in 100 µl of TE buffer. Total genomic DNA was quantified by measuring the absorbance at A260 and elec- trophoresed in 1% (w/v) agarose. For studying the effects of AEDF on genomic DNA, total genomic DNA from roots of Allium bulbs incubated only in distilled water was extracted as described above. 5 µg of DNA was incubated with increasing concentra- tions of AEDF for 12 hrs and electrophoresed in 1% (w/v) agarose. 3. Results 3.1. Analysis of Mitotic Activity and Changes in Phase Index of Allium Root Cells under Incubation with AEDF Incubation of 2-day-old onion roots in AEDF for 24 hr inhibited root growth by approximately 83% in com- parison with the negative controls (Figure 1(b)). The inhibition in root growth was comparable to that induced by the Fenton’s reagent [34] which is a commonly used inducer of oxidative stress. A dose response analysis of the effect of AEDF on root growth indicated that half maximal effective concentration (EC50) for inhibiton of root growth was observed at total phenolics concentra- tion of 0.4 mg/ml (Figure 1(c)). Analysis of mitotic ac- tivity indicated that incubation of roots in AEDF caused changes in the percentage of the distribution of particular phases’ in comparison to the roots in the control. While both the Fenton’s reagent and AEDF inhibited root growth along with alterations in the mitotic indices, the characteristic effect caused by AEDF, was an extensive increase in the metaphase index during the period of in- cubation (Figure 1(d)). Furthermore, incubation in AEDF induced clumping of chromosomes in all metaphase cells examined while the cells at interphase showed extensive vacuolisation of the cell nuclei (Figures 1(e)-(j)). The meristematic cells containing clumped chromosomes, accounted for 5% of the total cells examined which cor- responded to the percentage of cells in either metaphase or anaphase stage of mitotic division in the negative con- trols. 3.2. Transmission Electron Microscope Studies Roots of Allium bulbs incubated in AEDF for 24 hr showed inhibition of growth which was dependent on the concentration of AEDF used. In most of the roots, the inhibition in root growth was accompanied by swelling of the region near the root tips (Figures 2(a)-(g)). Trans- mission electron microscope studies of the roots incu- bated in AEDF for 24 hr showed extensive alterations in the ultra-structure of the cellular organelles investigated compared to the negative controls. The cell wall and cell membrane of the controls was intact and continuous. In contrast, in the experimental roots, while the integrity of Copyright © 2012 SciRes. AJPS 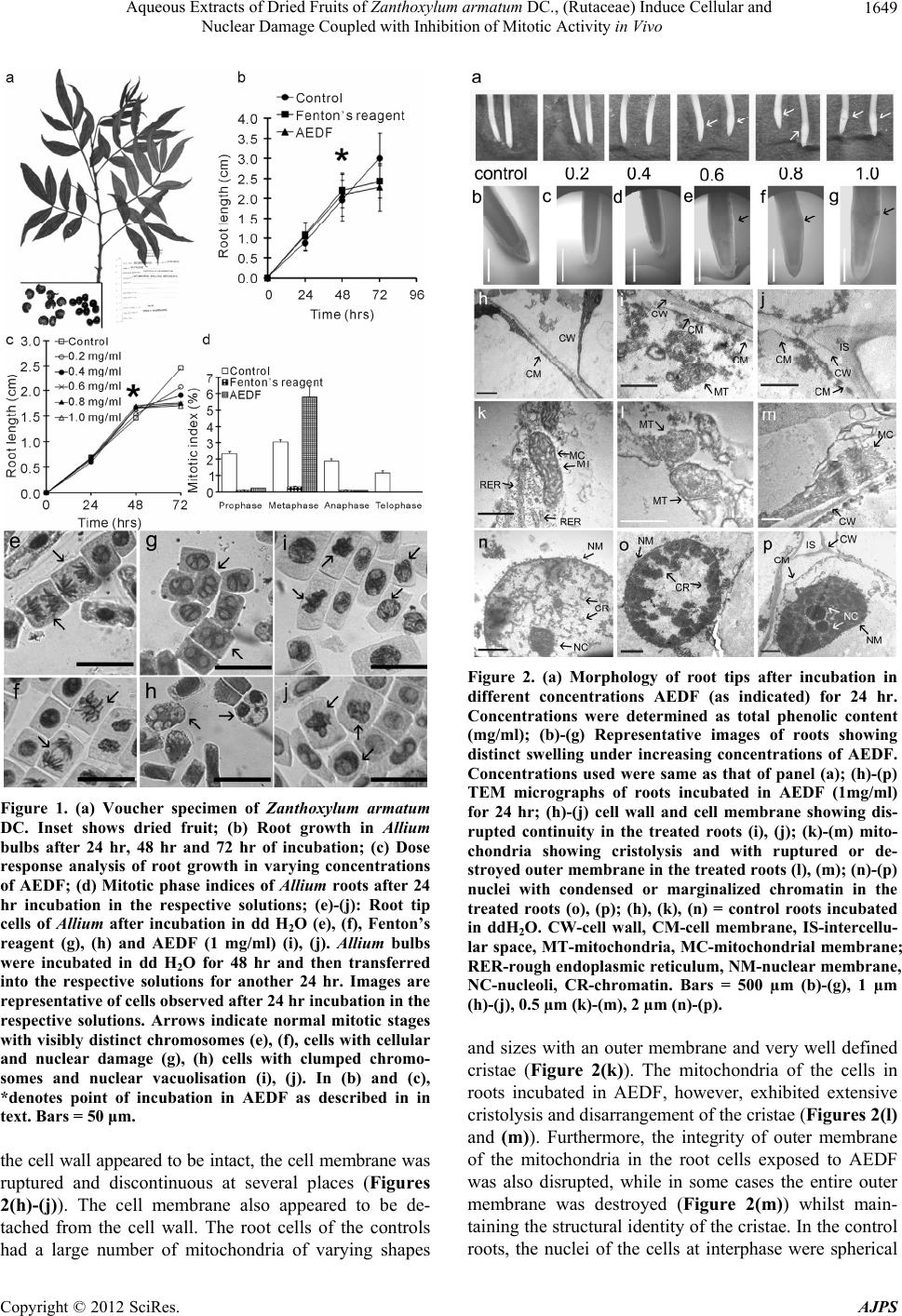 Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo 1649 Figure 1. (a) Voucher specimen of Zanthoxylum armatum DC. Inset shows dried fruit; (b) Root growth in Allium bulbs after 24 hr, 48 hr and 72 hr of incubation; (c) Dose response analysis of root growth in varying concentrations of AEDF; (d) Mitotic phase indices of Allium roots after 24 hr incubation in the respective solutions; (e)-(j): Root tip cells of Allium after incubation in dd H2O (e), (f), Fenton’s reagent (g), (h) and AEDF (1 mg/ml) (i), (j). Allium bulbs were incubated in dd H2O for 48 hr and then transferred into the respective solutions for another 24 hr. Images are representative of cells observed after 24 hr incubation in the respective solutions. Arrows indicate normal mitotic stages with visibly distinct chromosomes (e), (f), cells with cellular and nuclear damage (g), (h) cells with clumped chromo- somes and nuclear vacuolisation (i), (j). In (b) and (c), *denotes point of incubation in AEDF as described in in text. Bars = 50 μm. the cell wall appeared to be intact, the cell membrane was ruptured and discontinuous at several places (Figures 2(h)-(j)). The cell membrane also appeared to be de- tached from the cell wall. The root cells of the controls had a large number of mitochondria of varying shapes Figure 2. (a) Morphology of root tips after incubation in different concentrations AEDF (as indicated) for 24 hr. Concentrations were determined as total phenolic content (mg/ml); (b)-(g) Representative images of roots showing distinct swelling under increasing concentrations of AEDF. Concentrations used were same as that of panel (a); (h)-(p) TEM micrographs of roots incubated in AEDF (1mg/ml) for 24 hr; (h)-(j) cell wall and cell membrane showing dis- rupted continuity in the treated roots (i), (j); (k)-(m) mito- chondria showing cristolysis and with ruptured or de- stroyed outer membrane in the treated roots (l), (m); (n)-(p) nuclei with condensed or marginalized chromatin in the treated roots (o), (p); (h), (k), (n) = control roots incubated in ddH2O. CW-cell wall, CM-cell membrane, IS-intercellu- lar space, MT-mitochondria, MC-mitochondrial membrane; RER-rough endoplasmic reticulum, NM-nuclear membrane, NC-nucleoli, CR-chromatin. Bars = 500 µm (b)-(g), 1 µm (h)-(j), 0.5 µm (k)-(m), 2 µm (n)-(p). and sizes with an outer membrane and very well defined cristae (Figure 2(k)). The mitochondria of the cells in roots incubated in AEDF, however, exhibited extensive cristolysis and disarrangement of the cristae (Figures 2(l) and (m)). Furthermore, the integrity of outer membrane of the mitochondria in the root cells exposed to AEDF was also disrupted, while in some cases the entire outer membrane was destroyed (Figure 2(m)) whilst main- taining the structural identity of the cristae. In the control roots, the nuclei of the cells at interphase were spherical Copyright © 2012 SciRes. AJPS 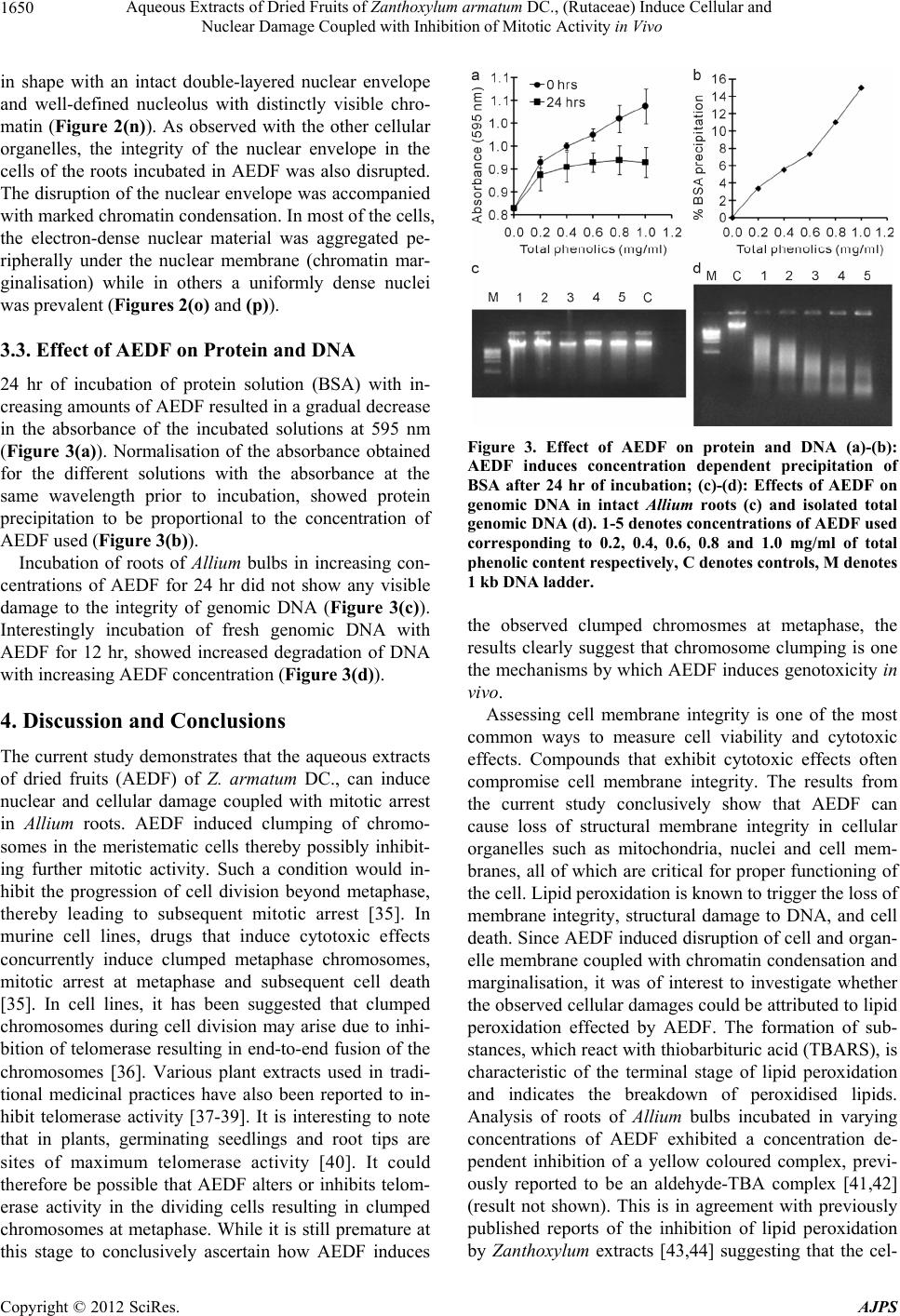 Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo 1650 in shape with an intact double-layered nuclear envelope and well-defined nucleolus with distinctly visible chro- matin (Figure 2(n)). As observed with the other cellular organelles, the integrity of the nuclear envelope in the cells of the roots incubated in AEDF was also disrupted. The disruption of the nuclear envelope was accompanied with marked chromatin condensation. In most of the cells, the electron-dense nuclear material was aggregated pe- ripherally under the nuclear membrane (chromatin mar- ginalisation) while in others a uniformly dense nuclei was prevalent (Figures 2(o) and (p)). 3.3. Effect of AEDF on Protein and DNA 24 hr of incubation of protein solution (BSA) with in- creasing amounts of AEDF resulted in a gradual decrease in the absorbance of the incubated solutions at 595 nm (Figure 3(a)). Normalisation of the absorbance obtained for the different solutions with the absorbance at the same wavelength prior to incubation, showed protein precipitation to be proportional to the concentration of AEDF used (Figure 3(b)). Incubation of roots of Allium bulbs in increasing con- centrations of AEDF for 24 hr did not show any visible damage to the integrity of genomic DNA (Figure 3(c)). Interestingly incubation of fresh genomic DNA with AEDF for 12 hr, showed increased degradation of DNA with increasing AEDF concentration (Figure 3(d)). 4. Discussion and Conclusions The current study demonstrates that the aqueous extracts of dried fruits (AEDF) of Z. armatum DC., can induce nuclear and cellular damage coupled with mitotic arrest in Allium roots. AEDF induced clumping of chromo- somes in the meristematic cells thereby possibly inhibit- ing further mitotic activity. Such a condition would in- hibit the progression of cell division beyond metaphase, thereby leading to subsequent mitotic arrest [35]. In murine cell lines, drugs that induce cytotoxic effects concurrently induce clumped metaphase chromosomes, mitotic arrest at metaphase and subsequent cell death [35]. In cell lines, it has been suggested that clumped chromosomes during cell division may arise due to inhi- bition of telomerase resulting in end-to-end fusion of the chromosomes [36]. Various plant extracts used in tradi- tional medicinal practices have also been reported to in- hibit telomerase activity [37-39]. It is interesting to note that in plants, germinating seedlings and root tips are sites of maximum telomerase activity [40]. It could therefore be possible that AEDF alters or inhibits telom- erase activity in the dividing cells resulting in clumped chromosomes at metaphase. While it is still premature at this stage to conclusively ascertain how AEDF induces Figure 3. Effect of AEDF on protein and DNA (a)-(b): AEDF induces concentration dependent precipitation of BSA after 24 hr of incubation; (c)-(d): Effects of AEDF on genomic DNA in intact Allium roots (c) and isolated total genomic DNA (d). 1-5 denotes concentrations of AEDF used corresponding to 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml of total phenolic content respectively, C denotes controls, M denotes 1 kb DNA ladder. the observed clumped chromosmes at metaphase, the results clearly suggest that chromosome clumping is one the mechanisms by which AEDF induces genotoxicity in vivo. Assessing cell membrane integrity is one of the most common ways to measure cell viability and cytotoxic effects. Compounds that exhibit cytotoxic effects often compromise cell membrane integrity. The results from the current study conclusively show that AEDF can cause loss of structural membrane integrity in cellular organelles such as mitochondria, nuclei and cell mem- branes, all of which are critical for proper functioning of the cell. Lipid peroxidation is known to trigger the loss of membrane integrity, structural damage to DNA, and cell death. Since AEDF induced disruption of cell and organ- elle membrane coupled with chromatin condensation and marginalisation, it was of interest to investigate whether the observed cellular damages could be attributed to lipid peroxidation effected by AEDF. The formation of sub- stances, which react with thiobarbituric acid (TBARS), is characteristic of the terminal stage of lipid peroxidation and indicates the breakdown of peroxidised lipids. Analysis of roots of Allium bulbs incubated in varying concentrations of AEDF exhibited a concentration de- pendent inhibition of a yellow coloured complex, previ- ously reported to be an aldehyde-TBA complex [41,42] (result not shown). This is in agreement with previously published reports of the inhibition of lipid peroxidation by Zanthoxylum extracts [43,44] suggesting that the cel- Copyright © 2012 SciRes. AJPS  Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo 1651 lular damages induced by AEDF possibly does not in- volve induction of lipid peroxidation and might occur via an alternative mechanism(s). Chromatin condensation and marginalisation are nu- clear events that have been associated with the disruption of cell division eventually resulting in cell death [45,46]. Chromatin condensation and nuclear DNA fragmentation occur in plant cells during senescence [47] and/or in the presence of cytotoxic agents [48] and prolonged expo- sure to higher concentrations of cytotoxic agents can induce loss of membrane integrity. Electron microscopy analyses indicate that AEDF drastically alters the mor- phology of the cellular organelles investigated. TEM results reveal ultrastructural morphological characteris- tics such as 1) electron-dense nuclei (marginalisation in the early phase); 2) disorganized cytoplasmic organelles; and 3) disrupted organelle membrane which indicate that the cytotoxic potential of AEDF could be attributed to its ability to possibly induce necrosis since the ultrastruc- tural alterations induced by AEDF bear striking resem- blances to typical necrotic hallmarks. The results presented in this study indicates that AEDF does not seem to induce any visible damage to the integ- rity of nuclear DNA under in vivo conditions which is in contrast to that reported for apoptotic cells or cells un- dergoing programmed cell death, but can induce degra- dation of isolated genomic DNA. The reason for such variation could be that under in vivo conditions, the pro- portion of DNA degraded by AEDF is much lesser com- pared to the intact genomic DNA, thereby masking the gentoxic potential of AEDF. This could be partially due to the reason that not all the cells are as easily accessible to AEDF as the meristematic cells present at the root tips. Since the meristematic cells constitute only a small per- centage of the total tissue from which the genomic DNA was isolated, therefore, the integrity of the nuclear DNA seemed unaffected by AEDF. However, under in vitro conditions when all the nuclear DNA present was acces- sible to AEDF, it induced extensive DNA degradation. This implies that AEDF does have the potential to induce acute gentoxic effects. In conclusion, the current study clearly demonstrates that the aqueous extracts of dried fruits (AEDF) of Zanthoxyllum armatum DC., which is used extensively in traditional herbal remedies exert its toxic potential in vivo by inducing membrane damage of cellular organelles, chromatin condensation, chromatin marginalisation, chromosome clumping and nuclear DNA damage resulting in subsequent mitotic arrest. 5. Acknowledgements The work was supported by the University Grants Com- mission, India research grant (Ref. No. F-5-5/2010-11/ MRP(NERO)/5348). REFERENCES [1] D. J. Newman and G. M. Cragg, “Natural Products as Sources of New Drugs over the last 25 Years,” Journal of Natural Products, Vol. 70, No. 3, 2007, pp. 461-477. doi:10.1021/np068054v [2] G. M. Cragg, D. G. I. Kingston and D. J. Newman, “Anticancer Agents from Natural Products,” In: D. G. I. Kingston, G. M. Cragg and D. J. Newman, Eds., CRC Taylor & Francis, New York, 2005, pp. 1-3. [3] P. B. Kaufman, A. Kirakosyan, M. McKenzie, P. Dayanandan, J. E. Hoyt and C. Li, “The Uses of Plant Natural Products by Humans and Risks Associated with Their Use,” In: L. J. Cseke, A. Kirakosyan, P. B. Kauf- man, S. Warber, J. A. Duke and H. L. Brielmann, Eds., Natural Products from Plants, CRC Taylor & Francis, New York, 2006, pp. 442-468. doi:10.1201/9781420004472.ch12 [4] S. M. Colegate and R. J. Molyneux, “An Introduction and Overview,” In: S. M. Colegate and R. J. Molyneux, Eds., Bioactive Natural Products: Detection, Isolation, and Structural Determination, 2nd Edition, CRC Press, New York, 2007, pp. 1-3. [5] C. Tringali, “Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties,” CRC Taylor & Francis, New York, 2001, pp. 9-10. [6] J. S. Negi, V. K. Bisht, A. K. Bhandari, P. Singh and R. C. Sundriyal, “Chemical Constituents and Biological Activi- ties of the Genus Zanthoxylum: A Review,” African Journal of Pure and Applied Chemistry, Vol. 5, No. 12, 2011, pp. 412-416. [7] L. O. J. Patiño, R. J. A. Prieto and S. L. E. Cuca, “Bioac- tive Compounds in Phytomedicine: Zanthoxylum Genus as Potential Source of Bioactive Compounds,” In: I. Ra- sooli, Ed., InTech Europe, Rijeka, 2012, pp. 185-218. [8] R. Diéguez, G. Garrido, S. Prieto, Y. Iznaga, L. González, J. Molina, M. Curini , F. Epifano and M. C. Marcotullio, “Antifungal Activity of Some Cuban Zanthoxylum Spe- cies,” Fitoterapia, Vol. 74, No. 4, 2003, pp. 384-386. doi:10.1016/S0367-326X(03)00048-0 [9] S. K. Adesina, “The Nigerian Zanthoxylum: Chemical and Biological Values,” African Journal of Traditional, Com- plimentary and Alternative Medicines, Vol. 2, No. 3, 2005, pp. 282-301. [10] S. Rochfort, J. Anthony, J. A. Parker and F. R. Dunshea, “Plant Bioactives for Ruminant Health and Productivity,” Phytochemistry, Vol. 69, No. 2, 2008, pp. 299-322. doi:10.1016/j.phytochem.2007.08.017 [11] S. S. Pereira, L. S. Lopes, R. B. Marques, K. A. Figueiredo, D. A. Costa, M. H. Chaves and F. R. C. Almeida, “Anti- nociceptive Effect of Zanthoxylum rhoifolium Lam. (Ru- taceae) in Models Acute Pain in Rodents,” Journal of Ethnopharmacology, Vol. 129, 2010, pp. 227-231. doi:10.1016/j.jep.2010.03.009 [12] S. Bhattacharya, M. K. Zaman and P. K. Haldar, “Anti- Copyright © 2012 SciRes. AJPS  Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo 1652 bacterial Activity of Stem Bark and Root of Indian Zanthoxylum nitidum,” Asian Journal of Pharmaceutical and Clinical Research, Vol. 2, No. 1, 2009, pp. 30-24. [13] L.-F. Shyur, J.-H. Tsung, J.-H. Chen, C.-Y. Chiu and C.-P. Lo, “Antioxidant Properties of Extracts from Medicinal Plants Popularly Used in Taiwan,” International Journal of Appllied Science and Engineering, Vol. 3, No. 3, 2005, pp. 195-202. [14] I. Elekwa, M. O. Monanu and E. O. Anosike, “Effects of Aqueous Extracts of Zanthoxylum macrophylla Roots on Membrane Stability of Human Erythrocytes of Different Genotypes,” Biokemistri, Vol. 17, No. 1, 2005, pp. 7-12. [15] E. Yamazaki, “Extraction of Antioxidants from Zanthoxy- lum piperitum DC. Fruit-Inhibition of Glycation with Polyphenols from Zanthoxylum piperitum DC. Fruit,” Reports of the Mie Prefectural Science and Technology, Promotion Center Industrial Research Division, Vol. 26, 2002, pp. 1-5. [16] A. H. Cahyana and L. Mardiana “The Use of Chemical Components Isolated from Andaliman (Zanthoxylum acanthopodium DC) Essential Oil as a Source of Natural Antioxidant,” Journal of Food Science and Technology, Vol. 1, No. 1, 2003, pp. 106-111. [17] Y.-C. Chung, C.-T. Chien, K.-Y. Teng and S.-T. Chou, “Antioxidative and Mutagenic Properties of Zanthoxylum ailanthoides Sieb & Zucc.,” Food Chemistry, Vol. 97, 2006, pp. 418-425. doi:10.1016/j.foodchem.2005.05.019 [18] T. P. Singh and O. M. Singh, “Phytochemical and Phar- macological Profile of Zanthoxylum armatum DC.—An Overview,” Indian Journal of Natural Products and Re- sources, Vol. 2, No. 3, 2011, pp. 275-285. [19] Barkatullah, M. Ibrar, N. Muhammad and L. Tahir, “An- timicrobial Evaluation, Determination of Total Phenolic and Flavoniod Contents in Zanthoxylum armatum DC,” Journal of Medicinal Plants Research, Vol. 6, No. 11, 2012, pp. 2105-2110. [20] G. Pachon, H. Rasoanaivo, A. Azqueta, J. C. Rakotozafy, A. Raharisololalao, A. L. De Cerain, J. De Lapuente, M. Borràs, S. Moukha, J. J. Centelles, E. E. Creppy and M. Cascante, “Anticancer Effect of a New Benzophenanth- ridine Isolated from Zanthoxylum madagascariense (Ru- taceline),” In Vivo, Vol. 21, No. 2, 2007, pp. 417-422. [21] G. Cebrián-Torrejón, S. A. Kahn, N. Lagarde, F. Castel- lano, K. Leblanc, J. Rodrigo, V. Molinier-Frenkel, A. Rojas de Arias, M. E. Ferreira, C. Thirant, A. Fournet, B. Figadère, H. Chneiweiss and E. Poupon, “Antiprolifera- tive Activity of Trans-Avicennol from Zanthoxylum chi- loperone var. Angustifolium against Human Cancer Stem Cells,” Journal of Natural Products, Vol. 75, No. 2, 2012, pp. 257-261. doi:10.1021/np2004165 [22] J. Y. Seo, M. Y. Park, T. Y. Jung, H. Y. Choi, D. Kim, H. S. Lee and S. K. Ku, “Genotoxicity Testing of Aqueous Extracts of Mahwangyounpae-Tang, a Polyherbal For- mula,” Food and Chemical Toxicology, Vol. 46, 2008, pp. 3827-3831. doi:10.1016/j.fct.2008.10.005 [23] C. J. van den Bout-van den Beukel, O. J. Hamza, M. J. Moshi, M. I. Matee, F. Mikx, D. M. Burger, P. P. Koop- mans, P. E. Verweij, W. G. Schoonen and A. J. van der Ven, “Evaluation of Cytotoxic, Genotoxic and CYP450 Enzymatic Competition Effects of Tanzanian Plant Ex- tracts Traditionally Used for Treatment of Fungal Infec- tions,” Basic & Clinical Pharmacology & Toxicology, Vol. 102, No. 6, 2008, pp. 515-526. doi:10.1111/j.1742-7843.2008.00225.x [24] J. Demma, E. Engidawork and B. Hellman, “Potential Genotoxicity of Plant Extracts Used in Ethiopian Tradi- tional Medicine,” Journal of Ethnopharmacology, Vol. 122, No. 1, 2009, pp. 136-142. doi:10.1016/j.jep.2008.12.013 [25] N. A. Spiridonov, D. A. Konovalov and V. V. Arkhipov, “Cytotoxicity of Some Russian Ethnomedicinal Plants and Plant Compounds,” Phytotherapy Research, Vol. 19, No. 5, 2005, pp. 428-432. doi:10.1002/ptr.1616 [26] N. J. Lee, J. H. Choi, B. S. Koo, S. Y. Ryu, Y. H. Han, S. I. Lee and D. U. Lee, “ Antimutagenicity and Cytotoxic- ity of the Constituents from the Aerial Parts of Rumex acetosa,” Biological and Pharmaceutical Bulletin, Vol. 28, No. 11, 2005, pp. 2158-2161. doi:10.1248/bpb.28.2158 [27] M. Gálvez, C. Martín-Cordero, M. López-Lázaro, F. Cortés and M. J. Ayuso, “Cytotoxic Effect of Plantago spp. on Cancer Cell Lines,” Journal of Ethnopharmacol- ogy, Vol. 88, No. 2-3, 2003, pp. 125-130. doi:10.1016/S0378-8741(03)00192-2 [28] S. R. Hynniewta and Y. Kumar, “Herbal Remedies among the Khasi Traditional Healers and Village Folk in Meghalaya,” Indian Journal of Traditional Knowledge, Vol. 7, No. 4, 2008, pp. 581-586. [29] V. Jaiswal, “Culture and Ethnobotany of Jaintia Tribal Community of Meghalaya, Northeast India—A Review,” Indian Journal of Traditional Knowledge, Vol. 9, No. 1, 2010, pp. 38-44. [30] H. P. S. Makkar, M. Bluemmel, N. K. Borowy and K. Becker, “Gravimetric Determination of Tannins and Their Correlations with Chemical and Protein Precipitation Me- thods,” Journal of the Science of Food and Agriculture, Vol. 61, No. 2, 1993, pp. 161-165. doi:10.1002/jsfa.2740610205 [31] Y. Choudhury and R. N. Sharan, “Ultrastructural Altera- tions in Liver of Mice Exposed Chronically and Trans- generationally to Aqueous Extract of Betel Nut: Implica- tions in Betel Nut-Induced Carcinogenesis,” Microscopy Research and Techique, Vol. 73, No. 5, 2010, pp. 530- 539. [32] M. Bradford, “A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding,” Analalytical Bio- chemistry, Vol. 72, 1976, pp. 248-254. doi:10.1016/0003-2697(76)90527-3 [33] M. Križman, J. Jakše, D. Baričevič, B. Javornik and M. Prošek, “Robust CTAB-Activated Charcoal Protocol for Plant DNA Extraction,” Acta Agriculturae Slovenica, Vol. 87, No. 2, 2006, pp. 427-433. [34] F. Haber and J. Weiss, “On the Catalysis of Hydroperox- ide,” Naturwissenschaften, Vol. 20, 1932, pp. 948-950. doi:10.1007/BF01504715 [35] A. S. Multani, C. Li, M. Ozen, M. Yadav, D. F. Yu, S. Copyright © 2012 SciRes. AJPS  Aqueous Extracts of Dried Fruits of Zanthoxylum armatum DC., (Rutaceae) Induce Cellular and Nuclear Damage Coupled with Inhibition of Mitotic Activity in Vivo Copyright © 2012 SciRes. AJPS 1653 Wallace and S. Pathak, “Paclitaxel and Water-Soluble Poly(L-Glutamic Acid)-Paclitaxel, Induce Direct Chro- mosomal Abnormalities and Cell Death in a Murine Me- tastatic Melanoma Cell Line,” Anticancer Research, Vol. 17, No. 6D, 1997, pp. 4269-4274. [36] W. C. Chou, A. L. Hawkins, J. F. Barrett, C. A. Griffin and C. V. Dang, “Arsenic Inhibition of Telomerase Tran- scription Leads to Genetic Instability,” Journal of Clini- cal Investigation, Vol. 108, No. 10, 2001, pp. 1541-1547 [37] S. Y. Lyu, S. H. Choi and W. B. Par, “Korean Mistletoe Lectin-Induced Apoptosis in Hepatocarcinoma Cells Is Associated with Inhibition of Telomerase via Mitochon- drial Controlled Pathway Independent of p53,” Archives of Pharmacal Research, Vol. 25, No. 1, 2002, pp. 93-101. doi:10.1007/BF02975269 [38] S. H. Choi, S. Y. Lyu and W. B. Park, “Mistletoe Lectin Induces Apoptosis and Telomerase Inhibition in Human A253 Cancer Cells through Dephosphorylation of Akt.,” Archives of Pharmacal Research, Vol. 27, No. 1, 2004, pp. 68-76. doi:10.1007/BF02980049 [39] M. Pourhassan, N. Zarghami, M. Rahmati, A. Alibakhshi and J. Ranjbari, “The Inhibitory Effect of Curcuma longa Extract on Telomerase Activity in A549 Lung Cancer Cell Line,” African Journal of Biotechnology, Vol. 9, No. 6, 2010, pp. 912-919. [40] K. Riha, J. Fajkus, J. Siroky and B. Vyskot, “Develop- mental Control of Telomere Lengths and Telomerase Ac- tivity in Plants,” The Plant Cell, Vol. 10, No. 10, 1998, pp. 1691-1698. [41] H. Kosugi, T. Kato and K. Kikugawa, “Formation of Yellow, Orange, and Red Pigments in the Reaction of Alk-2-Enals with 2-Thiobarbituric Acid,” Analytical Bio- chemistry, Vol. 165, No. 2, 1987, pp. 456-464. doi:10.1016/0003-2697(87)90296-X [42] Q. Sun, C. Faustman, A. Senecal, A. L. Wilkinson and H. Furr, “Aldehyde Reactivity with 2-Thiobarbituric Acid and TBARS in Freeze-Dried Beef during Accelerated Storage,” Meat Science, Vol. 57, No. 1, 2001, pp. 55-60. doi:10.1016/S0309-1740(00)00076-0 [43] E. J. Cho, T. Yokozawa, D. Y. Rhyu, H. Y. Kim, N. Shi- bahara and J. C. Park, “The Inhibitory Effects of 12 Me- dicinal Plants and Their Component Compounds on Lipid Peroxidation,” The American Journal of Chinese Medi- cine, Vol. 31, No. 6, 2003, pp. 907-917. doi:10.1142/S0192415X03001648 [44] J. M. Hur, J. G. Park, K. H. Yang, J. C. Park, J. R. Park, S. S. Chun, J. S. Choi and J. W. Choi, “Effect of Methanol Extract of Zanthoxylum piperitum Leaves and of Its Com- pound, Protocatechuic Acid, on Hepatic Drug Metaboliz- ing Enzymes and Lipid Peroxidation in Rats,” Bioscience Biotechnology Biochemistry, Vol. 67, No. 5, 2003, pp. 945-950. doi:10.1271/bbb.67.945 [45] N. Yao, Y. Tada, P. Park, H. Nakayashiki, Y. Tosa and S. Mayama, “Novel Evidence for Apoptotic Cell Response and Differential Signals in Chromatin Condensation and DNA Cleavage in Victorin-Treated Oats,” Plant Journal, Vol. 28, No. 1, 2001, pp. 13-26. doi:10.1046/j.1365-313X.2001.01109.x [46] K. Zheng, J.-W. Pan, L. Ye, Y. Fu, H.-Z. Peng, B.-Y. Wan, Q. Gu, H.-W. Bian, N. Han, J.-H. Wang, B. Kang, J.-H. Pan, H.-H. Shao, W.-Z. Wang and M.-Y. Zhu, “Pro- grammed Cell Death-Involved Aluminum Toxicity in Yeast Alleviated by Antiapoptotic Members with De- creased Calcium Signals,” Plant Physiology, Vol. 143, No. 1, 2007, pp. 38-49. doi:10.1104/pp.106.082495 [47] E. O’Brien, B. G. Murray, B. C. Baguley, B. A. Morris and I. B. Ferguson, “Major Changes in Chromatin Con- densation Suggest the Presence of an Apoptotic Pathway in Plant Cells,” Experimental Cell Research, Vol. 241, No. 1, 1998, pp. 46-54. doi:10.1006/excr.1998.4036 [48] S. Chakraborty, M. Roy, A. K. Taraphdar and R. K. Bhat- tacharya, “Cytotoxic Effect of Root Extract of Tiliacora racemosa and Oil of Semecarpus anacardium Nut in Human Tumour Cells,” Phytotherapy Research, Vol. 18, No. 8, 2004, pp. 595-600. doi:10.1002/ptr.1501.
|