Paper Menu >>
Journal Menu >>
 Intelligent Control and Automation, 2010, 1, 15-19 doi:10.4236/ica.201.11002 Published Online August 2010 (http://www.SciRP.org/journal/ica) Copyright © 2010 SciRes. ICA Optimal Control Strategy for a Fully Determined HIV Model Mohammad Shirazian1, Mohammad Hadi Farahi1,2 1Department of Applied Mathematics, Ferdowsi University of Mashhad, Mashhad, Iran 2The Center of Excellence in Modeling and Computations in Linear and Nonlinear Systems Ferdowsi University of Mashhad, Mashhad, Iran E-mail: mo.shirazian@stu-mail.um.ac.ir, farahi@math.um.ac.ir Received January 25, 2010; revised March 21, 2010; accepted June 22, 2010 Abstract This paper shows how mathematical methods can be implemented to formulate guidelines for clinical testing and monitoring of HIV/AIDS disease. First, a mathematical model for HIV infection is presented which the measurement of the CD4+T cells and the viral load counts are needed to estimate all its parameters. Next, through an analysis of model properties, the minimal number of measurement samples is obtained. In the sequel, the effect of Reverse Transcriptase enzyme Inhibitor (RTI) on HIV progression is demonstrated by using a control function. Also the total cost of treatment by this kind of drugs has been minimized. The nu- merical results are obtained by a numerical method in discretization issue, called AVK. Keywords: HIV/AIDS, Mathematical Modeling, System Identification, Control Theory, Immunotherapy 1. Introduction Despite tremendous effort for mathematical modeling of HIV/AIDS (for example, see [1-4]), estimation of model parameters has not been attended a lot. For example, in [2,5,6], only the virus clearance rate and the death rate of infected CD4+T cells have been estimated. The impor- tance of parameter estimation in models, is due to pre- dicting “set-points” in the early infection stage for mak- ing the desired treatment decisions (See [7]). One of the objectives of this paper is presenting a re- alistic model, i.e. the basic model of HIV, and estimating all its parameters. It is necessary to mention that one can identify all of the model parameters by using measured output (For more details see [4]). Another objective is to add a control function to the identified basic model which plays the role of reverse transcriptase enzyme inhibitor drug in disease progres- sion. In the sequel, the optimal control model of HIV will be solved by a method in discretization issue, called AVK. Numerical results are obtained using mathematical softwares, LINGO and MATLAB. 2. Translating Biological Knowledge to Ordinary Differential Equations (ODE) To make ODE’s from biological knowledge, first we need some syntax. For example, if we denote the count of uninfected and infected CD4+T helper cells, with a and b, respectively, the syntax “0a” can be used to present this biological descriptions: “Uninfected CD4+T cells die” and the syntax “ab bb” can present: “The reaction between two infected and uninfected CD4+T cells produces two infected CD4+T cells”. Now, for translating these syntaxes to the corresponding ODE’s, we use Mass action law. This law says: “The rate of change of products is proportional to the product of re- actants concentration”. So if the syntax “ab c” is obtained, according to the mass action law, we can write ckab , for k > 0, where dc dt is denoted by c . Two other reactions in the previous syntax is dying a and b reactants, while producing c. So we have also these two ODE's as: akab and bkab , for k > 0. Fi- nally, the desired ODE, corresponding to the syntax “ab c ” is 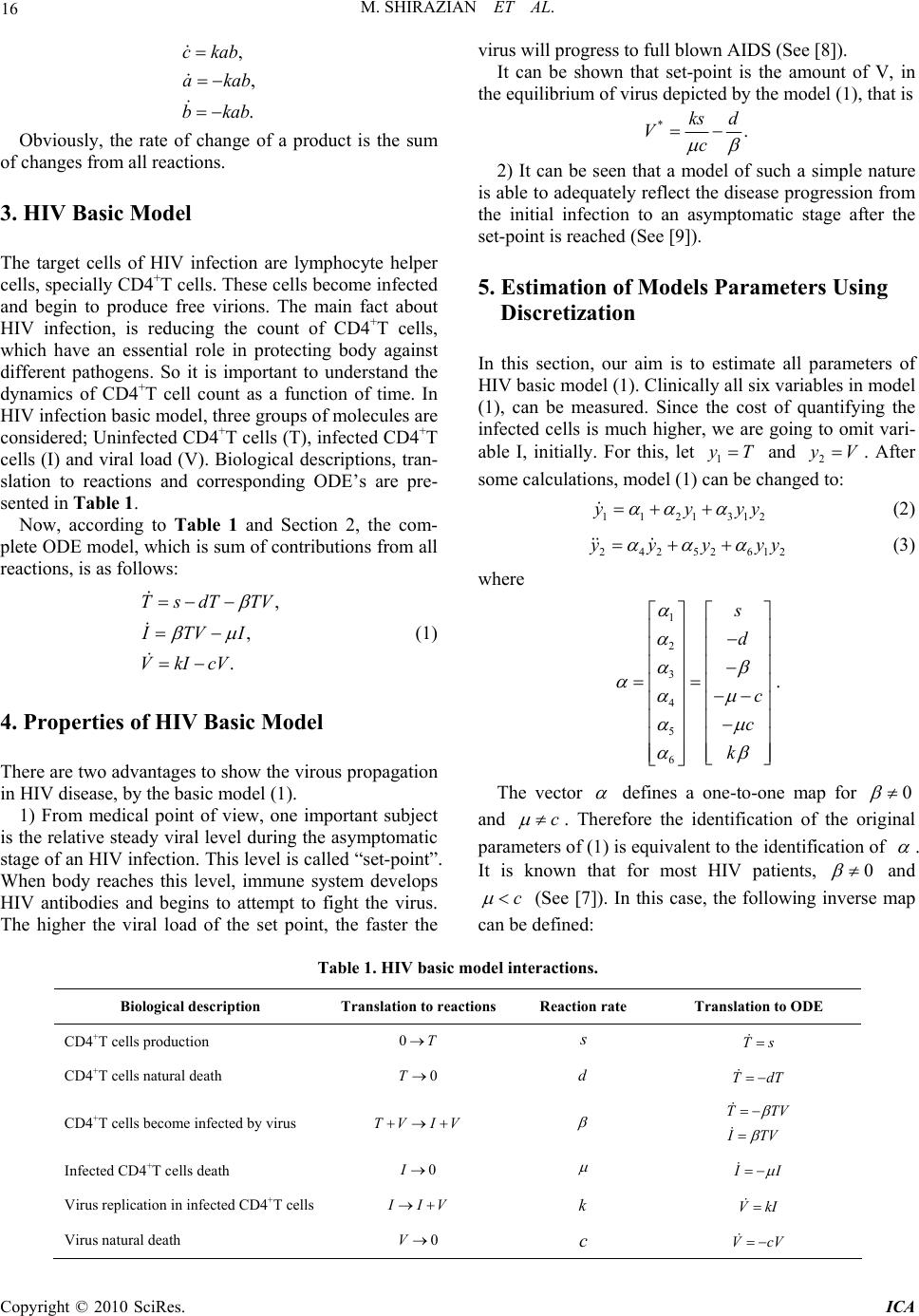 M. SHIRAZIAN ET AL. Copyright © 2010 SciRes. ICA 16 , , . ckab akab bkab Obviously, the rate of change of a product is the sum of changes from all reactions. 3. HIV Basic Model The target cells of HIV infection are lymphocyte helper cells, specially CD4+T cells. These cells become infected and begin to produce free virions. The main fact about HIV infection, is reducing the count of CD4+T cells, which have an essential role in protecting body against different pathogens. So it is important to understand the dynamics of CD4+T cell count as a function of time. In HIV infection basic model, three groups of molecules are considered; Uninfected CD4+T cells (T), infected CD4+T cells (I) and viral load (V). Biological descriptions, tran- slation to reactions and corresponding ODE’s are pre- sented in Table 1. Now, according to Table 1 and Section 2, the com- plete ODE model, which is sum of contributions from all reactions, is as follows: , , . TsdT TV ITVI VkIcV (1) 4. Properties of HIV Basic Model There are two advantages to show the virous propagation in HIV disease, by the basic model (1). 1) From medical point of view, one important subject is the relative steady viral level during the asymptomatic stage of an HIV infection. This level is called “set-point”. When body reaches this level, immune system develops HIV antibodies and begins to attempt to fight the virus. The higher the viral load of the set point, the faster the virus will progress to full blown AIDS (See [8]). It can be shown that set-point is the amount of V, in the equilibrium of virus depicted by the model (1), that is *. ks d Vc 2) It can be seen that a model of such a simple nature is able to adequately reflect the disease progression from the initial infection to an asymptomatic stage after the set-point is reached (See [9]). 5. Estimation of Models Parameters Using Discretization In this section, our aim is to estimate all parameters of HIV basic model (1). Clinically all six variables in model (1), can be measured. Since the cost of quantifying the infected cells is much higher, we are going to omit vari- able I, initially. For this, let 1 yT and 2 yV . After some calculations, model (1) can be changed to: 1121312 yyyy (2) 24252612 yyyyy (3) where 2 3 4 5 6 1s d c c k . The vector defines a one-to-one map for 0 and c . Therefore the identification of the original parameters of (1) is equivalent to the identification of . It is known that for most HIV patients, 0 and c (See [7]). In this case, the following inverse map can be defined: Table 1. HIV basic model interactions. Biological description Translation to reactionsReaction rate Translation to ODE CD4+T cells production 0T s Ts CD4+T cells natural death 0T d TdT CD4+T cells become infected by virus TV IV TTV I TV Infected CD4+T cells death 0I I I Virus replication in infected CD4+T cells I IV k VkI Virus natural death 0 V c VcV  M. SHIRAZIAN ET AL. Copyright © 2010 SciRes. ICA 17 1 2 3 2 44 5 2 44 5 6 3 4 2 4 2 s d c k . (4) Since there are three unknown parameters in each of Equation (2) and (3), it is necessary to generate at least two other equations based on each of them. This will be achieved by differentiating (2) and (3) more times, and produce upper derivatives of 1 y and 2 y. So one can concludes that at least four measurements of 1 y, CD4+T cell count, and five measurements of 2 y, viral load, are needed for a complete determination of model (1) pa- rameters (See [7]). Assume that the following measurements are avail- able. By discretization of Equations (2) and (3), and substi- tuting the approximated values of first derivative of 1 y and the first and second derivatives of 2 y, we found that 1 11 112123 1 ,0,1,2 ii iii i yy yyy i d (5) 1 22 425126 1 21 1 2222 12 1 1,0,1,2 ii iii i iiii ii i yy yyy d yyyy i dd d (6) Or in matrix form, we have 10 11 1 000 112121 111 11 1122 2 222 112 332 11 3 1 1 1 yy d yyy yy yyy d yyy yy d Similar matrix form can be obtained from (6). Thus, the variables i , i = 1, 2, ..., 6 and then from (4), all of the basic model parameters can be calculated. As an example, we considered the basic model (1), where the following estimated parameters are as Xia [7]. 7, 0.007,0.00000042163, 0.0999,0.2,90.67 . sd ck (7) Table 2. Available measurements for the count of CD4+T cells and viral load. Time (t) CD4+T cell count (1 y) Viral load (2 y) 0 t 0 1 y 0 2 y 01 td 1 1 y 1 2 y 012 tdd 2 1 y 2 2 y 0123 tdd d 3 1 y 3 2 y 01234 tdd dd – 4 2 y The solution of model (1) for [0,1000]t, with the initial values 01000T , 00I and 07000V, can be determined using the well-known numerical methods like RK4. The graphs of the propagation of healthy CD4+T cells, infected CD4+T cells and virous loads, re- spectively, are shown in Figure 1. 6. HIV Infection Optimal Control Model There are three convenient groups of drugs for AIDS retroviral therapy; Reverse transcriptase, Protease, and Integrase enzyme inhibitors. In this section, we study the role of reverse transcriptase inhibitors. The main action of this kind of drugs is preventing uninfected lymphocyte cells, to be infected by viral load. According to Table 1, Figure 1. The solution of basic model of HIV, model (1).  M. SHIRAZIAN ET AL. Copyright © 2010 SciRes. ICA 18 this action is equivalent to the reaction TV IV. So we control the first equation to prevent the transmis- sion of uninfected cells to infected ones. This control function is called ()ut, where 0()1ut. The most drug efficiency is in the case 1u which means CD4+T cells are not infected by viral load anymore. At the other side, 0u is the case which the drug does not change the disease progression. By above argument, the control system is as: (1 ), (1 ), . TsdT TV u ITVu I VkIcV (8) Using [10], consider the objective functional to be de- fined as: 0 2 1 ,() 2 f t t J TuTtut dt (9) where 110 . Our goal is maximizing the objective functional (9) subject to the control system (8); that is, maximizing the total count of CD4+T cells and minimiz- ing the costs of treatment by applying some RTI drugs. The solution of this optimal control problem should be calculated by numerical methods. We have used a special discretization method, called AVK. For a detailed explanation of this method, see [11]. In AVK method, for solving the optimal control prob- lem, 0 Min,, , f t t J xu gxtuttdt (10) Subject to: 0 00 ,,, , , f ff x tfxtuttttt xtxxtx (11) the following steps should be applied: Step 1. Form the total error function 1 E as: 0 1,,, f t t Exuxt fxtuttdt Step 2. Combine the total error function with the ob- jective functional (10) as follows: 01 2 00 Min, , ,, subjectto :, f t t ff gxtutt x tfxtuttdt xtxxtx (12) where nonnegative numbers 1 and 2 are two given weights and 12 1 . Step 3. In order to control the error, add the following constraint, 1,Exu to the optimal control problem in Step 2. So the modified optimal control problem (10)-(11) can be formulated as: 0 0 1 2 00 Min, , ,, subjectto : ,, , f f t t t t ff gxtutt x tfxtuttdt xtfxtuttdt xtxxtx (13) Step 4. Calculate i ut by minimizing the optimal control problem (13) using discretization method. For example, if the norm function ., is norm 1, then one can solve the following optimization problem: 0 1 21 0 1 0 1 1 0 Min ,,,, subject to : ,, , n iiii iii h n iiii h ff hgxutxfxut hx xtx xt u x fxt (14) where 0f tt hn , 0i ttih , () ii x xt, () ii uut and 1 () ii ii x x xxt h for 0,1,., 1in and n . Step 5. By the means of () i ut for every i t, from (11), it is easy to find () i x t, for any i, 0,1,., 1 in . We use this technique to solve the control problem (8) with the objective functional (9). The parameters used in the basic control model (8) are exactly as (7). Assume that the treatment begins when CD4+T cells reach their minimum count, in the absence of drug. According to Figure 1, (129) 363T is the mini- mum count of CD4+T cells. So the treatment interval is [129, 1000] day. Also, note that by Figure 1, at t = 129, we have (129) 57I and (129) 28860V. Now, we divide [129, 1000] into n parts with length h. The discretization form of (14) is: 12 1 0 2 000 1 Max 2 1 1 subjectto:,,0,01,0,1, 2,..., 363, 57,28860 n ii h iiiii iiiii iii iii i hTu TsdT TVu ITVuI VkIcV TIVu in TIV  M. SHIRAZIAN ET AL. Copyright © 2010 SciRes. ICA 19 Figure 1. The solution of optimal control problem (8)-(9), using AVK method. where assumed 12 1 2 . The results of this optimization problem which ob- tained by LINGO and MATLAB softwares for 200n and 6 10 , are depicted in Figure 2. 7. Conclusions In this paper, the parameter of the basic model of HIV/ AIDS is estimated only by measurement of the CD4+T cells and the viral load count. Since the suggested mod- els for HIV, or infectious diseases like consumption, cholera, influenza and etc., have unknown parameters which should be estimated, one can use the proposed method in this paper to estimate the parameters of such models. One of the most important kinds of drug treatments for HIV immunotherapy is assumed. One can investigate the effects of other drugs, like Protease enzyme inhibitors in preventing AIDS progression. In these cases, one can use the described discretization method for solving such op- timal control problems. 8. References [1] D. Covert and D. Kirschner, “Revisiting Early Models of the Host-Pathogen Interactions in HIV Infection,” Com- ments Theoretical Biology, Vol. 5, No. 6, 2000, pp. 383- 411. [2] M. A. Nowak and R.M. May, “Virus Dynamics: Mathe- matical Principles of Immunology and Virology,” Oxford University Press, New York, 2000. [3] A. S. Perelson and P. W. Nelson, “Mathematical Analysis of HIV-1 Dynamics in Vivo,” SIAM Review, Vol. 41, No. 1, 1999, pp. 3-44. [4] W.-Y. Tan and H. Wu, “Deterministic and Stochastic Models of AIDS Epidemics and HIV Infections with In- tervention,” World Scientific, Singapore, 2005. [5] M. A. Nowak and C. R. M. Bangham, “Population Dy- namics of Immune Responses to Persistent Viruses,” Sci- ence, Vol. 272, No. 5758, 1996, pp. 74-79. [6] X. Wei, S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch and J. D. Lifson, “Viral Dynamics in HIV-1 Infection”, Nature, Vol. 273, No. 6510, 1995, pp. 117-122. [7] X. Xia, “Estimation of HIV/AIDS parameters,” Auto- matica, Vol. 39, No. 11, 2003, pp. 1983-1988. [8] R. Pattman, M. Snow, P. Handy, K. N. Sankar and B. Elawad, “Oxford Handbook of Genitourinary Medicine, HIV and AIDS,” Oxford University Press, USA, 2005. [9] X. Xia, “Modelling of HIV Infection: Vaccine Readiness, Drug Effectiveness and Therapeutical Failures,” Journal of Process Control, Vol. 17, No. 3, 2007, pp. 253-260. [10] K. R. Fister and S. Lenhart, “Optimizing Chemotherapy in an HIV Model,” Journal of Differential Equations, Vol. 1998, No. 32, 1998, pp. 1-12. [11] K. P. Badakhshan and A. V. Kamyad, “Numerical Solu- tion of Nonlinear Optimal Control Problems Using Non- linear Programming,” Applied Mathematics and Compu- tation, Vol. 187, No. 2, 2007, pp. 1511-1519. |

