Paper Menu >>
Journal Menu >>
 Vol.2, No.8, 962-967 (2010) doi:10.4236/health.2010.28143 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ HEALTH The influence of DNA concentration in the experiment of DNA damage induced by 7Li ions and rays Fuquan Kong1, Xiao Wang1, Meinan Ni1, Li Sui1, Kui Zhao1,2,3* 1China Institute of Atomic Energy, Beijing, China 2School of Science, Hebei University of Technology, Tianjin, China 3Key Laboratory of Beam Technology and Modification of Ministry of Education, Beijing Normal University, Beijing, China; *Corresponding Author: kuiz@ciae.ac.cn Received 4 December 2009; revised 19 January 2010; accepted 23 January 2010. ABSTRACT To evaluate the influence of the DNA concentra- tion in the aqueous solution on DNA radiation damage, the plasmid DNA in the presence or absence of Mannitol (scavenger of free radical OH-) was irradiated by 7Li ions and γ rays at various DNA concentrations. Gel electrophore- sis analysis revealed that the DNA damage of single and double strand breaks induced by irradiation was dependent on DNA concentra- tion and became more severe at lower DNA concentration in the radiation experiment when all others parameters are the same. In the con- dition of -ray irradiation, most of double strand breaks (DSB) damage was neutralized and less associated with DNA concentration in the pre- sence of mannitol. However, under 7Li irradia- tion, the DSB damage could not be cleared by mannitol but was gradually aggravated with de- creasing DNA concentrations. Our study sheds light on the underlying mechanisms in the DNA radiation damage process. And there are poten- tial significances for the human space flight, cancer therapy by heavy ions as well as the ra- diation security assessment. Keywords: Heavy Ions; Plasmid DNA; DNA Concentration; Rays; Strand Break 1. INTRODUCTION DNA is considered to be the most important bio- macromolecule and target responsible for most of the biological effects in the cells. Many kinds of damage can be induced by radiation, e.g., base damage, single strand breaks (SSB), double strand breaks (DSB) and crosslink of DNA and protein [1]. DNA double strand breaks are considered to be the most important initial damage initi- ating serious biological consequences post-radiation. In particular, unrejoined DSBs may result in cell mutation or cell death. The DNA damage could be affected by radiation dose, quality of radiation, dose rate, etc. [2]. In contrast with the low Linear Energy Transfer (LET) ra- diations, the heavy-ion irradiation with higher LET and Bragg peak of dose distribution can produce more com- plicated track structure and stimulates biological havoc in organisms, tissues, cells, and DNA [3,4]. In previous experiments, it has been noticed that the DNA strand-break damage is related to DNA concentra- tion. Milligan et al. have measured the yield of single strand breaks G(SSB) induced by γ rays ranging from 0 Gy to 100 Gy and assayed the DNA samples by agarose gel electrophoresis in the presence or absence of scav- engers at various DNA concentrations. The result showed that the G(SSB) had small fluctuation over a wide range of DNA concentration in the absence of scavenger. In the presence of DMSO, G(SSB) was pro- portional to the DNA concentration, rising as the con- centration increasing. At higher DNA concentration, the G(SSB) approached a plateau value[5,6]. The similar result was obtained by SHAO et al. [7] in 2000. How- ever, these researches were focused on DNA damage induction at low-LET radiation. So in order to evaluate the influence of the DNA concentration in the aqueous solution on DNA radiation damage induced by low-LET and high-LET radiation, in this work, the high-LET heavy ions of 7Li was first employed in the plasmid DNA damage in addition to the low-LET γ rays with various DNA concentrations in the presence or absence of free radical scavenger (Mannitol).The distribution of DNA conformations and the number of DSB per DNA were statistically analyzed. It is found that the degree of DNA radiation damage has an obvious relationship with the DNA concentration, whereas this effect could be partially attenuated by scavenger at low-LET radiation. This work will redound to understanding of ionizing  F. Q. Kong et al. / HEALTH 2 (2010) 963-968 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 963 963 Openly accessible at radiation damage to the organisms and provides basic experimental data for the human space flight, cancer therapy by heavy ions as well as the radiation security assessment. 2. MATERIALS AND METHODS 2.1. DNA Sample The purified DNA sample, double-stranded pUC19 plasmid (2686-bp long) was purchased from TaKaRa Biotechnology Co., Ltd., (Dalian, China) at a concentra- tion of 500 ng/μl in TE buffer. The original forms of DNA sample are a mixture of supercoiled (SC) form (~90%) and open circular (OC) form. Mannitol was sup- plied by Beijing 306 Hospital at a concentration of 1.1 M and was diluted to 600 mM for experiment. 2.2. Rays and 7Li Ions Irradiation DNA samples in a series of concentrations were irradi- ated by γ rays of 60Co source located in China Institute of Atomic Energy (CIAE) at the dose of 482 Gy (dose rate = 10 Gy/min) at room temperature. Appling 37.3 MeV 7Li ions (LET = 70.2 keV/μm, in aqueous solution) generated by HI-13 heavy ion tandem accelerator of CIAE, the DNA samples in various concen- trations were irradiated at the dose of 500 Gy (48.4 Gy/ min) at room temperature. The range of 7Li ions in water is about 307 μm. For all irradiations, a 20 μl aliquot of DNA solution was dropped onto the middle of a 5 μm- thick Mylar film and sealed with another Mylar film. An Au-Si surface barrier semiconductor detector was de- ployed to monitor the number of incident ions, then radia- tion dose can be determined by formula (1). All presented values of energy, LET and dose are at the entrance surface of the solution. After irradiation, the samples were pre- served in the micro centrifuge tube at –20˚C prior to be used. The irradiated dose is defined as follows: Dose(Gy) = 1.6 × 10-9 × LET(keV/μm) × F(ions/cm2) × 1/ρ (g/cm3) (1) where F is the fluence of ions and ρ is the density of the medium. 2.3. Agarose Gel Electrophoresis The control and irradiated DNA samples were loaded on 1% agarose gel and electrophoresed for 90 min at 4V/cm. After electrophoresis, the gel was stained with ethidium bromide and visualized on a UV-transilluminator. The DNA bands were photographed and analyzed by Al- phaImager Imaging System (Alpha Innotech Corpora- tion, San Leandro, CA) to quantitate the fraction of the three kinds of DNA conformation (i.e., supercoiled, open circular and linear form of the DNA molecules). 3. RESULTS 3.1 The Result of γ Rays Irradiation DNA samples were irradiated by γ rays at a series of concentration from 10 to 100 ng/μl by the dose of 482 Gy. In Figure 1, lanes 1, 8 are unirradiated control sam- ples. From lane 2 to lane 7, the DNA concentrations are 100、50、40、30、20 and 10 ng/μl, respectively ( without mannitol) and samples in lane 9 to lane 14 are arranged in the same order (in the presence of 600 mM mannitol). It is shown that at lower DNA concentration, the SC form DNA disappears and the fraction of linear (L) form DNA increases. Meanwhile, the fraction of OC form DNA reduces with the decreasing concentration in the absence of mannitol in DNA solution. Moreover, the short linear DNA fragments appears when the concentra- tion is lower than 50 ng/μl, until all of DNA molecules are broken into very short fragments at the concentration of 10 ng/μl. This tendency indicates that DNA is dam- aged more seriously at lower DNA concentration. In Figure 1(b), 600 mM mannitol was added in the DNA solution. Comparing to Figure 1(a), the linear form dis- appears, on the contrast, most of the original SC form molecules are preserved and its fraction dropped by de- creasing DNA concentration. Nevertheless, the SC mo- lecules tends to transform into OC conformation under DNA concentration of 30 ng/μl, indicating that the SSB damage could not be eliminated by scavenger at lower DNA concentration. Generally, it is confirmed that DNA damage can be neutralied by mannitol to a great extent but is still depends on DNA concentration. The fractions of three kinds of DNA forms versus dif- ferent DNA concentrations without or with 600 mM mannitol are shown in Figure 2 and Figure 3 respec- tively. It is revealed in Figure 2 that the fraction of OC form dramatically rises from 0% to 75% and the fraction of linear form decreases from 100% to 25% with the increasing DNA concentrations in the absence of man- nitol. In the presence of 600 mM mannitol (Figure 3), Figure 1. Gel electrophoresis image of DNA after irradiated by γ rays at various concentrations. 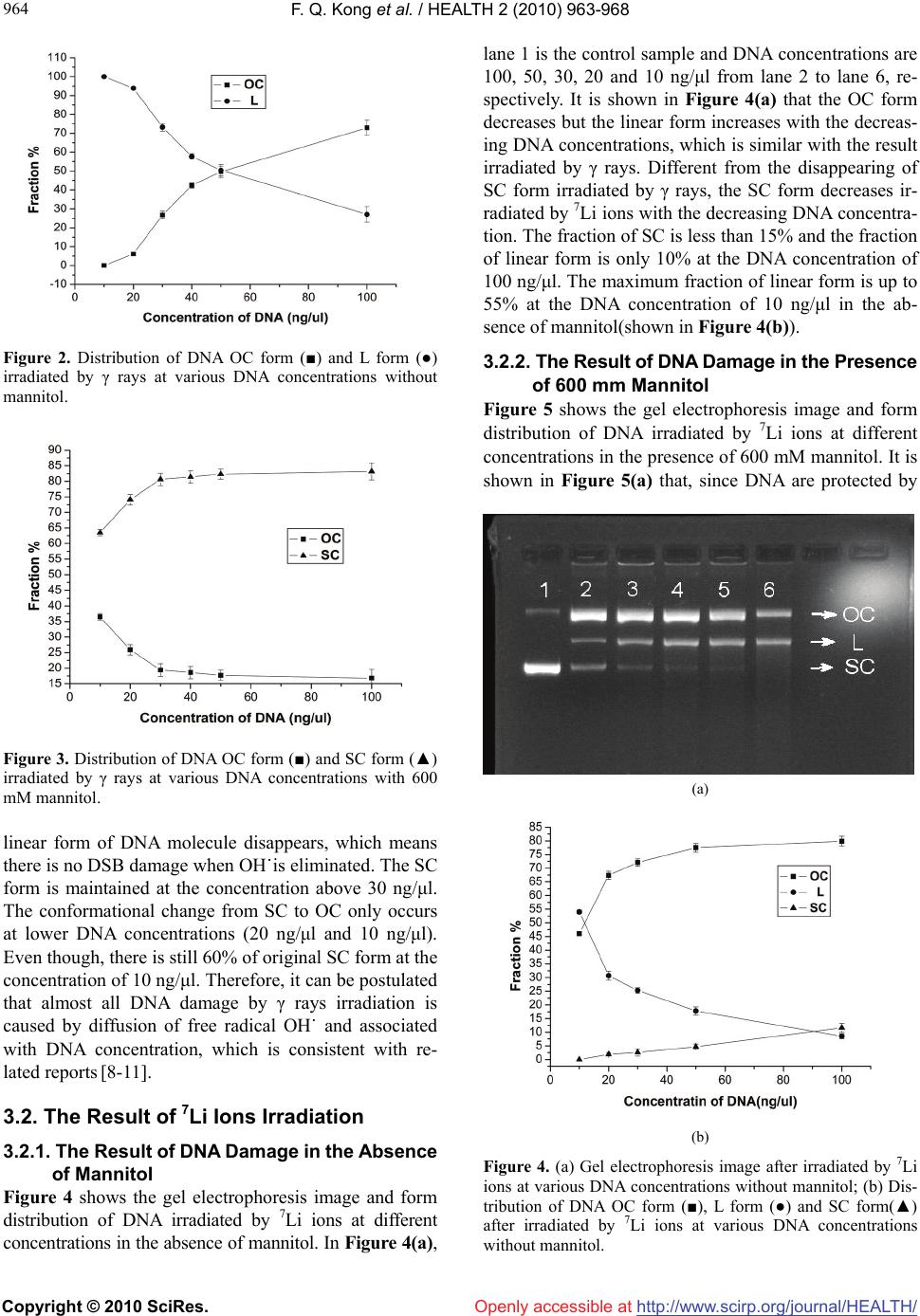 F. Q. Kong et al. / HEALTH 2 (2010) 963-968 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 964 Openly accessible at Figure 2. Distribution of DNA OC form (■) and L form (●) irradiated by γ rays at various DNA concentrations without mannitol. Figure 3. Distribution of DNA OC form (■) and SC form (▲) irradiated by γ rays at various DNA concentrations with 600 mM mannitol. linear form of DNA molecule disappears, which means there is no DSB damage when OH˙is eliminated. The SC form is maintained at the concentration above 30 ng/μl. The conformational change from SC to OC only occurs at lower DNA concentrations (20 ng/μl and 10 ng/μl). Even though, there is still 60% of original SC form at the concentration of 10 ng/μl. Therefore, it can be postulated that almost all DNA damage by γ rays irradiation is caused by diffusion of free radical OH˙ and associated with DNA concentration, which is consistent with re- lated reports [8-11]. 3.2. The Result of 7Li Ions Irradiation 3.2.1. The Result of DNA Damage in the Absence of Mannitol Figure 4 shows the gel electrophoresis image and form distribution of DNA irradiated by 7Li ions at different concentrations in the absence of mannitol. In Figure 4(a), lane 1 is the control sample and DNA concentrations are 100, 50, 30, 20 and 10 ng/μl from lane 2 to lane 6, re- spectively. It is shown in Figure 4(a) that the OC form decreases but the linear form increases with the decreas- ing DNA concentrations, which is similar with the result irradiated by γ rays. Different from the disappearing of SC form irradiated by γ rays, the SC form decreases ir- radiated by 7Li ions with the decreasing DNA concentra- tion. The fraction of SC is less than 15% and the fraction of linear form is only 10% at the DNA concentration of 100 ng/μl. The maximum fraction of linear form is up to 55% at the DNA concentration of 10 ng/μl in the ab- sence of mannitol(shown in Figure 4(b)). 3.2.2. The Result of DNA Damage in the Presence of 600 mm Mannitol Figure 5 shows the gel electrophoresis image and form distribution of DNA irradiated by 7Li ions at different concentrations in the presence of 600 mM mannitol. It is shown in Figure 5(a) that, since DNA are protected by (a) (b) Figure 4. (a) Gel electrophoresis image after irradiated by 7Li ions at various DNA concentrations without mannitol; (b) Dis- tribution of DNA OC form (■), L form (●) and SC form(▲) after irradiated by 7Li ions at various DNA concentrations without mannitol.  F. Q. Kong et al. / HEALTH 2 (2010) 963-968 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 965 965 Openly accessible at mannitol compared to the Figure 4(a), the influence of DNA concentration is still exit. However, different from the γ-ray result, the linear form still exists in the pres- ence of 600 mM mannitol, indicating that part of DSB damage comes from the direct ionizing energy deposi- tion onto the DNA molecules and does not disappear because of any radical scavenger. The data in Figure 5(b) shows that the severity of DNA damage is attenuated since the most of OH˙radicals are eliminated by manni- tol. Hence the maximum fraction of undamaged SC form reaches about 70% at higher DNA concentrations. How- ever the DNA damage is still aggravated with decreasing DNA concentration from the decreasing of SC form and increasing of linear form. 3.3. The DSBs/DNA Induced by Rays and 7Li Ions Based on the data presented in above, the number of DSB per single DNA molecule (DSBs/DNA) is calcu- lated by Eq.(2) [12]. DSBs/DNA = f(LI)/[1 – f(LI)] (2) where f(LI) is the fraction of linear form of DNA. 3.3.1. The DSBs/DNA Induced by Rays In Figure 6, the curve shows the DSBs/DNA at different DNA concentrations in the absence of mannitol except the concentration of 10 ng/ul since the fraction of linear form is 100% at this concentration. It is indicated that the yield of DSBs per DNA averagely increases as the decreasing DNA concentration, especially at the very lower DNA concentration. In the presence of 600 mM mannitol, the f(LI) is zero (shown in Figure 1 and Fig- ure 3) so the DSBs/DNA can not be calculated from the formula (2) which means that there is no DSB in DNA samples after irradiated by γ rays. It is indicated that the major damage from γ rays irradiation is the free radical and most free radicals can be neutralized by mannitol. So one DNA was attacked by more free radicals at very lower DNA concentration without mannitol. 3.3.2. The DSBs/DNA Induced by 7Li Ions In Figure 7, it is shown that the DSBs/DNA increase when the DNA concentrations decrease either with or without scavenger. Since the yield of DSB decreases in evidence when DNA samples are added 600 mM man- nitol compared with the yield of DSB without mannitol. But the DSB damage still considerable in the presence of mannitol, which implies that these DSB sites are resulted from the local multiple damage induced by direct ioniz- ing of 7Li ions, thus cannot be prevented completely by scavenger [13] and is dependent on DNA concentration. 4. DISCUSSIONS After irradiated by γ rays, the DNA concentration influ- ences the experimental result when all others parameters are the same. It would probably be result of interaction between DNA and free radical OH˙. The plentiful yield of OH˙is generated by the radiolysis of water when the DNA aqueous solution is irradiated by γ rays. During irradiation, the concentration of free radical in water could be assumed roughly steady and may be higher than the concentration of DNA molecules. At lower (a) (b) Figure 5. (a) Gel electrophoresis image after irradiated by 7Li ions at various DNA concentrations with 600 mM mannitol; (b) Distribution of DNA OC form (■), L form (●) and SC form(▲) after irradiated by 7Li ions at various DNA concentrations with 600mM mannitol. Figure 6. The DSBs/DNA at various DNA concentration irra- diated by γ rays. 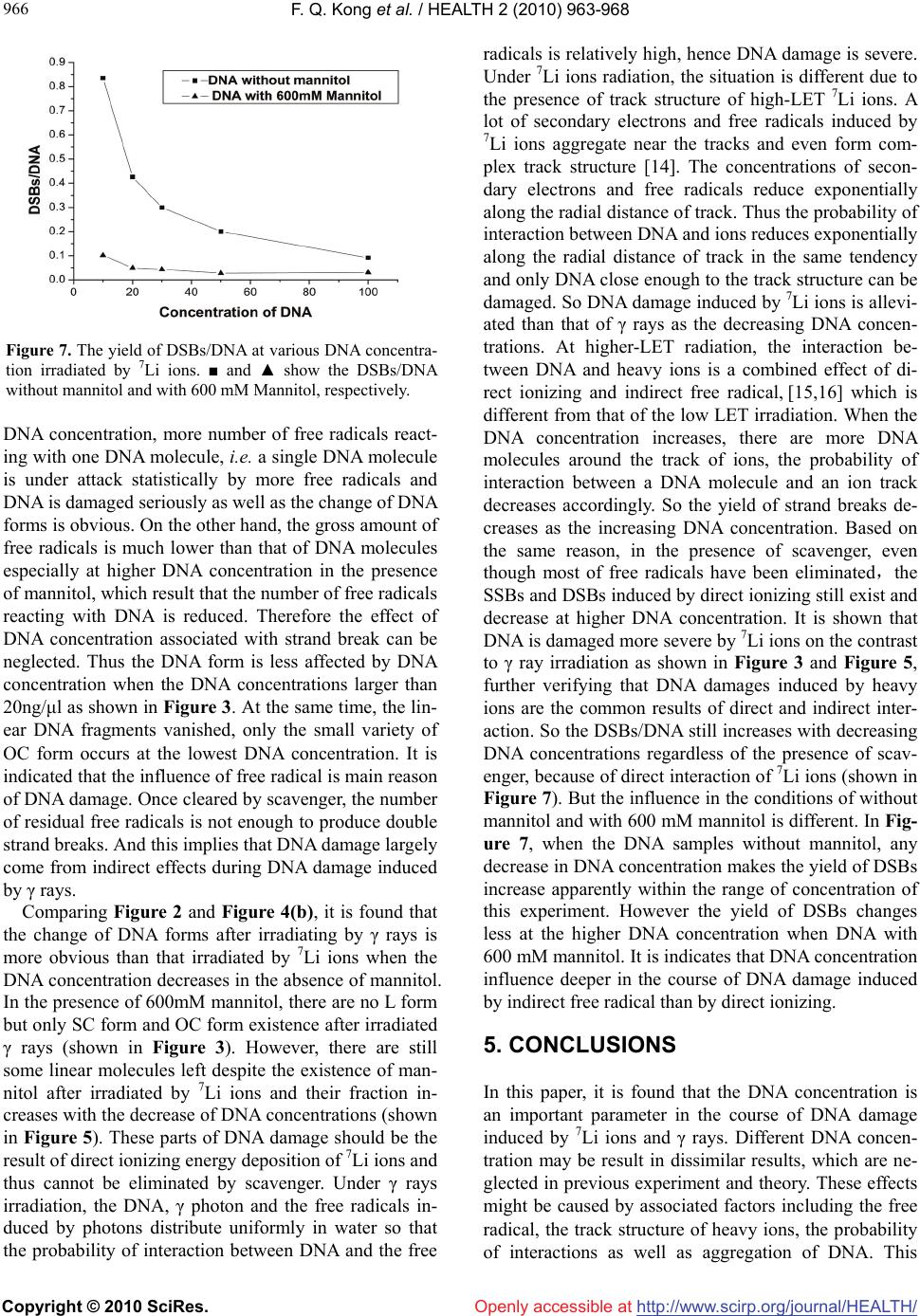 F. Q. Kong et al. / HEALTH 2 (2010) 963-968 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 966 Openly accessible at Figure 7. The yield of DSBs/DNA at various DNA concentra- tion irradiated by 7Li ions. ■ and ▲ show the DSBs/DNA without mannitol and with 600 mM Mannitol, respectively. DNA concentration, more number of free radicals react- ing with one DNA molecule, i.e. a single DNA molecule is under attack statistically by more free radicals and DNA is damaged seriously as well as the change of DNA forms is obvious. On the other hand, the gross amount of free radicals is much lower than that of DNA molecules especially at higher DNA concentration in the presence of mannitol, which result that the number of free radicals reacting with DNA is reduced. Therefore the effect of DNA concentration associated with strand break can be neglected. Thus the DNA form is less affected by DNA concentration when the DNA concentrations larger than 20ng/μl as shown in Figure 3. At the same time, the lin- ear DNA fragments vanished, only the small variety of OC form occurs at the lowest DNA concentration. It is indicated that the influence of free radical is main reason of DNA damage. Once cleared by scavenger, the number of residual free radicals is not enough to produce double strand breaks. And this implies that DNA damage largely come from indirect effects during DNA damage induced by γ rays. Comparing Figure 2 and Figure 4(b), it is found that the change of DNA forms after irradiating by γ rays is more obvious than that irradiated by 7Li ions when the DNA concentration decreases in the absence of mannitol. In the presence of 600mM mannitol, there are no L form but only SC form and OC form existence after irradiated γ rays (shown in Figure 3). However, there are still some linear molecules left despite the existence of man- nitol after irradiated by 7Li ions and their fraction in- creases with the decrease of DNA concentrations (shown in Figure 5). These parts of DNA damage should be the result of direct ionizing energy deposition of 7Li ions and thus cannot be eliminated by scavenger. Under γ rays irradiation, the DNA, γ photon and the free radicals in- duced by photons distribute uniformly in water so that the probability of interaction between DNA and the free radicals is relatively high, hence DNA damage is severe. Under 7Li ions radiation, the situation is different due to the presence of track structure of high-LET 7Li ions. A lot of secondary electrons and free radicals induced by 7Li ions aggregate near the tracks and even form com- plex track structure [14]. The concentrations of secon- dary electrons and free radicals reduce exponentially along the radial distance of track. Thus the probability of interaction between DNA and ions reduces exponentially along the radial distance of track in the same tendency and only DNA close enough to the track structure can be damaged. So DNA damage induced by 7Li ions is allevi- ated than that of γ rays as the decreasing DNA concen- trations. At higher-LET radiation, the interaction be- tween DNA and heavy ions is a combined effect of di- rect ionizing and indirect free radical, [15,16] which is different from that of the low LET irradiation. When the DNA concentration increases, there are more DNA molecules around the track of ions, the probability of interaction between a DNA molecule and an ion track decreases accordingly. So the yield of strand breaks de- creases as the increasing DNA concentration. Based on the same reason, in the presence of scavenger, even though most of free radicals have been eliminated,the SSBs and DSBs induced by direct ionizing still exist and decrease at higher DNA concentration. It is shown that DNA is damaged more severe by 7Li ions on the contrast to γ ray irradiation as shown in Figure 3 and Figure 5, further verifying that DNA damages induced by heavy ions are the common results of direct and indirect inter- action. So the DSBs/DNA still increases with decreasing DNA concentrations regardless of the presence of scav- enger, because of direct interaction of 7Li ions (shown in Figure 7). But the influence in the conditions of without mannitol and with 600 mM mannitol is different. In Fig- ure 7, when the DNA samples without mannitol, any decrease in DNA concentration makes the yield of DSBs increase apparently within the range of concentration of this experiment. However the yield of DSBs changes less at the higher DNA concentration when DNA with 600 mM mannitol. It is indicates that DNA concentration influence deeper in the course of DNA damage induced by indirect free radical than by direct ionizing. 5. CONCLUSIONS In this paper, it is found that the DNA concentration is an important parameter in the course of DNA damage induced by 7Li ions and γ rays. Different DNA concen- tration may be result in dissimilar results, which are ne- glected in previous experiment and theory. These effects might be caused by associated factors including the free radical, the track structure of heavy ions, the probability of interactions as well as aggregation of DNA. This  F. Q. Kong et al. / HEALTH 2 (2010) 963-968 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 967 967 [7] Shao, C.L., Yu, Z.L. and Masahiro, S. (2000) Reaction rate coefficients of hydroxyl radical-induced DNA single and α-type double-strand breaks. Radiation and Envi- ronmental Biophysics, 39(2), 121-124. finding there are potential significances for the human health especially for the space flight, cancer therapy by heavy ions as well as the radiation security assessment and would be a challenge to the theoretic research and strongly calls for further experiments. [8] Yang, M.J, Kong, F.Q., Zhan, Y., et al. (2007) A study of protective action of different scavengers on DNA damage induced by γ ray. Nuclear Techniques, 30, 255- 258. 6. ACKNOWLEGEMENTS [9] Aslam Siddiqi, M. and Bothe, E. (1987) Single- and dou- ble- strand break formation in DNA irradiation in aque- ous sloution: Dependence on dose and OH radical scav- enger concentration. Radiation Research, 112(3), 449- 463. The authors thank the crew of HI-13 Tandem accelerator for their cooperation during DNA irradiation process. The authors thank pro- fessor Yizhong Zhuo and professor Zhongwen Wang for their valuable criticism. [10] Hutchinson, F. (1985) Chemical changes induced in DNA by ionizing radiation. Progress in Nucleic Acid Re- search and Molecular Biology, 32, 115-154. [11] Zhou, L.J., Fang, Y.Z., Zhang, Y.P., et al. (1993) The effect of γ-ray irradiation on plasmid pBR 322 DNA. Journal of Radiation Research and Radiation Processing, 11(1), 28-30 REFERENCES [1] Yang, C.X. and Mei, M.T. (1995) Space radiobiology (in Chinese). Zhongshan University Press, Guangzhou, 23- 24 [12] Spotheim-Maurizot, M., Charlier, M. and Sabattier, R. (1990) DNA radiolysis by fast neutrons. International Journal of Radiation Biology, 57(2), 301-313. [2] Xia, S. X., Chen, J. P., et al. (1998) Radiobiology (in Chinese). The Academy of Military Medical Science Press, Beijing, 1-21. [13] Zhao, K., Sui, L., Kong, F.Q., et al. (2007) Investigation of DNA damage induced by high LET 7Li ions. Pro- ceeding of China-Korea Joint Symposium on Nuclear Technique Application in Agriculture and Life Science, Institute of Nuclear Agricultural Sciences, Zhejiang University, Hangzhou, 174-177. [3] Du, H.B., Qiu, G.Y. and Du, Y.H. (1997) Study on plas- mid supercoiled DNA damage induced by three types of radiation, Acta Biochimica et Biophysica Sinica, 13(2), 261-266. [14] Cao, T.G., Ma, Y.Z. and Zhuo, Y. (2004) Track structure of proton and α particle in water. High Energy Physics and Nuclear Physics, 28(4), 377-381. [4] Y u, Z. L. and Huo, Y. P. (1994) Review in low energy ion biology. Journal of Anhui Agricultural University, 21(3), 221-225 [15] Sui, L., Guo, J.Y., Kong, F.Q., et al. (2007) Investigation of direct interaction of DNA damage induced by high LET 7Li ions. Nuclear Techniques, 30, 250-254. [5] Milligan, J.R., Aguilerra, J.A. and Ward, J.F. (1993) Variation of single-strand break yield with scavenger concentration for plasmid DNA irradiated in aqueous so- lution. Radiation Research, 133(2), 151-157. [16] Y u, X., Ye, C.Q. and Zhou, P.K. (1997) The effect of DNA strand breaks induced by heavy ions. Space Medi- cine & Medical Engineering, 10(4), 310-312. [6] Milligan, J.R. and Ward, J.F. (1994) Yield of single- strand breaks due to attack on DNA by scavenger- derived radicals. Radiation Research, 137(3), 295-299. Openly accessible at |

