 American Journal of Plant Sciences, 2012, 3, 1573-1580 http://dx.doi.org/10.4236/ajps.2012.311190 Published Online November 2012 (http://www.SciRP.org/journal/ajps) 1573 Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) Angelo Gismondi, Mariagiovanna Serio, Lorena Canuti, Antonella Canini* Department of Biology, University of Rome “Tor Vergata”, Roma, Italy. Email: *canini@uniroma2.it Received August 9th, 2012; revised September 18th, 2012; accepted October 19th, 2012 ABSTRACT Saffron, the most expensive spice in the world, is got by Crocus sativus L. stigmas. The production of this substance has attracted human interest, since ancient cultures, for its medicinal and culinary properties. Because of saffron high eco- nomic value, sometimes, since Middle Ages, it is adulterated with other vegetal materials, dyes or synthetic molecules. Object of this work was the study of one of the best world saffron: Civitaretenga (AQ, Central Italy) C. sativus. Taste, color and aroma of Civitaretenga spice were determined, according to international standards (ISO/Technical Specifica- tion 3632), to define its high quality. A biochemical approach was then applied to obtain a secondary metabolite profile of this product. So, crocins, total phenolic content, flavonoids and phenolic acids were detected by HPLC-DAD and spectrophotometric analysis. Moreover, in vitro antioxidant properties and in vivo antineoplastic effects, on highly me- tastatic murine B16-F10 melanoma cell line, were successfully revealed in Civitaretenga C. sativus extract. All these data confirmed the elevated quality of Civitaretenga saffron and its highly reducing and chemopreventive activity. Keywords: Crocus sativus L.; Saffron; Plant Secondary Metabolites; Antioxidant; Antineoplastic 1. Introduction Crocus sativus L. is an herbaceous species of the Irida- ceae family. It is a monocot plant very diffused in the Mediterranean Basin and Western Asia [1]. The infertil- ity of this species has been associated to its triploid ge- nome (3n), whose origin has not been clarified yet: lit- erature data suppose that it would be generated from the evolution, or the hybridization, of other Crocus exem- plars [2]. Consequently, C. sativus only propagates vege- tatively [3-5]. Its flower is made up of six violet tepals, three yellow stamens and a single pistil whose stigma, defined by three red filaments, is the source of the saf- fron [6]. This spice is the most expensive in the world because C. sativus bloom occurs, in a very short period, only once a year and its harvest should be performed manually [7]. C. sativus stigmas are characterized by the presence of sugars, minerals, fats, vitamins and second- dary metabolites: terpenes, flavonoids, anthocyanins and carotenoids. Between them, carotenoids are the most important molecules because they determinate color and taste of the spice. From this class of compounds, in C. sativus, we can essentially find lycopene, α- and β-caro- tene, zeaxanthin and crocetin that are liposoluble and the hydrosoluble crocins, derived by crocetin esterification with sugars [8-10]. Principal saffron crocins are trans-cro- cetin di-(β-D-gentiobiosil) ester (named trans-4-GG), trans- crocetin (β-D-glucosil)-(β-D-gentiobiosil) ester (named trans-3-Gg), trans-crocetin (β-D-gentiobiosil) ester (named trans-2-G), cis-crocetin di-(β-D-gentiobiosil) ester (named cis-4-GG), trans-crocetin di-(β-D-glucosil) ester (named trans-2-gg) and cis-crocetin(β-D-glucosil)-(β-D-gentio- biosil) ester (named cis-3-Gg) [11]. High temperatures and humidity levels induce crocin oxidation and degra- dation and consequently the decrease of spice attributes [12]. Aroma, on the other hand, is determined by the amount of safranal, a terpenic aldehyde, and picrocrocin, a glycosidic form of the safranal [9-13]. Saffron has cap- tured human attention since when past populations em- ployed it as drug, perfume, dye and aroma [14-17]. Its adulteration, motivated by the high economic value, has been performed, since Middle Ages, with natural or syn- thetic substances [18,19]. For these reasons, international standards [20] were framed to scientifically determine saffron quality, to find out spice fraudulent alterations and for its valorization. According to these conventions, spectrophotometric and chromatographic investigations are able to determine spice color, taste and aroma [21-23]. Object of this work has been the identification of Civi- taretenga (AQ, Central Italy) C. sativus biochemical components and the quality classification of the saffron *Corresponding author. Copyright © 2012 SciRes. AJPS  Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) 1574 derived from its stigmas, according to ISO/Technical Specification 3632 standards. As C. sativus extract is re- ported in literature showing a large amount of flavonoids, especially kaempferol, quercetin and naringenin [24,25], in this study, we also analyzed its antioxidant power and antiproliferative effects on murine B16-F10 melanoma cells. 2. Materials and Methods 2.1. Plant Material Crocus sativus bulbs were obtained from Civitaretenga cultivars (AQ, Central Italy) and grown in the Botanical Garden of the University of Rome “Tor Vergata”. For spice production stigmas of C. sativus were dehydrated at 103˚C for 2, 8 or 16 hours. 2.2. Spice Quality Determination Spice quality was established, in relation to its taste (picrocrocin content), aroma (safranal amount) and color (crocin level),with respect to ISO/TS 3632 standards [20]. Briefly, 5 mg of saffron were resuspended in 20 mL of ultrapure water at room temperature in the dark for 1 hour and then filtered by a 0.45 µm cellulose filter. Spice quality was analyzed by spectrophotometric method us- ing the following formulas: spice taste-1% 1 cm E 257 nm Abs20000100% moisture content , safranal content-1% 1 cm E 330 nm Abs20000100% moisture content , color intensity- 1% 1 cm E 440 nm Abs20000100% moisture content ; where is 1% molar extinction coefficient of the solution measured in 1 cm cuvette and 257 nm, 330 nm and 440 nm are absorption wavelengths respectively of picrocrocin, safranal and crocins. 1% 1 cm E 2.3. Secondary Metabolite Extraction Secondary metabolites extraction was performed on dried (16 h) stigmas according to [22] method. 50 mg of dried stigmas were powdered with liquid nitrogen. The extraction was performed in 10 mL of ddH2O (or metha- nol) in agitation at 4˚C, in the dark, for 24 hours. After centrifugation at 30.000 g for 20 minutes, the supernatant was filtered and analyzed. 2.4. Total Phenolic Content Total polyphenol determination was carried out by Folin- Ciocalteau assay [26]. 9 mL of ddH2O (or methanol) and 1 mL of Folin-Ciocalteau reagent (Sigma-Aldrich) were added to 1 mL of each secondary metabolite extract. Af- ter 5 minutes of incubation, 10 mL of Na2CO3 7% (w/v) and 4 mL of ddH2O (or methanol) were added. The solu- tion was vortexed and incubated at room temperature for 1 hour in the dark. Absorption was determined at 760 nm by a UV-visible spectrophotometer Cary 50 (Bio Varian). Total polyphenol concentration was calculated with re- spect to a caffeic acid calibration curve (20 - 100 mg/L) and results were expressed as µg of caffeic acid equiva- lents (µg CAE/mg DW) of dried stigmas. 2.5. HPLD-DAD Analysis Crocins, flavonoids and phenolic acids, extracted (in wa- ter) as described before, were identified and quantized by a high performance liquid chromatography (HPLC, Shi- madzu) instrument associated with DAD detector (SPD-M20A), multisolvent delivery system (LC-10AD), auto-sampler (SIL-10AD), controller module (SCL-10A) and Class VP 5.02 software. Crocin analysis was per- formed according to Alonso et al. [27] with some modi- fications. A Hamilton PRP-10 column (10 µm, 4.6 × 150 mm) was used and the elution gradient was performed with 10% (v/v) acetonitrile (A) and methanol (B). The gradient system was 100% A to 90% A in 10 minutes and then 0% A to 50 minutes, at flow rate of 1 mL/min. Column re-equilibration between runs was of 10 minutes. The column was kept at 30˚C. Crocins (trans-4-GG, trans-3-Gg, cis-4-GG, trans-2-G, cis-3-Gg and trans-2-G) were measured at 440 nm. Flavonoids and phenolic acids were analyzed according to Canini et al. [28] with a Phenomenex Gemini NX C18 column (5 µm, 4.6 × 150 mm). Flavoniods (myricetin, quercetin and kaempferol) were monitored at 280 nm while phenolic acids (gallic, chlorogenic and caffeic acids) were monitored at 320 nm. The eluition gradient was performed with tricloroacetic acid pH 2.5 (A) and acetonitrile (B). The gradient system was 85% A to 65% A in 20 minutes and then 20% A in 8 minutes and maintained at this concentration for 5 min- utes, at flow rate of 1 mL/min. The column was kept at 35˚C. Column re-equilibration between runs was of 10 minutes. For each analysis, 20 μL of each sample was injected. Peak identification was assured according to their retention times and by co-elution with authentic standards (Fluka). 2.6. DPPH Radical Scavenging Test Aqueous saffron extract antioxidant activity was deter- mined on the basis of its scavenging activity on the stable DPPH free radical (Merck). According to Brand-Wil- liams et al. [29], we monitored absorbance decrease of a 100 µM DPPH methanolic solution, at 517 nm, after 30 Copyright © 2012 SciRes. AJPS  Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) 1575 minutes of sample addition. Sample antiradical activity was calculated by the following ratio: Abs controlAbs sampleAbs control100 ; where Abs control is DPPH solution absorption and Abs sample is DPPH solution absorption after sample addi- tion. Antioxidant activity was expressed as IC50 (sample concentration producing 50% of DPPH activity decrease with respect to the control). 2.7. FRAP Assay FRAP assay measures absorbance change at 593 nm ow- ing to the formation of a blue-colored Fe II—tripyridyl- triazine compound (Merck), from the colorless oxidized Fe III form, by the action of electron donating antioxi- dants [30]. 200 µL of sample was added to 1.8 mL freshly prepared and pre-warmed (37˚C) FRAP reagent (10 mM TPTZ in 40 mM HCl, 20 mM FeCl3, 0.3 M ace- tate buffer pH 3.6; 1:1:10 v/v/v) and incubated at 37˚C for 10 minutes. Fe II standard solution (50 - 500 µM) was obtained from ferrous sulphate (FeSO4 in ddH2O). Results were expressed as mM ferric ions reduced to ferrous form per gram of sample dried weight, according to Woidylo et al. [31]. 2.8. Cell Cultures and Proliferation Assays Highly metastatic murine B16-F10 melanoma cell line was propagated under standard culture conditions [32]. Briefly, cells were cultured in Dulbecco’s modified Ea- gle’s medium (D-MEM), supplemented with 10% fetal calf serum (FCS), 200 mM glutamine, 100 U/mL penicil- lin and 0.1 mg/mL streptomycin, and maintained in hu- midified atmosphere with 5% CO2 at 37˚C. To test C. sativus antineoplastic property, B16-F10 cells were seeded and grown in 35-mm dishes and treated with aqueous stigma extracts (250, 500 and 1000 µg/ml) for 24, 48, and 72 hours. Cell growth was determinated by MTT based kit (Sigma). This product was designed for the spectrophotometric measurement of cell proliferation rate as a function of mitochondrial activity in living cells. On the other hand, the proliferation was also analyzed by counting cells with a Neubauer modified chamber, after Trypan Blue staining (1%, w/v) for cytotoxicity evalua- tion. Cell morphology was observed by optical micro- scope (20×) (Nikon, TE2000-PFS). 2.9. Statistical Analysis Each experiment was repeated at least three times. Analy- sis of variance was conducted using one-way ANOVA test with SPSS (ver.19 ita) for Microsoft and means were compared by Duncan tests. 3. Results 3.1. Saffron Quality Determination Stigmas of Civitaretenga C. sativus were dehydrated, powdered and subjected to aqueous extraction. The dry- ing process was conducted at different times (2, 8 and 16 hours). To evaluate saffron taste, aroma and color, the extract was analyzed according to ISO/TS 3632 method. Saffron values, obtained by spectrophotometric analysis at different wavelengths, were reported in Table 1: all values remained inside ISO/TS 3632 standard ranges, except picrocrocin level after 2h of desiccation. 8 and 16 h dehydration processes decreased picrocrocin amount, respectively of 20% and 37.3%, and increased safranal content, of 7.4% and 22.2%, with respect to 2 h procedure. No considerable variation was observed in crocin amount. 1% 1 cm E 3.2. Secondary Metabolite Analysis Aqueous or methanolic extraction was performed on C. sativus stigmas. Total phenolic content was measured in both extracts: methanolic solution presented 53.52 ± 1.75 µg CAE/mg DW of total polyphenols with respect to the aqueous one that was only 31.46 ± 1.05 µg CAE/mg DW. HPLC-DAD analysis permitted crocin identification in saffron aqueous extract (Figures 1 and 2): trans-4-GG, trans-3-Gg, trans-2-gg, cis-4-GG, cis-3-Gg and trans- 2-G isomers were detected, at specific retention times (tR, min), and quantified (Q, mg/g) with respect to authentic standards, as reported in Table 2. Trans-4-GG and trans- 3-Gg molecules were the most abundant identified cro- cins. The chromatographic method was also applied to reveal the presence, or the absence, of principal saffron flavonoids (kampferol, quercetin, genistein and myricetin) and phenolic acids (cumaric, caffeic, chlorogenic and gallic acid) in the sample (Table 3). Table 1. Civitaretenga saffron values at 257, 330 and 440 nm for different spice dehydration times. In the lower part are also reported ISO 3632 standard value ranges. E1% 1cm E1% 1cm Saffron quality evaluation Saffron dehydration time (h) 1% 1 cm E 257 1% 1 cm E 330 1% 1 cm E 440 2 h 75 ± 0.02 27 ± 0.02 94 ± 0.07 8 h 60 ± 0.01 29 ± 0.01 95 ± 0.004 16 h 47 ± 0.02 33 ± 0.01 92 ± 0.03 ISO 3632 standard range 30 < X < 70 20 < X < 50 80 < X < 190 Copyright © 2012 SciRes. AJPS 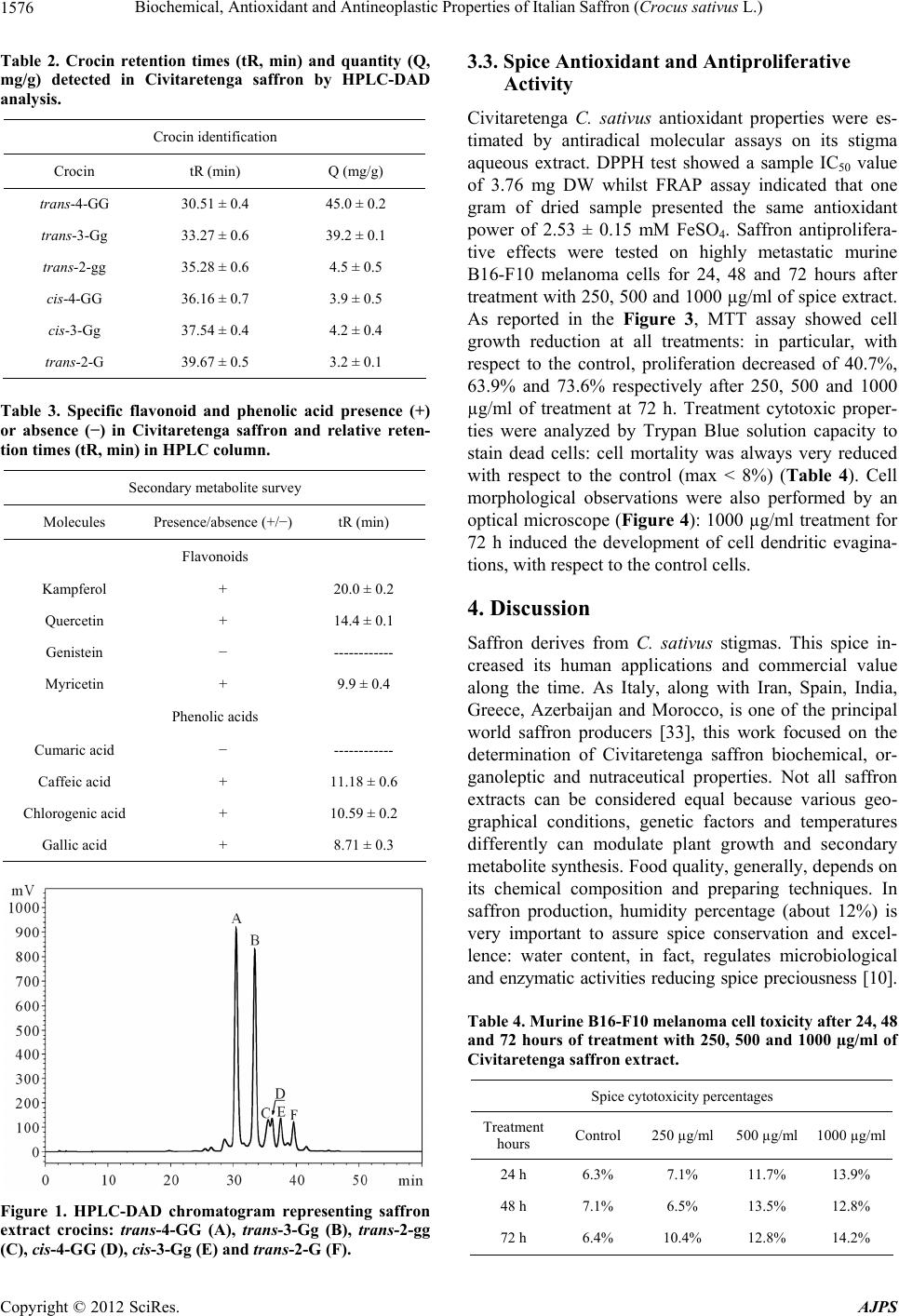 Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) 1576 Table 2. Crocin retention times (tR, min) and quantity (Q, mg/g) detected in Civitaretenga saffron by HPLC-DAD analysis. Crocin identification Crocin tR (min) Q (mg/g) trans-4-GG 30.51 ± 0.4 45.0 ± 0.2 trans-3-Gg 33.27 ± 0.6 39.2 ± 0.1 trans-2-gg 35.28 ± 0.6 4.5 ± 0.5 cis-4-GG 36.16 ± 0.7 3.9 ± 0.5 cis-3-Gg 37.54 ± 0.4 4.2 ± 0.4 trans-2-G 39.67 ± 0.5 3.2 ± 0.1 Table 3. Specific flavonoid and phenolic acid presence (+) or absence (−) in Civitaretenga saffron and relative reten- tion times (tR, min) in HPLC column. Secondary metabolite survey Molecules Presence/absence (+/−) tR (min) Flavonoids Kampferol + 20.0 ± 0.2 Quercetin + 14.4 ± 0.1 Genistein − ------------ Myricetin + 9.9 ± 0.4 Phenolic acids Cumaric acid − ------------ Caffeic acid + 11.18 ± 0.6 Chlorogenic acid + 10.59 ± 0.2 Gallic acid + 8.71 ± 0.3 Figure 1. HPLC-DAD chromatogram representing saffron extract crocins: trans-4-GG (A), trans-3-Gg (B), trans-2-gg (C), cis-4-GG (D), cis-3-Gg (E) and trans-2-G (F). 3.3. Spice Antioxidant and Antiproliferative Activity Civitaretenga C. sativus antioxidant properties were es- timated by antiradical molecular assays on its stigma aqueous extract. DPPH test showed a sample IC50 value of 3.76 mg DW whilst FRAP assay indicated that one gram of dried sample presented the same antioxidant power of 2.53 ± 0.15 mM FeSO4. Saffron antiprolifera- tive effects were tested on highly metastatic murine B16-F10 melanoma cells for 24, 48 and 72 hours after treatment with 250, 500 and 1000 µg/ml of spice extract. As reported in the Figure 3, MTT assay showed cell growth reduction at all treatments: in particular, with respect to the control, proliferation decreased of 40.7%, 63.9% and 73.6% respectively after 250, 500 and 1000 µg/ml of treatment at 72 h. Treatment cytotoxic proper- ties were analyzed by Trypan Blue solution capacity to stain dead cells: cell mortality was always very reduced with respect to the control (max < 8%) (Table 4). Cell morphological observations were also performed by an optical microscope (Figure 4): 1000 µg/ml treatment for 72 h induced the development of cell dendritic evagina- tions, with respect to the control cells. 4. Discussion Saffron derives from C. sativus stigmas. This spice in- creased its human applications and commercial value along the time. As Italy, along with Iran, Spain, India, Greece, Azerbaijan and Morocco, is one of the principal world saffron producers [33], this work focused on the determination of Civitaretenga saffron biochemical, or- ganoleptic and nutraceutical properties. Not all saffron extracts can be considered equal because various geo- graphical conditions, genetic factors and temperatures differently can modulate plant growth and secondary metabolite synthesis. Food quality, generally, depends on its chemical composition and preparing techniques. In saffron production, humidity percentage (about 12%) is very important to assure spice conservation and excel- lence: water content, in fact, regulates microbiological and enzymatic activities reducing spice preciousness [10]. Table 4. Murine B16-F10 melanoma cell toxicity after 24, 48 and 72 hours of treatment with 250, 500 and 1000 µg/ml of Civitaretenga saffron extract. Spice cytotoxicity percentages Treatment hours Control 250 µg/ml 500 µg/ml 1000 µg/ml 24 h 6.3% 7.1% 11.7% 13.9% 48 h 7.1% 6.5% 13.5% 12.8% 72 h 6.4% 10.4% 12.8% 14.2% Copyright © 2012 SciRes. AJPS 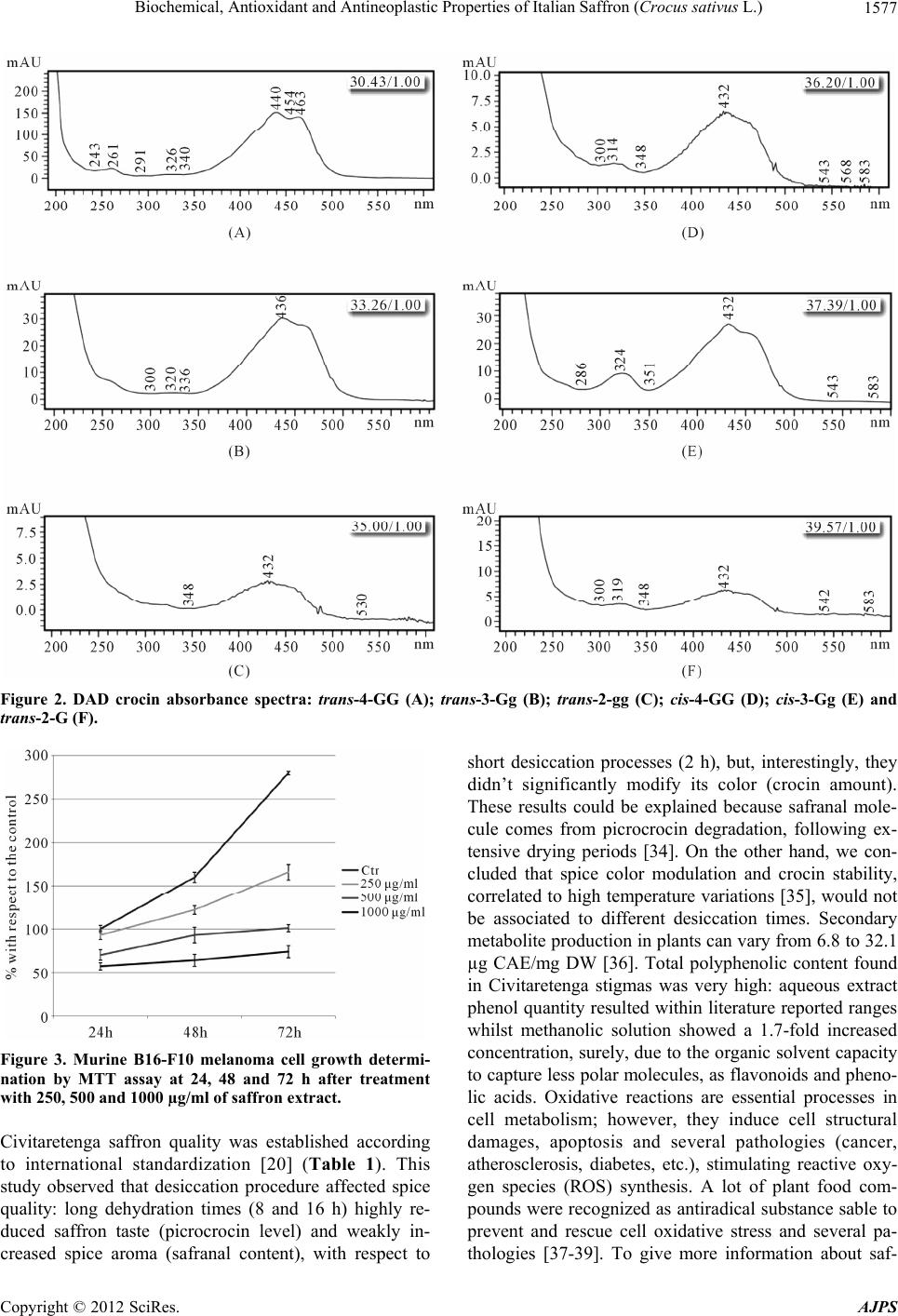 Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) Copyright © 2012 SciRes. AJPS 1577 Figure 2. DAD crocin absorbance spectra: trans-4-GG (A); trans-3-Gg (B); trans-2-gg (C); cis-4-GG (D); cis-3-Gg (E) and trans-2-G (F). Figure 3. Murine B16-F10 melanoma cell growth determi- nation by MTT assay at 24, 48 and 72 h after treatment with 250, 500 and 1000 µg/ml of saffron extract. Civitaretenga saffron quality was established according to international standardization [20] (Table 1). This study observed that desiccation procedure affected spice quality: long dehydration times (8 and 16 h) highly re- duced saffron taste (picrocrocin level) and weakly in- creased spice aroma (safranal content), with respect to short desiccation processes (2 h), but, interestingly, they didn’t significantly modify its color (crocin amount). These results could be explained because safranal mole- cule comes from picrocrocin degradation, following ex- tensive drying periods [34]. On the other hand, we con- cluded that spice color modulation and crocin stability, correlated to high temperature variations [35], would not be associated to different desiccation times. Secondary metabolite production in plants can vary from 6.8 to 32.1 µg CAE/mg DW [36]. Total polyphenolic content found in Civitaretenga stigmas was very high: aqueous extract phenol quantity resulted within literature reported ranges whilst methanolic solution showed a 1.7-fold increased concentration, surely, due to the organic solvent capacity to capture less polar molecules, as flavonoids and pheno- lic acids. Oxidative reactions are essential processes in cell metabolism; however, they induce cell structural damages, apoptosis and several pathologies (cancer, atherosclerosis, diabetes, etc.), stimulating reactive oxy- gen species (ROS) synthesis. A lot of plant food com- pounds were recognized as antiradical substance sable to prevent and rescue cell oxidative stress and several pa- thologies [37-39]. To give more information about saf- 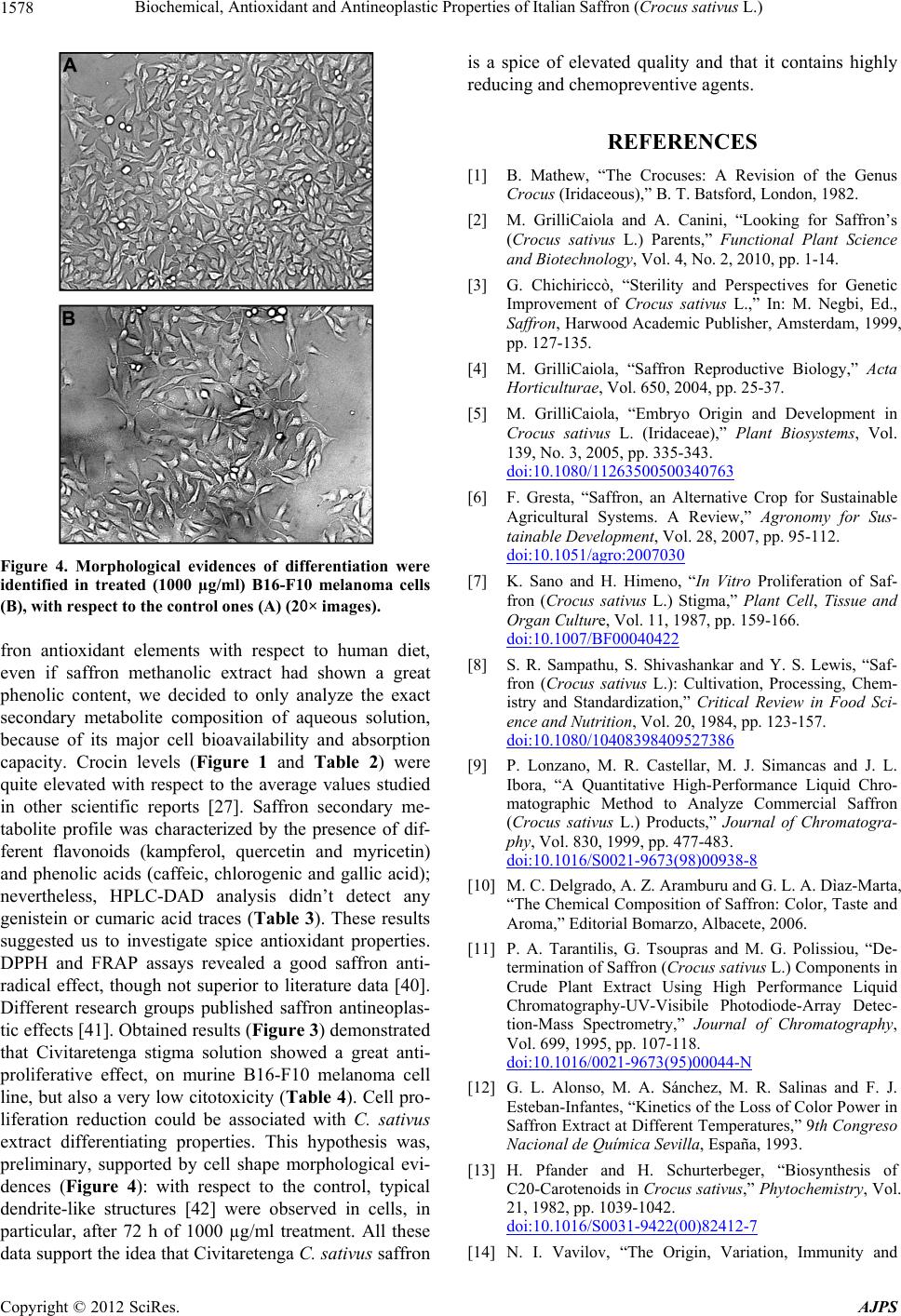 Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) 1578 Figure 4. Morphological evidences of differentiation were identified in treated (1000 µg/ml) B16-F10 melanoma cells (B), with respect to the control ones (A) (20× images). fron antioxidant elements with respect to human diet, even if saffron methanolic extract had shown a great phenolic content, we decided to only analyze the exact secondary metabolite composition of aqueous solution, because of its major cell bioavailability and absorption capacity. Crocin levels (Figure 1 and Table 2) were quite elevated with respect to the average values studied in other scientific reports [27]. Saffron secondary me- tabolite profile was characterized by the presence of dif- ferent flavonoids (kampferol, quercetin and myricetin) and phenolic acids (caffeic, chlorogenic and gallic acid); nevertheless, HPLC-DAD analysis didn’t detect any genistein or cumaric acid traces (Table 3). These results suggested us to investigate spice antioxidant properties. DPPH and FRAP assays revealed a good saffron anti- radical effect, though not superior to literature data [40]. Different research groups published saffron antineoplas- tic effects [41]. Obtained results (Figure 3) demonstrated that Civitaretenga stigma solution showed a great anti- proliferative effect, on murine B16-F10 melanoma cell line, but also a very low citotoxicity (Table 4). Cell pro- liferation reduction could be associated with C. sativus extract differentiating properties. This hypothesis was, preliminary, supported by cell shape morphological evi- dences (Figure 4): with respect to the control, typical dendrite-like structures [42] were observed in cells, in particular, after 72 h of 1000 µg/ml treatment. All these data support the idea that Civitaretenga C. sativus saffron is a spice of elevated quality and that it contains highly reducing and chemopreventive agents. REFERENCES [1] B. Mathew, “The Crocuses: A Revision of the Genus Crocus (Iridaceous),” B. T. Batsford, London, 1982. [2] M. GrilliCaiola and A. Canini, “Looking for Saffron’s (Crocus sativus L.) Parents,” Functional Plant Science and Biotechnology, Vol. 4, No. 2, 2010, pp. 1-14. [3] G. Chichiriccò, “Sterility and Perspectives for Genetic Improvement of Crocus sativus L.,” In: M. Negbi, Ed., Saffron, Harwood Academic Publisher, Amsterdam, 1999, pp. 127-135. [4] M. GrilliCaiola, “Saffron Reproductive Biology,” Acta Horticulturae, Vol. 650, 2004, pp. 25-37. [5] M. GrilliCaiola, “Embryo Origin and Development in Crocus sativus L. (Iridaceae),” Plant Biosystems, Vol. 139, No. 3, 2005, pp. 335-343. doi:10.1080/11263500500340763 [6] F. Gresta, “Saffron, an Alternative Crop for Sustainable Agricultural Systems. A Review,” Agronomy for Sus- tainable Development, Vol. 28, 2007, pp. 95-112. doi:10.1051/agro:2007030 [7] K. Sano and H. Himeno, “In Vitro Proliferation of Saf- fron (Crocus sativus L.) Stigma,” Plant Cell, Tissue and Organ Culture, Vol. 11, 1987, pp. 159-166. doi:10.1007/BF00040422 [8] S. R. Sampathu, S. Shivashankar and Y. S. Lewis, “Saf- fron (Crocus sativus L.): Cultivation, Processing, Chem- istry and Standardization,” Critical Review in Food Sci- ence and Nutrition, Vol. 20, 1984, pp. 123-157. doi:10.1080/10408398409527386 [9] P. Lonzano, M. R. Castellar, M. J. Simancas and J. L. Ibora, “A Quantitative High-Performance Liquid Chro- matographic Method to Analyze Commercial Saffron (Crocus sativus L.) Products,” Journal of Chromatogra- phy, Vol. 830, 1999, pp. 477-483. doi:10.1016/S0021-9673(98)00938-8 [10] M. C. Delgrado, A. Z. Aramburu and G. L. A. Dìaz-Marta, “The Chemical Composition of Saffron: Color, Taste and Aroma,” Editorial Bomarzo, Albacete, 2006. [11] P. A. Tarantilis, G. Tsoupras and M. G. Polissiou, “De- termination of Saffron (Crocus sativus L.) Components in Crude Plant Extract Using High Performance Liquid Chromatography-UV-Visibile Photodiode-Array Detec- tion-Mass Spectrometry,” Journal of Chromatography, Vol. 699, 1995, pp. 107-118. doi:10.1016/0021-9673(95)00044-N [12] G. L. Alonso, M. A. Sánchez, M. R. Salinas and F. J. Esteban-Infantes, “Kinetics of the Loss of Color Power in Saffron Extract at Different Temperatures,” 9th Congreso Nacional de Química Sevilla, España, 1993. [13] H. Pfander and H. Schurterbeger, “Biosynthesis of C20-Carotenoids in Crocus sativus,” Phytochemistry , Vol. 21, 1982, pp. 1039-1042. doi:10.1016/S0031-9422(00)82412-7 [14] N. I. Vavilov, “The Origin, Variation, Immunity and Copyright © 2012 SciRes. AJPS  Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) 1579 Breeding of Cultivated Plants,” The Cronica Botanica, Co., Waltham, 1951. [15] F. Tammaro, “Crocus sativus L.—cv. Piano di Navelli (L’Aquila Saffron): Environment, Cultivation, Mor- phometric Characteristics, Active Principle, Uses,” In: F. Tammaro and L. Marra, Eds., Proceedings of the Interna- tional Conference on Saffron (Crocus sativus L.), L’Aquila, 1990, pp. 47-57. [16] F. I. Abdullaev, J. Espinosa and J. Aguirre, “Biomedical Properties of Saffron and Its Potential Use in Cancer Therapy and Chemoprevention Trials,” Cancer Detection and Prevention, Vol. 28, No. 6, 2004, pp. 426-432. doi:10.1016/j.cdp.2004.09.002 [17] J. Tavakkol-Afshari, A. Brook and S. H. Mousavi, “Study of Cytotoxic and Apoptogenic Properties of Saffron Ex- tract in Human Cancer Cell Lines,” Food and Chemical Toxicology, Vol. 46, No. 11, 2008, pp. 3443-3447. doi:10.1016/j.fct.2008.08.018 [18] J. M. Maish, “On the Adulteration of Saffron,” The Ana- lyst, 1885, pp. 200-203. doi:10.1039/an8851000200 [19] M. Carmona and G. L. Alonso, “Advances in the Control of Saffron Quality,” 1st International Symposium on Saf- fron Biology and Biotechnology, Albacete, 27 October 2003, pp. 26-27. [20] “ISO/Technical Specification 3632,” 2003. http://www.iso.org/iso/iso_catalogue/catalogue_ics/catalo gue_detail_ics.htm?ics1=67&ics2=220&ics3=10&csnum ber=44523 [21] A. Zalacain, S. A. Ordoudi, E. M. Diaz-Plaza, M. Car- mona, I. Blazquez, M. Z. Tsimidou and G. L. Alonso, “Near-Infrared Spectroscopy in Saffron Quality Control: Determination of Chemical Composition and Geographi- cal Origin,” Journal of Agricultural and Food Chemistry, Vol. 53, 2005, pp. 9337-9341. doi:10.1021/jf050846s [22] H. Caballero-Ortega, R. Pereda-Miranda and F. I. Abdul- laev, “HPLC Quantification of Major Active Components from 11 Different Saffron (Crocus sativus L.) Sources,” Food Chemistry, Vol. 100, 2007, pp. 1126-1131. doi:10.1016/j.foodchem.2005.11.020 [23] L. Sabatino, M. Scordino, M. Gargano, A. Belligno, P. Traulo and G. Gagliano, “HPLC/PDA/ESI-MS Evalua- tion of Saffron (Crocus sativus L.) Adulteration,” Natural Product Communication, Vol. 6, No. 12, 2011, pp. 1873- 1876. [24] M. Carmona, A. M. Sanchez, F. Ferreres, A. Zalacain, F. Tomas-Berberan and G. L. Alonso, “Identification of the Flavonoid Fraction in Saffron Spice by LC/DAD/MS/MS: Comparative Study of Samples from Different Geo- graphical Origin,” Food Chemistry, Vol. 100, 2007, pp. 445-450. doi:10.1016/j.foodchem.2005.09.065 [25] A. Termentzi and E. Kokkalou, “LC-DAD-MS (ESI+) Analysis and Antioxidant Capacity of Crocus sativus Petal Extracts,” Planta Medica, Vol. 74, No. 5, 2008, pp. 573-581. doi:10.1055/s-2008-1074498 [26] V. L. Singleton and J. A. Rossi Jr., “Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents,” American Journal of Enology and Viticulture, Vol. 16, 1965, pp. 144-158. [27] G. L. Alonso, M. R. Salinas, J. Garijo and M. A. Sànchez, “Composition of Crocins and Picrocrocins from Spanish Saffron (Crocus sativus L.),” Food Quality, Vol. 24, No. 3, 2001, pp. 219-233. doi:10.1111/j.1745-4557.2001.tb00604.x [28] E. Pichichero, L. Canuti, and A. Canini, “Characterisation of the Phenolic and Flavonoid Fractions and Antioxidant Power of Italian Honeys of Different Botanical Origin,” Journal of the Science of Food and Agriculture, Vol. 89, No. 4, 2009, pp. 609-616. doi:10.1002/jsfa.3484 [29] W. Brand-Williams, M. E. Cuvelier and C. Berset, “Use of a Free Radical Method to Evaluate Antioxidant Activ- ity,” Food Science and Techonology, Vol. 28, No. 1, 1995, pp. 25-30. [30] I. F. F. Benzie and J. J. Strain, “The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay,” Analytical Biochemistry, Vol. 239, No. 1, 1996, pp. 70-76. doi:10.1006/abio.1996.0292 [31] A. Wojdyło, J. Oszmianski and R. Czemerys, “Antioxi- dant Activity and Phenolic Compounds in 32 Selected Herbs,” Food Chemistry, Vol. 105, No. 3, 2007, pp. 940- 949. doi:10.1016/j.foodchem.2007.04.038 [32] I. J. Fidler, “Selection of Successive Tumor Cell Lines for Metastasis,” Nature, Vol. 245, 1973, pp. 148-149. [33] M. Negbi, “Saffron Cultivation: Past, Present and Future Prospects,” In: M. Negbi, Ed., Saffron Crocus sativus L., Harwood Academic Publishers, Amsterdam, 1999, pp. 1- 19. [34] M. Bolandi, F. Shahidi, N. Sedaghat, R. Farhoush and H. Mousavi-Nik, “Shelf-Life Determination of Saffron Stig- ma: Water Activity and Temperature Studies,” World Ap- plied Sciences Journal, Vol. 5, No. 2, 2008, pp. 132-136. [35] P. Winterhalter and M. Straubinger, “Saffron-Renewed Interest in an Ancient Spice,” Food Reviews International, Vol. 16, 2000, pp. 39-59. doi:10.1081/FRI-100100281 [36] M. Bajpai, A. Mishra and D. Prakash, “Antioxidant and Free Radical Scavenging Activities of Some Leafy Vege- tables,” International Journal of Food Sciences and Nutr- ition, Vol. 56, No. 7, 2005, pp. 473-481. doi:10.1080/09637480500524299 [37] S. A. Ordoudi, C. D. Befani, N. Nenadis, G. G. Koliakos and M. Z. Tsimidou, “Further Examination of Antiradical Properties of Crocus sativus Stigmas Extract Rich in Crocins,” Journal Agricultural Food Chemistry, Vol. 57, No. 8, 2009, pp. 3080-3086. doi:10.1021/jf804041g [38] M. Sengul, H. Yildiz, N. Gungor, B. Cetin, Z. Eser and S. Ercisli, “Total Phenolic Content, Antioxidant and Antim- icrobial Activities of Some Medicinal Plants,” Pakistan Journal of Pharmaceutical Sciences, Vol. 22, No. 1, 2009, pp. 102-106. [39] S.-C. Ho and P.-W. Chang, “Inhibitory Effects of Several Spices on Inflammation Caused by Advanced Glycation End Products,” American Journal of Plant Sciences, Vol. 3, 2012, pp. 995-1002. doi:10.4236/ajps.2012.327118 [40] M. A. Papandreou, C. D. Kanakis, M. G. Polissiou, S. Efthimiopoulos, P. Cordopatis, M. Margary and F. N. Lamari, “Inhibitory Activity on Amyloid-β-Aggregation and Antioxidant Properties of Crocus sativus Stigmas Extract and Its Crocin Constituents,” Journal Agricultural Copyright © 2012 SciRes. AJPS  Biochemical, Antioxidant and Antineoplastic Properties of Italian Saffron (Crocus sativus L.) Copyright © 2012 SciRes. AJPS 1580 Food Chemistry, Vol. 54, 2006, pp. 8162-8168. doi:10.1021/jf061932a [41] D. G. Chryssanthi, F. N. Lamari, G. Iatrou, A. Pylara, K. N. Karamanos and P. Cordopatis, “Inhibition of Breast Cancer Cell Proliferation by Style Constituents of Dif- ferent Crocus Species,” Anticancer Research, Vol. 27, No. 1, 2007, pp. 357-362. [42] A. Gismondi, A. Lentini, C. Tabolacci, B. Provenzano and S. Beninati, “Transglutaminase-Dependent Antipro- liferative and Differentiative Properties of Nimesulide on B16-F10 Mouse Melanoma Cells,” Amino Acids, Vol. 38, 2010, pp. 257-262. doi:10.1007/s00726-009-0244-9
|