 American Journal of Plant Sciences, 2012, 3, 1568-1572 http://dx.doi.org/10.4236/ajps.2012.311189 Published Online November 2012 (http://www.SciRP.org/journal/ajps) Insecticidal Toxicity of Spilanthol from Spilanthes acmella Murr. against Plutella xylostella L. Anuradha Sharma1, Vishal Kumar2, Rameshwar Singh Rattan1, Neeraj Kumar2, Bikram Singh2* 1Entomology and Pesticide Residue Laboratory, Hill Area Tea Sciences (HATS) Division, Palampur, India; 2Natural Plant Product Division, Institute of Himalayan Bioresource Technology (CSIR), Palampur, India. Email: rsrattan@scientist.com, *bikram_npp@rediffmail.com Received August 12th, 2012; revised September 16th, 2012; accepted October 15th, 2012 ABSTRACT The present study explored the Spilanthes acmella Murr. for insecticidal principle, a plant of high value. The seed ex- tract showed insecticidal activity against Plutella xylostella. Further, bioassay guided isolation of bioactive compounds resulted in insecticidal active molecule, which was identified with the help of ESI-MS and NMR. Highest activity of 95 - 100 percent was observed at low dose of 2 g/l with spilanthol, while 60 - 70 and 80 - 90 percent mortality at 5 g/l in crude seed extracts prepared in methanol and hexane after 48 hours exposure, respectively. LC50 of 1.49, 5.14, 5.04, 11.75 g/l was observed with spilanthol, crude seed extract of methanol, hexane, deltamethrin, respectively. The findings indicate the potential of S. acmella with potent insecticidal toxicity for the management of P. xylostella and other in- sects of agricultural importance. Keywords: Spilanthes acmella; Insecticidal Activity; Plutella xylostella; Spilanthol; Alkamides; Extracts; Toxicity 1. Introduction Spilanthes acmella Murr. is an annual herb belonging to the compositae family distinguishable from other species by its yellow flower head; the leaves and flower have pungent taste accompanied by tingling and numbness on the tongue. The flower heads and leaves have been used for the treatment of toothache and skin diseases [1]. Sev- eral bioactive constituents including spilanthol have been isolated from this species [2,3]. Phytochemical investiga- tions of the genus Spilanthes, Acmella ciliata was found to contain 20 amides [4,5]. The extracts or its constitu- ents S. acmella showed toxic activity against insects [6- 8], bacteria [9-11], fungi [12-15] and nematodes [16]. The diamondback moth (DBM), Plutella xylostella (L.) (Lepidoptera: Plutellidae), is one of the most destructive insect pests of crucifers worldwide. Larvae of diamond- back moth, P. xylostella feed on the foliage of the cruci- ferous plants from the seedling stage to harvest, and greatly reduce the yield and quality of produce. P. xylos- tella has only become a significant pest relatively re- cently, with major problems observed in the 1970s ap- parently caused at least in part by the evolution of insec- ticide resistance [17]. It is an oligophagous species that feeds on plant species of the family Brassicaceae [18], which include economically important crops such as cabbage, cauliflower, broccoli, canola and Brussels sp- routs. As such it is a worldwide pest, costing over the past US$1 billion to control annually. Pesticides have dominated attempts to control P. xylostella for more than 40 years [19-23]. It was the first crop insect reported to be resistant to DDT and now in many crucifer-producing regions. It has shown significant resistance to almost every insecticide applied in field including biopesticides such as crystal toxins from B. thuringiensis and spino- syns from Saccharopolyspora spinosa under field condi- tions [24,25]. Insecticide resistance associated crop fail- ure has been reported in many parts of the World [26,27]. A large number of insecticides with different modes of action are available for the control of susceptible P. xy- lostella but resistance has been observed to all but the newest modes of action in one or more regions. Largely, because of the negative impact of pesticides and the in- creasing difficulty encountered in controlling diamond- back moth populations, much effort has been devoted to find alternative control measures for this pest. Botanical insecticides can influence the behavior and development of the herbivorous insects that search for or use the plant for their reproduction. The present investigations were focused on exploring the insecticidal activity of S. acme- lla extract and bioassay-guided isolation of its con- *Corresponding author. Copyright © 2012 SciRes. AJPS  Insecticidal Toxicity of Spilanthol from Spilanthes acmella Murr. against Plutella xylostella L. 1569 stituents and structural analysis. 2. Materials and Methods 2.1. Plant Material and Extraction The mature flower heads/leaves from the S. acmella Murr. were collected for preparation of extract. The air- dried flower heads of S. acmella (500 g) were ground to fine powder and extracted with hexane (20 ml/g of dry weight of the material, 20 hr), followed by filtration. The extract was fractionated by dry column chromatography over silica-gel to separate twelve fractions (A-L). The combined fractions D-F eluted with 30% ethyl acetate in hexane were rechromatographed on a silica-gel column. The column was eluted with hexane and gradually in- creasing the polarity with ethyl acetate. Eluting with hexane-ethyl acetate (9:1) yielded compound 1 (490 mg), which was identified with the help of ESI-MS and NMR analysis. The filtrates of the first extraction were com- bined and evaporated in vacuo to give a crude hexane extract (9.9 g). Similarly, the extraction was performed using ethyl acetate and methanol to obtain the corre- sponding ethyl acetate (2.5 g) and methanol (28.1 g) ex- tracts, respectively. 2.2. Screening Bioassay Leaf dip method was used to evaluate the activity of plant extract/fraction against test insect. The cabbage leaves were cut into circular shape with 34 cm2 area (di- ameter 6.5 cm). The leaves were allowed to dry at ambi- ent condition. The circular leaf discs were treated for about 30 seconds. The 2nd instar larvae were released on circular leaf discs placed in Petri plate. The Petri plates were sealed with Parafilm®. Bioassay was conducted on laboratory reared 2nd instar larvae of P. xylostella. The test was repeated 4 times (10 larvae/replicate) with three replications. The mortality was observed after 24 and 48 hours. Each experiment was repeated with negative con- trol. Toxicity effects were reported as LC50 and LC90 representing the concentration in g/l with 50 and 90 per- cent larval mortality rate after 48 hours of exposure. All experiment were conducted in a growth chamber set at 25˚C ± 2˚C, 16:8 h (dark:light) photoperiod and 65% RH. The larval mortality data were corrected for control mor- tality by the formula of Abbott [28]. LC50, LC90 and 95% confidence limits (upper and lower) were analyzed using EPA Probit analysis (Version 1.5) software based on Finney’s Probit Analysis [29,30]. 2.3. Experimental NMR spectra were recorded on a Bruker Avance-300 spectrometer. Mass spectra were recorded on QTOF- Micro of Waters Micromass. TLC was performed on silica gel 60 F254 plates (60 - 120 mesh) for column chro- matography (Sisco Research Laboratories Pvt. Ltd.). 3. Results and Discussion The present study revealed the toxicity of botanical ex- tract from the flower heads of Spilanthes acmella against 2nd instar larvae of P. xylostella. Compound 1 i.e. spi- lanthol showed the 95 - 100 percent mortality as com- pared to crude extracts of hexane (70 - 80) and methanol (60% - 70%) after 48 hour exposure. The LC50 of spi- lanthol, crude seed extracts in hexane and methanol was 1.49, 5.14, and 5.04 g/l, respectively (Table 1). Del- tamethrin (Decis®) was used as reference standard in the studies (LC50: 11.75). In preliminary studies, leaf extracts in hexane showed only 2 - 3 percent mortality against 2nd instar of P. xylostella larvae after 48 hours of expo- sure. During the experiment, it was observed that there is a striking difference between the levels of mortality caused by these extracts. i.e. 70 - 80 per cent larvae ob- served as moribund with in one hour of exposure to spi- lanthol at a concentration of 2 g/l. The insecticidal activ- ity of plant extracts could be attributed to either the ma- or compound of oil, or to the synergistic/or antagonistic effects of all the components present in the mixture [31,32]. Volatile compounds extracted from plants and their constituents have been shown to be potent source of botanical pesticide [33-35]. On the other hand, the chemical composition could also vary depending on the geographical area, the collection season, the parts of the plant used for distillation (leaf, stem, flowers, roots), and the presence of chemotypes or chemical components present within the same species [36,37]. Twelve fractions (A-L) were collected and combined. Fractions D-F were eluted with hexane:ethyl acetate (7:3). The bioactive fractions (D-F) were purified by column chromatography over silica-gel eluting with hexane and gradually increasing the polarity by ethyl acetate. Eluting with hexane-ethyl acetate (9:1) yielded compound 1 (490 mg), which was identified as spilanthol with the help of ESI-MS and NMR analysis [38]. 4. Discussion Spilanthol was found active against P. xylostella. This is the first report of the compound with insecticidal activity against P. xylostella from the flower heads of S. acmella. Previously, the extracts from Spilanthes acmella have been mostly identified toxic against different mosquito species (i.e. Anapheles, Culex and Aedes). Electrophysio- logical studies showed immediate hyperexcitation fol- lowed by complete inhibition of cercal nerve activity [39]. Maximum mortality occurred with flower head ex- tracts (Table 1). The authors concluded that spilanthol and alkamides are responsibe for toxic effects [32]. Be- l Copyright © 2012 SciRes. AJPS 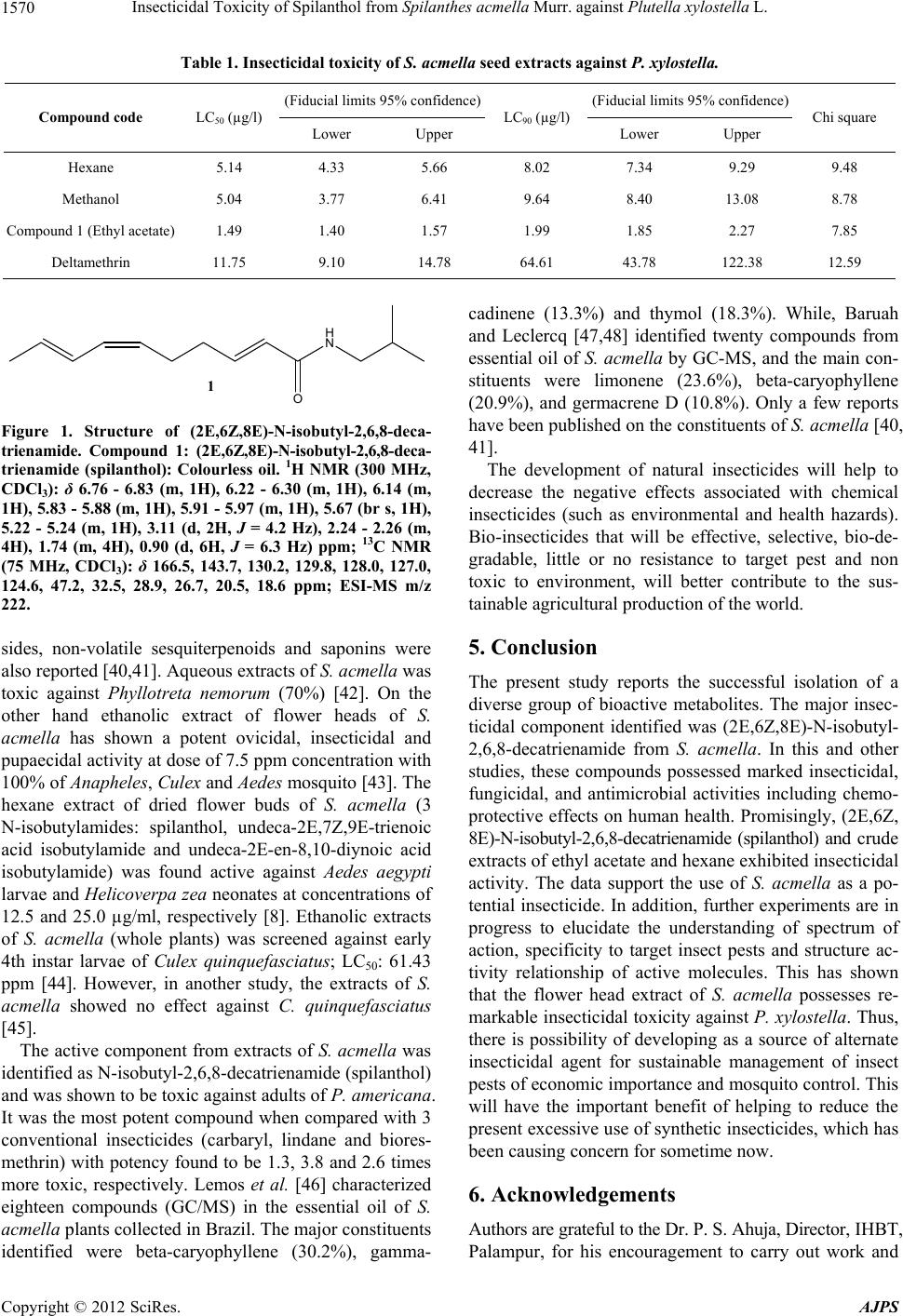 Insecticidal Toxicity of Spilanthol from Spilanthes acmella Murr. against Plutella xylostella L. Copyright © 2012 SciRes. AJPS 1570 Table 1. Insecticidal toxicity of S. acmella seed extracts against P. xylostella. (Fiducial limits 95% confidence)(Fiducial limits 95% confidence) Compound code LC50 (µg/l) Lower Upper LC90 (µg/l) Lower Upper Chi square Hexane 5.14 4.33 5.66 8.02 7.34 9.29 9.48 Methanol 5.04 3.77 6.41 9.64 8.40 13.08 8.78 Compound 1 (Ethyl acetate) 1.49 1.40 1.57 1.99 1.85 2.27 7.85 Deltamethrin 11.75 9.10 14.78 64.61 43.78 122.38 12.59 H N O 1 Figure 1. Structure of (2E,6Z,8E)-N-isobutyl-2,6,8-deca- trienamide. Compound 1: (2E,6Z,8E)-N-isobutyl-2,6,8-deca- trienamide (spilanthol): Colourless oil. 1H NMR (300 MHz, CDCl3): δ 6.76 - 6.83 (m, 1H), 6.22 - 6.30 (m, 1H), 6.14 (m, 1H), 5.83 - 5.88 (m, 1H), 5.91 - 5.97 (m, 1H), 5.67 (br s, 1H), 5.22 - 5.24 (m, 1H), 3.11 (d, 2H, J = 4.2 Hz), 2.24 - 2.26 (m, 4H), 1.74 (m, 4H), 0.90 (d, 6H, J = 6.3 Hz) ppm; 13C NMR (75 MHz, CDCl3): δ 166.5, 143.7, 130.2, 129.8, 128.0, 127.0, 124.6, 47.2, 32.5, 28.9, 26.7, 20.5, 18.6 ppm; ESI-MS m/z 222. sides, non-volatile sesquiterpenoids and saponins were also reported [40,41]. Aqueous extracts of S. acmella was toxic against Phyllotreta nemorum (70%) [42]. On the other hand ethanolic extract of flower heads of S. acmella has shown a potent ovicidal, insecticidal and pupaecidal activity at dose of 7.5 ppm concentration with 100% of Anapheles, Culex and Aedes mosquito [43]. The hexane extract of dried flower buds of S. acmella (3 N-isobutylamides: spilanthol, undeca-2E,7Z,9E-trienoic acid isobutylamide and undeca-2E-en-8,10-diynoic acid isobutylamide) was found active against Aedes aegypti larvae and Helicoverpa zea neonates at concentrations of 12.5 and 25.0 µg/ml, respectively [8]. Ethanolic extracts of S. acmella (whole plants) was screened against early 4th instar larvae of Culex quinquefasciatus; LC50: 61.43 ppm [44]. However, in another study, the extracts of S. acmella showed no effect against C. quinquefasciatus [45]. The active component from extracts of S. acmella was identified as N-isobutyl-2,6,8-decatrienamide (spilanthol) and was shown to be toxic against adults of P. americana. It was the most potent compound when compared with 3 conventional insecticides (carbaryl, lindane and biores- methrin) with potency found to be 1.3, 3.8 and 2.6 times more toxic, respectively. Lemos et al. [46] characterized eighteen compounds (GC/MS) in the essential oil of S. acmella plants collected in Brazil. The major constituents identified were beta-caryophyllene (30.2%), gamma- cadinene (13.3%) and thymol (18.3%). While, Baruah and Leclercq [47,48] identified twenty compounds from essential oil of S. acmella by GC-MS, and the main con- stituents were limonene (23.6%), beta-caryophyllene (20.9%), and germacrene D (10.8%). Only a few reports have been published on the constituents of S. acmella [40, 41]. The development of natural insecticides will help to decrease the negative effects associated with chemical insecticides (such as environmental and health hazards). Bio-insecticides that will be effective, selective, bio-de- gradable, little or no resistance to target pest and non toxic to environment, will better contribute to the sus- tainable agricultural production of the world. 5. Conclusion The present study reports the successful isolation of a diverse group of bioactive metabolites. The major insec- ticidal component identified was (2E,6Z,8E)-N-isobutyl- 2,6,8-decatrienamide from S. acmella. In this and other studies, these compounds possessed marked insecticidal, fungicidal, and antimicrobial activities including chemo- protective effects on human health. Promisingly, (2E,6Z, 8E)-N-isobutyl-2,6,8-decatrienamide (spilanthol) and crude extracts of ethyl acetate and hexane exhibited insecticidal activity. The data support the use of S. acmella as a po- tential insecticide. In addition, further experiments are in progress to elucidate the understanding of spectrum of action, specificity to target insect pests and structure ac- tivity relationship of active molecules. This has shown that the flower head extract of S. acmella possesses re- markable insecticidal toxicity against P. xylostella. Thus, there is possibility of developing as a source of alternate insecticidal agent for sustainable management of insect pests of economic importance and mosquito control. This will have the important benefit of helping to reduce the present excessive use of synthetic insecticides, which has been causing concern for sometime now. 6. Acknowledgements Authors are grateful to the Dr. P. S. Ahuja, Director, IHBT, Palampur, for his encouragement to carry out work and  Insecticidal Toxicity of Spilanthol from Spilanthes acmella Murr. against Plutella xylostella L. 1571 gratefully acknowledge CSIR for financial grant. REFERENCES [1] D. M. Verma and N. P. Balakrishnana and R. D. Dixit, “Flora of Madhya Pradesh,” Botanical Survey of India, Kolkotta, Vol. 1, 1993, pp. 612-613. [2] V. G. Gokhale and B. V. Bhide, “Chemical Investigation of Spilanthes acmella,” Journal of Indian Chemical Soci- ety, Vol. 22, 1945, pp. 250-252. [3] S. Prachayasittikul, S. Suphapong, A. Worachartcheewan, R. Lawung, S. Ruchirawat and V. Prachayasittikul, “Bio- active Metabolites from Spilanthes acmella Murr.,” Mo- lecules, Vol. 14, No. 2, 2009, pp. 850-867. doi:10.3390/molecules14020850 [4] R. Martin and H. Becker, “Spilanthol-Related Amides from Acmella ciliate,” Phytochemistry, Vol. 23, No. 8, 1984, pp. 1781-1783. doi:10.1016/S0031-9422(00)83490-1 [5] R. Martin and H. Becker, “Amides and Other Constitu- ents from Acmella ciliate,” Phytochemistry, Vol. 24, No. 10, 1985, pp. 2295-2300. doi:10.1016/S0031-9422(00)83030-7 [6] A. M. Broussalis, G. E. Ferraro, V. S. I. Martino, R. Pin- zon, J. D. Coussio and J. C. Alvarez, “Argentine Plants as Potential Source of Insecticidal Compounds,” Journal of Ethnopharmacology, Vol. 67, No. 2, 1999, pp. 219-223. doi:10.1016/S0378-8741(98)00216-5 [7] V. Pandey, V. Agrawal, K. Raghavendra and A. P. Dash, “Strong Insecticidal Activity of Three Species of Spilan- thes (Akarkara) against Malaria (Anopheles stephensi Liston, Anopheles culicifacies, species C) and Filaria Vector (Culex quinquefasciatus Say),” Parasitology Re- search, Vol. 102, 2007, pp. 171-174. doi:10.1007/s00436-007-0763-9 [8] R. S. Ramsewak, A. J. Erickson and M. G. Nair, “Bioac- tive N-Isobutylamides from the Flower Buds of Spilan- thes acmella,” Phytochemistry, Vol. 51, 1999, pp. 729- 732. doi:10.1016/S0031-9422(99)00101-6 [9] W. Fabry, P. Okemo and R. Ansorg, “Activity of East African Medicinal Plants against Helicobacter pylori,” Chemotherapy Basel, Vol. 42, No. 5, 1996, pp. 315-317. [10] S. A. Rani and S. U. Murty, “Evaluation of Antimicrobial Activity of Spilanthes acmella Flower Head Extract,” Journal of Natural Remedies, Vol. 5, No. 2, 2005, pp. 170-171. [11] G. V. M. Sharma, T. Shekharam and V. Upender, “Stere- oconvergent Synthesis of a Potent Mosquito Larvicide: (2E,4E,8E,10Z)-N-(2-methylprpyl)-2,4,8,10-dodecatetrae neamide,” Tetrahedron, Vol. 46, 1990, pp. 5665-5672. doi:10.1016/S0040-4020(01)87765-6 [12] W. Fabry, P. Okemo and R. Ansorg, “Fungistatic and Fungicidal Activity of East African Medicinal Plants,” Mycoses, Vol. 39, No. 1-2, 1996, pp. 67-70. doi:10.1111/j.1439-0507.1996.tb00087.x [13] M. K. Rai, A. Verma and A. K. Pandey, “Antifungal Ac- tivity of Spilanthes calva after Inoculation of Pirifor- maspora indica,” Mycoses, Vol. 47, 2002, pp. 479-481. doi:10.1111/j.1439-0507.2004.01045.x [14] S. Phongpaichit, S. Subhadhirasakul and C. Wattanapi- romsakul, “Antifungal Activities of Extracts from Thai Medicinal Plants against Opportunistic Fungal Pathogens Associated with AIDS Patients,” Mycoses, Vol. 48, No. 5, 2005, pp. 333-338. doi:10.1111/j.1439-0507.2005.01142.x [15] S. A. Rani and S. U. Murty, “Antifungal Potential of Flower Head Extract of Spilanthes acmella Linn.,” Afri- can Journal of Biomedical Research, Vol. 9, No. 1, 2006, pp. 67-68. [16] R. Pandey, “Application of Botanicals for Management of Root-Knot Nematode Disease of Ammi majus L.,” Indian Journal of Nematology, Vo. 32, No. 2, 2002, pp. 198-200. [17] N. S. Talekar and A. M. Shelton, “Biology, Ecology and Management of the Diamondback Moth,” Annual Review of Entomology, Vol. 38, No. 1, 1993, pp. 275-301. doi:10.1146/annurev.en.38.010193.001423 [18] A. J. Thorsteinson, “The Chemotactic Responses That Determine Host Specificity in an Oligophagous Insect (Plutella maculipennis (Curt.) Lepidoptera),” Canadian Journal of Zoology, Vol. 31, No. 1, 1953, pp. 52-72. doi:10.1139/z53-006 [19] A. R. Syed, “Insecticide Resistance in the Diamondback Moth in Malaysia,” Proceedings of 2nd International Workshop, Taiwan, 10-14 December 1990, 603 p. [20] A. M. Shelton, F. V. Sances, J. Hawley, J. D. Tang, V.M. Boune, et al., “Assessment of Insecticide Resistance after the Outbreak of Diamondback Moth (Lepidoptera: Plutel- lidae) in California in 1997,” Journal of Economic Ento- mology, Vol. 93, No. 3, 2000, pp. 931-936. doi:10.1603/0022-0493-93.3.931 [21] A. M. Shelton, J. A. Wyman, N. L. Cushing, K. Apfel- beck, T. J. Dennehy, et al., “Insect Resistance of Dia- mondback Moth (Lepidoptera: Plutellidae) in North America,” Journal of Economic Entomology, Vol. 86, No. 1, 1993, pp. 11-19. [22] G. P. Georghiou, “The Occurrence of Resistance to Pesti- cides in Arthropods. An Index of Cases Reported through 1980,” Accession No: XF8227745, FAO, Rome, 1981, pp. 25-30. [23] T. Saito, T. Miyata, N. Sinchaisri and A. Vattanatangum, “Management of Brown Plant Hopper and Resistance of Diamondback Moth,” Nagoya University Corp. Press, Nagoya, 1995, pp. 128-147. [24] B. E. Tabashnik, Y. Carriere, T. J. Dennehy, S. Morin, M. S. Sisterson, et al., “Insect Resistance to Transgenic Bt crops: Lessons from the Laboratory and Field,” Journal of Economic Entomology, Vol. 96, No. 4, 2003, pp. 1031- 1038. doi:10.1603/0022-0493-96.4.1031 [25] M. Sarfraz and B. A. Keddie, “Conserving the Efficacy of Insecticides against Plutella xylostella (L.) (Lep., Plutel- lidae),” Journal of Applied Entomology, Vol. 129, No. 3, 2005, pp. 149-157. doi:10.1111/j.1439-0418.2005.00930.x [26] N. Endersby and P. Ridland, “Insecticide Resistance in Victorian Populations of Diamondback Moth, Plutella xylostella (L.),” Australian Entomological Society, 1994, Copyright © 2012 SciRes. AJPS  Insecticidal Toxicity of Spilanthol from Spilanthes acmella Murr. against Plutella xylostella L. Copyright © 2012 SciRes. AJPS 1572 p. 31. [27] C. N. Sun, “Insecticide Resistance in Diamondback Moth. In Diamondback Moth and Other Crucifera Pests,” Pro- ceedings of 2nd International Workshop, Taiwan, 10-14 December 1990, 1992, pp. 419-426. [28] W. S. Abbott, “A method of Computing the Effectiveness of an Insecticide,” Journal of Economic Entomology, Vol. 18, 1925, pp. 265-267. [29] E. P. A., “Probit Analysis,” Version 1.5, 2012. http://www.epa.gov/nerleerd/stat2.htm. [30] D. J. Finney, “Probit Analysis,” 3rd Edition, Cambridge University Press, Cambridge, 1971. [31] M. Vollinger, “The Possible Development of Resistance against Neem Seed Kernel Extract and Deltamethrin in Plutella xylostella,” In: H. Schmutterer and K. R. S. Ascher, Eds., Natural Pesticides from the Neem Tree (Azadirachta indica A. Juss) and Other Tropical Plants, Proceedings of 3rd International Neem Conference, Ger- man Agency for Technical Cooperation (GTZ), Eschborn, 1987, pp. 543-554. [32] H. Schmutterer, “Potential of Azadirachtin Containing Pesticides for Integrated Pest Control in Developing and Industrialized Countries,” Journal of Insect Physiology, Vol. 34, 1988, pp. 713-719. doi:10.1016/0022-1910(88)90082-0 [33] E. Guenther, “The Essential Oil,” Vol. 1, D. Van Nos- trand Co. Inc., New York, 1948. [34] S. K. Shaaya, M. Kostjukovski, J. Eilberg and C. Suk- prakarn, “Plant Oils as Fumigants & Contact Insecticides for the Control of Stored Product Insects,” Journal of Stored Products Research, Vol. 33, No. 1, 1997, pp. 7-15. doi:10.1016/S0022-474X(96)00032-X [35] M. B. Isman, “Plant Essential Oils for Pest and Disease Management,” Crop Protection, Vol. 19, No. 8, 2000, pp. 603-608. doi:10.1016/S0261-2194(00)00079-X [36] R. Granget and J. Passet, “Thymus vilgaris Spontane de France: Races Chimiques et Chemotaxonomie,” Phyto- chemistry, Vol. 12, 1973, pp. 1683-1691. doi:10.1016/0031-9422(73)80388-7 [37] B. Benjilali and H. Richards, “Etude de Quelques Peu- plements d’Armoise Blanche du Maroc,” Rivista Italiana, Vol. 62, 1980, pp. 69-74. [38] N. Nakatani and M. Nagashima, “Pungenet Alkamides from Spilanthes acmella L. var Oleraceae Clark,” Bio- sciences, Biotechnology and Biochemistry, Vol. 56, 1992, pp. 759-762. doi:10.1271/bbb.56.759 [39] H. A. Kadir, M. B. Zakaria, A. A. Kechil and M. S. Azirun, “Toxicity and Electrophysiological Effects of Spilanthes acmella Murr. Extracts on Periplaneta ameri- cana L.,” Pesticide Science, Vol. 25, No. 4, 1989, pp. 329-335. doi:10.1002/ps.2780250402 [40] N. R. Krishnaswamy, S. Prasanna, T. R. Seshadri and T. N. C. Vedantham, “α and β-Amyrin Esters and Sitosterol Glucoside from Spilanthes acmella,” Phytochemistry, Vol. 14, 1975, pp. 1666-1667. doi:10.1016/0031-9422(75)85386-6 [41] D. K. Mukharya and A. H. Ansari, “Olean-12-en-3-O- beta-D-galactopyranosyl(1→4)-O-alpha-L-rhamnopyrano side: A New Triterpenoidal Saponin from the Roots of Spilanthes acmella (Murr.),” Indian Journal of Chemistry B, Vol. 26, 1987, pp. 81-87. [42] I. P. Subedi and K. Vaidya, “Control of Flea Beetle, Phyllotreta nemorum L. (Coleoptera: Crysomelidae) Using Available Natural Resources,” Himalayan Journal of Sciences, Vol. 1, No. 2, 2003, pp. 111-114. [43] D. K. Saraf and V. K. Dixit, “Spilanthes acmella Murr.: Study on Its Extract Spilanthol as Insecticidal Com- pound,” Asian Journal of Experimental Sciences, Vol. 16, No. 1-2, 2002, pp. 9-19. [44] B. Pitasawat, W. Choochote, D. Kanjanapothi, A. Pan- thong, A. Jitpakdi and U. Chaithong, “Screening for In- secticidal Activity of Ten Carminative Plants,” Southeast Asian Journal of Tropical Medicine and Public Health, Vol. 29, 1998, pp. 660-662. [45] P. Samiron and M. C. Kalita, “Effect of Some Indigenous Plants Extracts of N. E. India against Aedes aegypti and Culex quinquefasciatus,” Medical Entomology & Zoology, Vol. 55, No. 4, 2004, pp. 325-327. [46] T. L. G. Lemos, O. D. L. Pessoa, F. J. A. Matos, J. W. Alencar and A. A. Craveiro, “The Essential Oil of Spi- lanthes acmella Murr,” Journal Essential Oil Research, Vol. 3, 1991, pp. 369-370. doi:10.1080/10412905.1991.9697962 [47] R. N. Baruah and P. A. Leclercq, “Characterization of the Essential Oil from Flower Heads of Spilanthes acmella,” Journal Essential Oil Research, Vol. 5, 1993, pp. 693- 695. doi:10.1080/10412905.1993.9698310 [48] A. H. Ansari, D. K. Mukharya and V. K. Saxena, “Anal- gesic study of N-isobutyl-4,5-decadienamide Isolated from the Flowers of Spilanthes acmella (Murr),” Indian Journal of Pharmaceutical Sciences, Vol. 50, 1988, 106 p.
|