Comparison of Photosynthetic Pigment Contents of the Resurrection Plants Ramonda serbica and
Ramonda nathaliae of Some Different Populations from Kosovo, Albania and Macedonia
1592
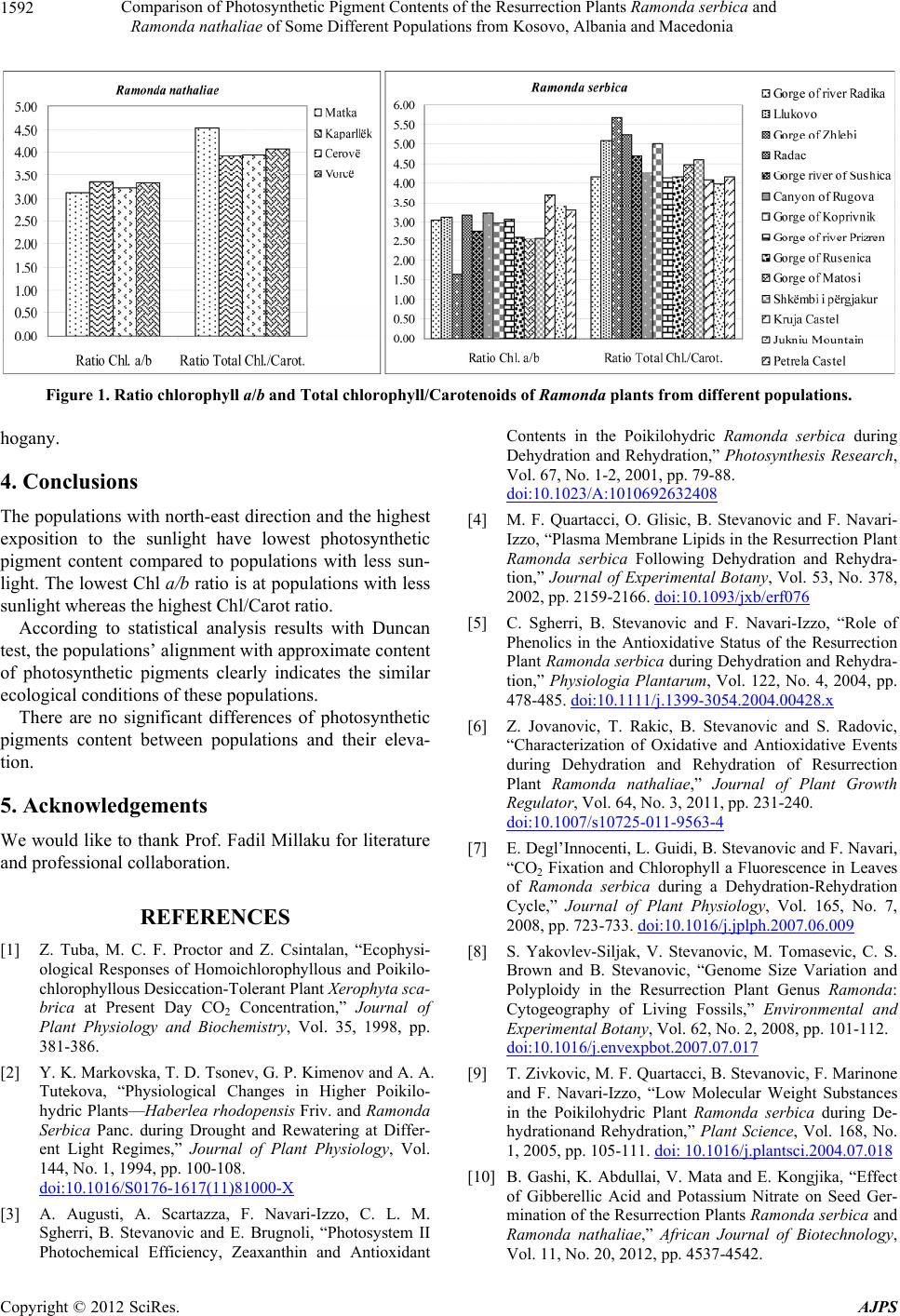
Figure 1. Ratio chlorophyll a/b and Total chlorophyll/Carotenoids of Ramonda plants from different populations.
hogany.
4. Conclusions
The populations with north-east direction and the highest
exposition to the sunlight have lowest photosynthetic
pigment content compared to populations with less sun-
light. The lowest Chl a/b ratio is at populations with less
sunlight whereas the highest Chl/Carot ratio.
According to statistical analysis results with Duncan
test, the populations’ alignment with approximate content
of photosynthetic pigments clearly indicates the similar
ecological conditions of these populations.
There are no significant differences of photosynthetic
pigments content between populations and their eleva-
tion.
5. Acknowledgements
We would like to thank Prof. Fadil Millaku for literature
and professional collaboration.
REFERENCES
[1] Z. Tuba, M. C. F. Proctor and Z. Csintalan, “Ecophysi-
ological Responses of Homoichlorophyllous and Poikilo-
chlorophyllous Desiccation-Tolerant Plant Xerophyta sca-
brica at Present Day CO2 Concentration,” Journal of
Plant Physiology and Biochemistry, Vol. 35, 1998, pp.
381-386.
[2] Y. K. Markovska, T. D. Tsonev, G. P. Kimenov and A. A.
Tutekova, “Physiological Changes in Higher Poikilo-
hydric Plants—Haberlea rhodopensis Friv. and Ramonda
Serbica Panc. during Drought and Rewatering at Differ-
ent Light Regimes,” Journal of Plant Physiology, Vol.
144, No. 1, 1994, pp. 100-108.
doi:10.1016/S0176-1617(11)81000-X
[3] A. Augusti, A. Scartazza, F. Navari-Izzo, C. L. M.
Sgherri, B. Stevanovic and E. Brugnoli, “Photosystem II
Photochemical Efficiency, Zeaxanthin and Antioxidant
Contents in the Poikilohydric Ramonda serbica during
Dehydration and Rehydration,” Photosynthesis Research,
Vol. 67, No. 1-2, 2001, pp. 79-88.
doi:10.1023/A:1010692632408
[4] M. F. Quartacci, O. Glisic, B. Stevanovic and F. Navari-
Izzo, “Plasma Membrane Lipids in the Resurrection Plant
Ramonda serbica Following Dehydration and Rehydra-
tion,” Journal of Experimental Botany, Vol. 53, No. 378,
2002, pp. 2159-2166. doi:10.1093/jxb/erf076
[5] C. Sgherri, B. Stevanovic and F. Navari-Izzo, “Role of
Phenolics in the Antioxidative Status of the Resurrection
Plant Ramonda serbica during Dehydration and Rehydra-
tion,” Physiologia Plantarum, Vol. 122, No. 4, 2004, pp.
478-485. doi:10.1111/j.1399-3054.2004.00428.x
[6] Z. Jovanovic, T. Rakic, B. Stevanovic and S. Radovic,
“Characterization of Oxidative and Antioxidative Events
during Dehydration and Rehydration of Resurrection
Plant Ramonda nathaliae,” Journal of Plant Growth
Regulator, Vol. 64, No. 3, 2011, pp. 231-240.
doi:10.1007/s10725-011-9563-4
[7] E. Degl’Innocenti, L. Guidi, B. Stevanovic and F. Navari,
“CO2 Fixation and Chlorophyll a Fluorescence in Leaves
of Ramonda serbica during a Dehydration-Rehydration
Cycle,” Journal of Plant Physiology, Vol. 165, No. 7,
2008, pp. 723-733. doi:10.1016/j.jplph.2007.06.009
[8] S. Yakovlev-Siljak, V. Stevanovic, M. Tomasevic, C. S.
Brown and B. Stevanovic, “Genome Size Variation and
Polyploidy in the Resurrection Plant Genus Ramonda:
Cytogeography of Living Fossils,” Environmental and
Experimental Botany, Vol. 62, No. 2, 2008, pp. 101-112.
doi:10.1016/j.envexpbot.2007.07.017
[9] T. Zivkovic, M. F. Quartacci, B. Stevanovic, F. Marinone
and F. Navari-Izzo, “Low Molecular Weight Substances
in the Poikilohydric Plant Ramonda serbica during De-
hydrationand Rehydration,” Plant Science, Vol. 168, No.
1, 2005, pp. 105-111. doi: 10.1016/j.plantsci.2004.07.018
[10] B. Gashi, K. Abdullai, V. Mata and E. Kongjika, “Effect
of Gibberellic Acid and Potassium Nitrate on Seed Ger-
mination of the Resurrection Plants Ramonda serbica and
Ramonda nathaliae,” African Journal of Biotechnology,
Vol. 11, No. 20, 2012, pp. 4537-4542.
Copyright © 2012 SciRes. AJPS