Paper Menu >>
Journal Menu >>
 Vol.2, No.8, 857-861 (2010) doi:10.4236/health.2010.28129 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ HEALTH Openly accessible at Potential mechanism for the effects of dexamethasone on growth of human melanoma cells in vitro Abdel-Moneim M. Osman1*, Omimah A. Nasseir2, Naglaa R. Ismail3 1College of Medicine, Pharmacology Department, King Abdulaziz University, Jeddah, Saudi Arabia; *Corresponding Author: moneimosman@hotmail.com 2Science college of Girsl, King Abdulziz University, Jeddah, Saudi Arabia 3 Department of Zoology, Faculty of Science, Fayoum University, Fayoum, Egypt Received 20 March 2010; revised 7 April 2010; accepted 10 April 2010. ABSTRACT This study deals with the inhibitory effects of dexamethsone on the proliferation of a human melanoma cell line in vitro. A retarded cell pro- liferation was observed in M-5A cells with the presence of specific high affinity glucocorticoid steroid receptors. There was a correlation be- tween the number of glucococrticoid binding sites and the reduction of cell proliferation in term of reduced plating efficiency. Arrest or accumula- tion of M-5A cells in G1 phase or both in G1 and G2 phase appeared to be involved in the growth inhibitory effect by dexamethsone. The magni- tude and duration of cell cycle arrest up to 72 hours were dose dependent. There was a cor- relation between the duration of the disturbance of the cell cycle progression in M-5A cells after dexamethsone treatment and the inhibition of cell proliferation. Synthesis of receptor protein was not specifically stimulated or inhibited relative to the effect on cellular protein content in general. This may conclude that in the mela- noma M-5A cell, death after glucocorticoid treat- ment is somehow related to the glucocorticoid receptor content and to the disturbance of cell cycle distribution. Keywords: Melanoma Cells; Dexamethasone; Glucocorticoid Receptors 1. INTRODUCTION Although malignant melanoma has not generally been regarded a hormonally responsive neoplasm, there is some evidence suggesting that melanoma may respond to the hormonal condition of the host. Pregnancy was reported to be associated with unfavorable prognosis in stage II disease [1]. However, adrenalectomy for mela- noma metastatic to the adrenal gland provides good pal- liation of complete regression of distant metastatic melanoma after bilateral adrenalectomy, suggesting a possible role for adrenal hormones in modifying mela- noma progression in certain patients [2]. A few reports have been published about the moderate sensitivity of human as well as animal melanoma to the action of glu- cocorticoid steroid hormones. Bregman et al. [3] found that dexamethsone at a concentration of 1 uM inhibited the colony formation in a human melanoma cell strain (C8146c) by 60%. Moreover, Ramirez et al. [4], showed 85% tumor rejection in mice challenged with B16 melanoma after administration of anti glucocorticoid- induced TNF receptor family related protein. Recently, Banciu et al. [5] reported that a glucocorticoid predniso- lone phosphate encapsulated in long-circulating lipo- somes exerts antitumor activity through the inhibition of tumor angiogenesis. Therefore the aim of our study was directed to investigate to what extent glucocorticoid in term of dexamethasone affect human melanoma cells in vitro. Moreover, to study whether human melanoma cells contain glucocorticoid receptors and, if so, is there a relation between the sensitivity to glucocorticoid and the amount of receptors present. 2. MATERIALS AND METHODS This study is partly conducted in the King Fahd Medical center, College of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia and partly in Pharmacology Unit, National Cancer Institute, Cairo University. 2.1. Cells and Cell Culture A human melanoma cell line M-5A was received at un- known passage level from liquid nitrogen store National Cancer Institute, Cairo University. Its characteristics have been described [6].  A. M. M. Osman et al. / HEALTH 2 (2010) 857-861 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 858 Cells were grown in modified minimum essential Ea- gle’s medium which penicillin (100 i.u./ml) and strep- tomycin (100 ug/ml) had been added to the medium contained 10% newborn calf serum inactivated by heat- ing at 56˚C for 30 min. Cells were cultured at 37˚C in humidified 5% CO2 atmosphere. 2.2. Evaluation of Drug Effect To study the effect of dexamethasone on cell prolifera- tion, a colony forming assay has been used. Exponen- tially growing cells were trypsinized off the substratum and counted in a hemocytometer. Sufficient numbers of cells (500-5000) were plated in 25 cm2 Greiner flasks to yield between 15 and 300 colonies per flaks at the time of evaluation. The number of cells required for each ex- periment was determined in preliminary studies. After treatment for one hour, the incubation medium was re- placed by fresh medium. After a time interval corres- ponding with nearly 5 to 7 doubling times of untreated cells, the number of colonies was counted and expressed as percent of the controls. To asses the cytotoxic action the per cent reduction in plating efficiency was calcu- lated from the equation (C-T)/C × 100, where C and T are the final numbers of colonies in control and treated cultures, respectively. 2.3. Glucocorticoid Receptor Assay Binding study for dexamethasone was carried out using a whole cell assay adapted from Rosner and Cristofalo [7] .Single cell suspensions were harvested from expo- nentially growing cultures, transfereed in triplicate to 60 mm Falcon dishes, 1 × 106 cells per dish, and incubated in growth medium. Twenty-four hours later, the medium was removed and cells were washed once with PBS at 22˚C. Fresh medium was added with (3H) dexamethsone (40 Ci/mmole, Amersham International Ltd, England) or (3H) dexamethsone in the presence of 1000-fold excess concentration of unlabeled dexamethsone to measure total and nonspecific binding of dexamethasone to the cells, respectively. The cells were incubated for 60 or 90 min in a humidified 5% CO2 atmosphere at 37˚C. Cells were then solubilized in 2 ml of 0.5 M sodium hydroxide at 50˚C for one hour. After cooling the cells they were neutralized with 0.16 ml of 6 N hydrochloric acid. And the cells transferred to scintillation vial and 15 ml of Insta-gel scintillation liquid (Packard) was added. Ra- dioactivity of the samples was determined in dpm in a Packard Model 3385 scintillation counter with an aver- age efficiency for tritium of 34%. Specific binding of dexamethsone was calculated from the difference between the total binding of (3H) dexamethsone at the saturating concentration of 4 × 10-8 M dexamethasone and the nonspecific binding that oc- curred in the presence of excess unlabeled dexamethasone. The molar amount of specifically bound (3H) dexa- methsone was calculated from its specific radioactivity and the dpm/cell was measured. Conversion by Avo- gadro’s number gave the number of specific binding sites per cells. Protein content was determined according to Lowry et al. [8]. 2.4. Flowcytometric Analysis Cell cycle analysis was performed using a flowcytomter ICP II (Phywe AG. Gottingen) equipped with a sheatflow cell essentially as described by Smets et al. [9] .Cells were stained by 2 ml of solution which contained 2 mg ethidium bromide, 0.8 mg Hoechst stain 33258, 0.5 g. bovine serum albumin and 2.4 g Tris/100 ml. The per- centage of cells with DNA per cell values corresponding with G1, S or G2/M phase were determined by plani- metry of the histograms. 3. RESULTS 3.1. Glucocorticoid Binding Sites Table 1 shows the relation between the duration of in- cubation with (3H) dexamethsone and the specific and nonspecific binding by 106 cells. Binding of the radioac- tive dexamethasone started rapidely as observed after 30 seconds of incubation. Maximum specific binding was achieved after 60-90 min. Prolongation of incubation to 120 min reduced the specific binding of glucocrticoid and increased the nonspecific binding. This decrease appeared not to be caused by the lower dexamethasone concentration during prolongation of the incubation (13 nM instead of 40 nm) since 13 nM must be considered nearly saturating according to the date in Figure 1. We have investigated the number of glucocorticoid binding site in melanoma M-5A cells after treatment with dexa- methsone 2.5 uM for one hour (Table 2). The number of dexamethasone binding site per cell was determined by the whole cell assay in control and treated culture. There was 31% increase in number of binding sites after 24 hours. The number of binding sites remained higher than in controls until the time that the treated cells resumed proliferation. The increase in the number of binding site/cells after dexamethsone treatment was dose de- pendant (Table 3). When measured 9 days after treat- ment with 2.5 or 12.5 uM of dexamethsone a 2.8-fold increase was observed at the higher concentration. Throughout these studies, it was noticed that the M-5A melanoma cells became larger during the growth delay induced by treatment with dexamethsone. Their total protein content was determined in order to see whether the increase in specific glucorticoid binding sites went parallel to a general increase in the amount of protein per cell. Table 4 showed a dose dependent increase in cellu- lar protein content after dexamethsone treatment. 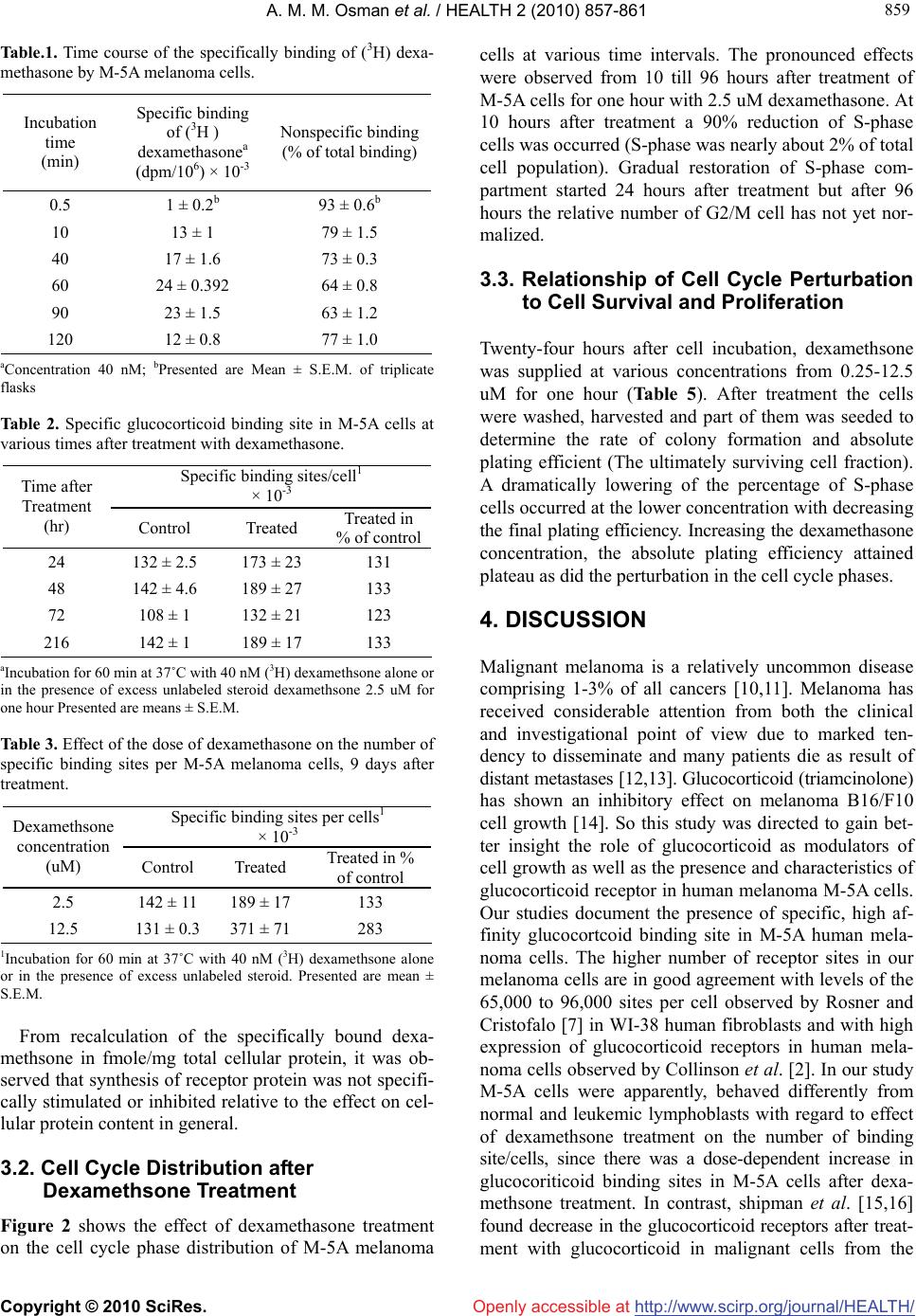 A. M. M. Osman et al. / HEALTH 2 (2010) 857-861 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 859 859 Table.1. Time course of the specifically binding of (3H) dexa- methasone by M-5A melanoma cells. Incubation time (min) Specific binding of (3H ) dexamethasonea (dpm/106) × 10-3 Nonspecific binding (% of total binding) 0.5 1 ± 0.2b 93 ± 0.6b 10 13 ± 1 79 ± 1.5 40 17 ± 1.6 73 ± 0.3 60 24 ± 0.392 64 ± 0.8 90 23 ± 1.5 63 ± 1.2 120 12 ± 0.8 77 ± 1.0 aConcentration 40 nM; bPresented are Mean ± S.E.M. of triplicate flasks Table 2. Specific glucocorticoid binding site in M-5A cells at various times after treatment with dexamethasone. Specific binding sites/cell1 × 10-3 Time after Treatment (hr) Control Treated Treated in % of control 24 132 ± 2.5 173 ± 23 131 48 142 ± 4.6 189 ± 27 133 72 108 ± 1 132 ± 21 123 216 142 ± 1 189 ± 17 133 aIncubation for 60 min at 37˚C with 40 nM (3H) dexamethsone alone or in the presence of excess unlabeled steroid dexamethsone 2.5 uM for one hour Presented are means ± S.E.M. Table 3. Effect of the dose of dexamethasone on the number of specific binding sites per M-5A melanoma cells, 9 days after treatment. Specific binding sites per cells1 × 10-3 Dexamethsone concentration (uM) Control Treated Treated in % of control 2.5 142 ± 11 189 ± 17 133 12.5 131 ± 0.3 371 ± 71 283 1Incubation for 60 min at 37˚C with 40 nM (3H) dexamethsone alone or in the presence of excess unlabeled steroid. Presented are mean ± S.E.M. From recalculation of the specifically bound dexa- methsone in fmole/mg total cellular protein, it was ob- served that synthesis of receptor protein was not specifi- cally stimulated or inhibited relative to the effect on cel- lular protein content in general. 3.2. Cell Cycle Distribution after Dexamethsone Treatment Figure 2 shows the effect of dexamethasone treatment on the cell cycle phase distribution of M-5A melanoma cells at various time intervals. The pronounced effects were observed from 10 till 96 hours after treatment of M-5A cells for one hour with 2.5 uM dexamethasone. At 10 hours after treatment a 90% reduction of S-phase cells was occurred (S-phase was nearly about 2% of total cell population). Gradual restoration of S-phase com- partment started 24 hours after treatment but after 96 hours the relative number of G2/M cell has not yet nor- malized. 3.3. Relationship of Cell Cycle Perturbation to Cell Survival and Proliferation Twenty-four hours after cell incubation, dexamethsone was supplied at various concentrations from 0.25-12.5 uM for one hour (Table 5). After treatment the cells were washed, harvested and part of them was seeded to determine the rate of colony formation and absolute plating efficient (The ultimately surviving cell fraction). A dramatically lowering of the percentage of S-phase cells occurred at the lower concentration with decreasing the final plating efficiency. Increasing the dexamethasone concentration, the absolute plating efficiency attained plateau as did the perturbation in the cell cycle phases. 4. DISCUSSION Malignant melanoma is a relatively uncommon disease comprising 1-3% of all cancers [10,11]. Melanoma has received considerable attention from both the clinical and investigational point of view due to marked ten- dency to disseminate and many patients die as result of distant metastases [12,13]. Glucocorticoid (triamcinolone) has shown an inhibitory effect on melanoma B16/F10 cell growth [14]. So this study was directed to gain bet- ter insight the role of glucocorticoid as modulators of cell growth as well as the presence and characteristics of glucocorticoid receptor in human melanoma M-5A cells. Our studies document the presence of specific, high af- finity glucocortcoid binding site in M-5A human mela- noma cells. The higher number of receptor sites in our melanoma cells are in good agreement with levels of the 65,000 to 96,000 sites per cell observed by Rosner and Cristofalo [7] in WI-38 human fibroblasts and with high expression of glucocorticoid receptors in human mela- noma cells observed by Collinson et al. [2]. In our study M-5A cells were apparently, behaved differently from normal and leukemic lymphoblasts with regard to effect of dexamethsone treatment on the number of binding site/cells, since there was a dose-dependent increase in glucocoriticoid binding sites in M-5A cells after dexa- methsone treatment. In contrast, shipman et al. [15,16] found decrease in the glucocorticoid receptors after treat- ment with glucocorticoid in malignant cells from the  A. M. M. Osman et al. / HEALTH 2 (2010) 857-861 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/Openly accessible at 860 peripheral blood of leukemic patients and in blood from normal volunteers, respectively. That could be due to an internal regulation of receptor levels in different respon- sive tumor. Cell cycle analysis of M-5A cells after dexamethasone treatment showed depletion of S-phase 10 hours later with accumulation of the cells in G2/M phase (Figure 2). Moreover there was a good correlation between depletion of S-phase which, mean arrest in G1 phase and the cyto- toxic activity of dexamethasone (Table 5). Early study by Smets et al. [9] has shown that prolonged glucocorti- coid treatment of L1210 leukemic cells potentiated cy- tolysis by accumulation of the cells in the early G1 phase. So, the overall proposed that the antiproliferative effect of dexamethsone against growth of M-5A cells could be due to G1 arrest which enhanced the probability of tran- sit to the G0 compartment in which dexamethasone me- diated cytolysis would occur. Such effect may be medi- Table 4. Specific glucocorticoid binding in relation to protein content of M-5A cells, 9 days after treatment with dexameth- sone for one hour. Dexameth- asone Con- centration (uM) Specific binding sites/cella,b × 10-3 Nonspe- cific/total specific bindinga,b (%) Total cellular proteina,c (ug/106 cells) Specific binding/ mg cellular protein (fmol) 0 131 59 362 ± 55d 601 ± 92d,e 2.5 172 60 554 ± 16 515 ± 15e 12.5 300 61 1000 ± 59 489 ± 29e a Incubation for 60 min at 37˚C with 40 nM (3H) dexamethsone alone or in the presence of excess unlabeled steroid dexamethsone 2.5,12.5 uM for one hour; bAverage number obtained from triplicate for total and nonspecific binding, respectively; cMean data from two sister flasks; dresented are mean ± S.E.M.; eNot significantly different. Table 5. Relationship between cell cycle phase distribution proliferation rate and plating efficiency of M-5A cells after treatment with various concentration of dexamethasone. Cell cycle distribution 24b hours after treatment Dosea (uM) G1% S% G2+M% No. of visi- ble colonies 8 days after treatment (% of con- trol) Final plating efficiency (% of control) 0 63 ± 4 21 ± 4 16 ± 6100 ± 7 100 ± 4 0.25 66 ± 5 10 ± 2 24 ± 771 ± 7 96 ± 4 1.25 63 ± 3 7 ± 1 30 ± 451 ± 2 78 ± 4 6.25 66 ± 2 7 ± 3 27 ± 135 ± 4 60 ± 2 12.5 62 ± 11 7 ± 3 31 ± 820 ± 1 56 ± 2 aDexamethsone was supplied for one hour and 24 hours later the cells were collected as described in the materials and methods; bPresented are mean ± S.E.M. of two separate experiment, 3 flasks for each ex- periment. Figure 1. Binding of (3H) dexamethasone by M-5A melanoma cells (dpm/106 cells) at different drug concentration. Average data (M ± S.E.M.) from four experiment.Incubations were for 60 mininutes at 37°C. • total binding, º nonspecific binding, ◊ specific binding. Figure 2. Effect of dexamethsone treatment (2.5 uM for 60 min) on cell cycle phase distribution of M-5A cells at the indi- cated hours post-treatment. X-axis, channel number (relative DNA/cell); Y-axis, cell/channel (× 10-4). Channels 45-120 (S and G2/M) amplified 4-fold. Relative compositions are given in the figure.  A. M. M. Osman et al. / HEALTH 2 (2010) 857-861 Copyright © 2010 SciRes. http://www.scirp.org/journal/HEALTH/ 861 861 [5] Banciu, M., Metselaar, J.M., Schiffelers, R.M., Storm, G. (2008) Antitumor activity of liposomal prednisolone phosphate depends on the presence of functional tu- mor-associated macrophages in tumor tissue. Neoplasia, 10(2), 108-117. ated by glucocorticoid receptors since Ramirez et al. [4] have reported activation of TNF receptor family related gene which overcomes tolerance/ignorance to melanoma differentiation antigen and enhanced antitumor immunity. In addition, Dobos [17] reported that 2-methoxyestradiol effectively inhibited melanoma cell proliferation by in- ducing apoptosis and an arrest in the G2/M phase. The mechanism of action involved microtubules, mitochon- drial damage and caspase activation as well. [6] Liao, S.K., Dent, P.B. and McCulloch, P.B. (1971) Char- acterizatiof of human malignant melanoma cell lines. I. morphology and growth characteristics in culture. Jour- nal of the National Cancer Institute, 54(5), 1037-1044. [7] Rosner, B.A. and Cristofalo, V.J. (1981) Changes in spe- cific dexamethsone binding during aging in WI-38 cells. Endocrinology, 108(5), 1965-1971. In SCID mice, 2-methoxyestradiol was effective in reducing primary tumor weight and the number of liver colonies after intrasplenic injection of human melanoma cells, and causing significantly higher rate of apoptotic cells in colonies. [8] Lowry, O.H., Rosenbrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193(1), 265- 275 [9] Smets, L.A., Bout, B., Brouwer, M. and Tulp, A. (1983) Cytotoxic effect of dexamethsone restricted to noncycling, early G1 phase cells of L1210 leukemia. Journal of Cel- lular Physiology, 116(3), 397-403. Although a study from a single established human melanoma cell line, no general conclusion can be drawn neither with respect to the exact basis for the observed cytotoxic activity in the M-5A cell line nor to clinical implications, the results as presented might stimulate the development of new approaches in the systemic chemo- therapy for malignant melanoma. [10] McCarthy, W.H., Black, A.L. and Milton, G.W. (1980) Melanoma in New South Wales: An epidemiologic sur- vey 1970-1976. Cancer, 46(2), 427-432. [11] Nathanson, L. (1983) Epidemiologic and etiologic con- siderations in malignant melanoma. In: Costanzi, J.J., Ed., Malignant Melanoma 1, Cancer Treatment and Research, 9, 1-27. REFERENCES [12] Comis, R.L. (1976) DTIC (NSC-45388) in malignant melanoma: A perspective. Cancer Treatment Reports, 60(2), 165-176. [1] Shiu, M.H., Schottenfeld, D., Maclean, B. and Fortner, J.G. (1976) Adverse effect of pregnancy on melanoma. A reappraisal. Cancer, 37(1), 181-187. [13] Costanzi, J.J. (1983) The chemotherapy of malignant melanoma. In: Costanzi, J.J., Ed., Malignant melanoma 1. Cancer Treatment and Research, 9, 259-274. [2] Collinson, F.J., Lam, T.K., Bruijn, W.M., De Wilt, J.H., Lamont, M., Thompson, J.K. and Kefford, R.F. (2008) Long-term survival and occasional regression of distat melanoma metastases after adrenal metastectomy. Annals Surgical Oncology, 15(6), 1741-1749. [14] Ristic-Fira, A., Vujcic, M., Krstic-Demonacos, M. and Kanazir, D. (1999) Identification and characterization of glucocorticoid receptors in B16 mouse melanoma cells. Endocrine Regulations, 33(3), 109-115. [3] Bregman, M.D., Peters, E., Sander, D. and Meyskens, F.L. Jr. (1983) Dexamethsone, prostaglandin A and reti- noic acid modulation of murine and human melanoma cells grown in soft agar. Journal of the National Cancer Institute, 71(5), 927-932. [15] Shipman, G.F., Bloomfield, C.D., Smith, K.A., Peterson, B.A. and Munck, A. (1981). The effect of glucocorticoid therapy on glucocorticoid receptors in leukemia and lymphoma. Blood, 58(6), 1198-1202. [16] Shipman, G.F., Bloomfield, C.D., Gajl-Peczalaska, K.L., Munck, A.U. and Smith, K.A. (1983) Glucocrticoid and lymphocytes.III. Effect of glucocoorticoid administration on lymphocyte glucocorticoid receptors. Blood, 61(6), 1086-1090. [4] Ramirez, M.T, Chow, A., Hirschhorn, C.D., Terwey, T.H., Hochma, A.A., Lu, S., Miles, R.C., Sakaguchi, S., Houghton, A.N. and Van den Brink, M.R. (2006) Gluco- corticopid-induced TNF receptor family related gene ac- tivation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immu- nity. Journal of Immunology, 176(11), 6434-6442. [17] Dobos, J. (2009) Endocrine factors influencing mela- noma progression. Magyar Onkologia Journal, 53(1), 47-50. Openly accessible at |

