Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 168-176 doi:10.4236/msa.2010.13027 Published Online August 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Hydrogen Absorption and Electrochemical Properties of As-Quenched Nanocrystalline Mg20Ni10 – xCux (x = 0 – 4) Alloys Jinliang Gao1, Zhonghui Hou2, Qilu Ge1, Dongliang Zhao1, Shihai Guo1, Yanghuan Zhang1,2 1Department of Functional Material Research, Central Iron and Steel Research Institute, Beijing, China; 2School of Material, Inner Mongolia University of Science and Technology, Baotou, China. Email: zyh59@yahoo.com.cn Received April 20th, 2010; revised May 18th, 2010; accepted May 19th, 2010. ABSTRACT Nanocrystalline Mg2Ni-type alloys with nominal compositions of Mg20Ni10 – xCux (x = 0, 1, 2, 3, 4) were synthesized by rapid quenching technique. The microstructures of the as-cast and quenched alloys were characterized by XRD, SEM and HRTEM. The hydrogen absorption and desorption kinetics of the alloys were measured using an automatically controlled Sieverts apparatus. The electrochemical hydrogen storage performances were tested by an automatic gal- vanostatic system. The results show that all the as-quenched alloys hold a typical nanocrystalline structure, and the rapid quenching does not change the major phase Mg2Ni. The hydrogen absorption and desorption capacities of the alloys significantly increase with rising quenching rate. Additionally, the rapid quenching significantly improves the electrochemical hydrogen storage capacity of the alloys, but it slightly impairs the cycle stability of the alloys. Keywords: Mg2Ni-type Alloy, Rapid Quenching, Nanocrystalline, Hydrogen Absorption, Electrochemical Properties 1. Introduction Among the known alloys with a potential use in hydrog- en storage, Mg and Mg-based metallic hydrides are consi- dered to be more promising materials for hydrogen stor- age because of their highest hydrogen capacity and low price [1,2]. Unfortunately, some shortcomings of these kinds of metal hydrides, such as slow sorption/desorption kinetics, high dissociation temperature and poor electroc- hemical cycling properties, limit their practical applica- tion. Therefore, finding ways of improving the hydration kinetics of Mg-based alloys has been one of the main ch- allenges faced by researchers in this area. Various attem- pts, involving mechanical alloying (MA) [3], GPa hydr- ogen pressure method [4], melt spinning [5], gravity ca- sting [6], polyol reduction [7], hydriding combustion sy- nthesis [8], surface modification [9], alloying with other elements [10,11], adding catalysts [12] etc, have been un- dertaken to improve the activation and hydriding proper- ties. Zaluska et al. [13] reported that a milled mixture of Mg2NiH4 and MgH2 shows excellent absorption/desorp- tion kinetics at 220-240˚C and a maximum hydrogen concentration of more than 5 wt.%. Hanada et al. [14] obtained a hydrogen storage capacity of 6.5 wt.% after doping MgH2 with nanosized-Ni in a temperature range of 150-250˚C. Recham et al. [15] found that the hydrog- en absorption property of ball-milled MgH2 can be enha- nced by adding NbF5, and MgH2 + 5wt.%NbF5 compos- ite desorbs 3 wt.% of H2 at 150˚C. Dobrovolsky et al. [16] synthesized a MgH2 (50 wt.%) + TiB2 (50 wt.%) com- posite by intensive mechanical milling and found that TiB2 addition decreases the dissociation temperature of the MgH2 hydride by about 50˚C. The result obtained by Cui et al [17] confirmed that amorphous and/or nanocr- ystalline Mg-Ni-based alloys can electrochemically abs- orb and also desorb large amounts of hydrogen already at room temperature. Kohno et al. [18] obtained a large discharge capacity of 750 mAh/g at a current density of 20 mA/g for modified Mg2Ni alloys. Ball-milling, indubitably, is a quite powerful method for the preparation of nanocrystalline and amorphous Mg and Mg-based alloys. Particularly, it is suitable to solubi- lize particular elements into MgH2 or Mg2NiH4 above the thermodynamic equilibrium limit. This is helpful to des- tabilize MgH2 or Mg2NiH4 [19]. However, the milled Mg and Mg-based alloys show very poor hydrogen absorbing and desorbing stability due to the fact that the metastable  Hydrogen Absorption and Electrochemical Properties of As-Quenched 169 Nanocrystalline Mg20Ni10 - xCux (x = 0 - 4) Alloys structures formed by ball milling tended to vanish during multiple hydrogen absorbing and desorbing cycles [20]. Alternatively, rapid quenching technique can overcome the above mentioned shortcoming and effective avoiding the significant degradation of hydrogen absorbing and de- sorbing cycle properties of Mg and Mg-based [21]. Ad- ditionally, the rapid quenching technique is an effective method to obtain a nanocrystalline structure and is very suitable for mass-production of nanocrystalline Mg-bas- ed alloys. It was also confirmed that nanocrystalline al- loys produced by rapid quenching could have excellent hydriding characteristics even at room temperature, sim- ilar to the alloys produced by the MA process. Spassov et al. [22] prepared Mg2(Ni,Y) hydrogen storage alloy with exact composition Mg63Ni30Y7 by rapid solidifica- tion, and its maximum hydrogen absorption capacity (about 3.0 wt.%) and hydrogenation kinetics of the as- quenched Mg2(Ni, Y) were found to exceed those of the conventionally prepared polycrystalline Mg2Ni alloys and to be comparable to the hydrogen absorption charac- teristics of nanocrystalline ball-milled Mg2Ni. Huang et al. [23] found that amorphous and nanocrystalline Mg- based alloy (Mg60Ni25)90Nd10 prepared by rapid quenching ob- tained the highest discharge capacity of 580 mAh/g and the maximum hydrogen capacity of 4.2 wt.% H. The objective of this work is to produce the Mg-Ni- based ternary nanocrystalline alloys by rapid quenching and to examine the hydrogen absorption and electro- chemical properties of the nanocrystalline Mg20Ni10-xCux (x = 0 – 4) alloys. 2. Experimental The nominal compositions of the experimental alloys were Mg20Ni10 – xCux (x = 0, 1, 2, 3, 4). For convenience, the alloys were denoted with Cu content as Cu0, Cu1, Cu2, Cu3 and Cu4, respectively. The alloy ingots were pre- pared using a vacuum induction furnace in a helium at- mosphere at a pressure of 0.04 MPa. Part of the as-cast alloys was re-melted and quenched by melt-spinning with a rotating copper roller. The quenching rate was approximately expressed by the linear velocity of the copper roller because it is too difficult to measure a real quenching rate i.e. cooling rate of the sample during quenching. The quenching rates used in the experiment were 15, 20, 25 and 30 m/s, respectively. The phase structures of the as-cast and quenched al- loys were determined by XRD diffractometer (D/max/ 2400). The diffraction, with the experimental parameters of 160 mA, 40 kV and 10˚/min respectively, was per- formed with CuKα1 radiation filtered by graphite. The thin film samples of the as-quenched alloys were pre- pared by ion etching for observing the morphology with high resolution transmission electronic microscope (HR- TEM) (JEM-2100F, operated at 200 kV), and for deter- mining the crystalline state of the samples with electron diffraction (ED). The morphologies of the as-cast alloys were examined by scanning electronic microscope (SEM) (Philips QUANTA 400). The hydrogen absorption and desorption kinetics of the alloys were measured by an automatically controlled Sieverts apparatus. The hydrogen absorption was con- ducted at 1.5MPa and the hydrogen desorption in a vac- uum (1 × 10-4 MPa) at 200˚C. The alloy ribbons were pulverized and then mixed with carbonyl nickel powder in a weight ratio of 1:4. The mixture was cold pressed into round electrode pellets of 10 mm in diameter and total mass of about 1 g with a pressure of 35 MPa. A tri-electrode open cell, consisting of a metal hydride electrode, a sintered NiOOH/Ni(OH)2 counter electrode and a Hg/HgO reference electrode, was used for testing the electrochemical characteristics of the experimental alloy electrodes. A 6 M KOH solution was used as electr- olyte. The voltage between the negative electrode and the reference electrode was defined as the discharge voltage. In every cycle, the alloy electrode was first charged at a current density of 20 mA/g, after resting for 15 min, it was discharged at the same current density to –0.500 V cut-off voltage. The environment temperature of the me- asurement was kept at 30˚C. 3. Results and Discussion 3.1 Microstructure Characteristics The XRD profiles of the as-cast and quenched Cu2 and Cu4 alloys are presented in Figure 1, showing that all the as-cast and quenched alloys display a single phase struc- ture. The rapid quenching does not change the phase str- ucture. Listed in Table 1 are the lattice parameters, the cell volume and the full width at half maximum (FWHM) values of the main diffraction peaks of the as-cast and quenched Cu2 and Cu4 alloys which were calculated by software of Jade 6.0. It can be derived from Table 1 that the rapid quenching causes the FWHM values of the main diffraction peaks of the alloys significantly incre- ased and the lattice parameters and cell volume of the alloys cleverly enlarged, which is doubtless attributed to the refinement of the average grain size and stored stress in the grains produced by the rapid quenching. The crys- tallite size < Dhkl > (Ǻ) of the as-quenched alloy was calculated from the FWHM values of the broad diffrac- tion peak (203) in Figure 1(b), using Scherrer’s equation. The grain sizes of the as-quenched alloys are in a range of 2-6 nm, consistent with results reported by Friedlmeier et al. [24]. It is important to notice that < D > values were calculated on the same peak having Miller indices (203) due to better possibility of mutual comparison. Figure 2 shows the HRTEM micrographs and electron Copyright © 2010 SciRes. MSA 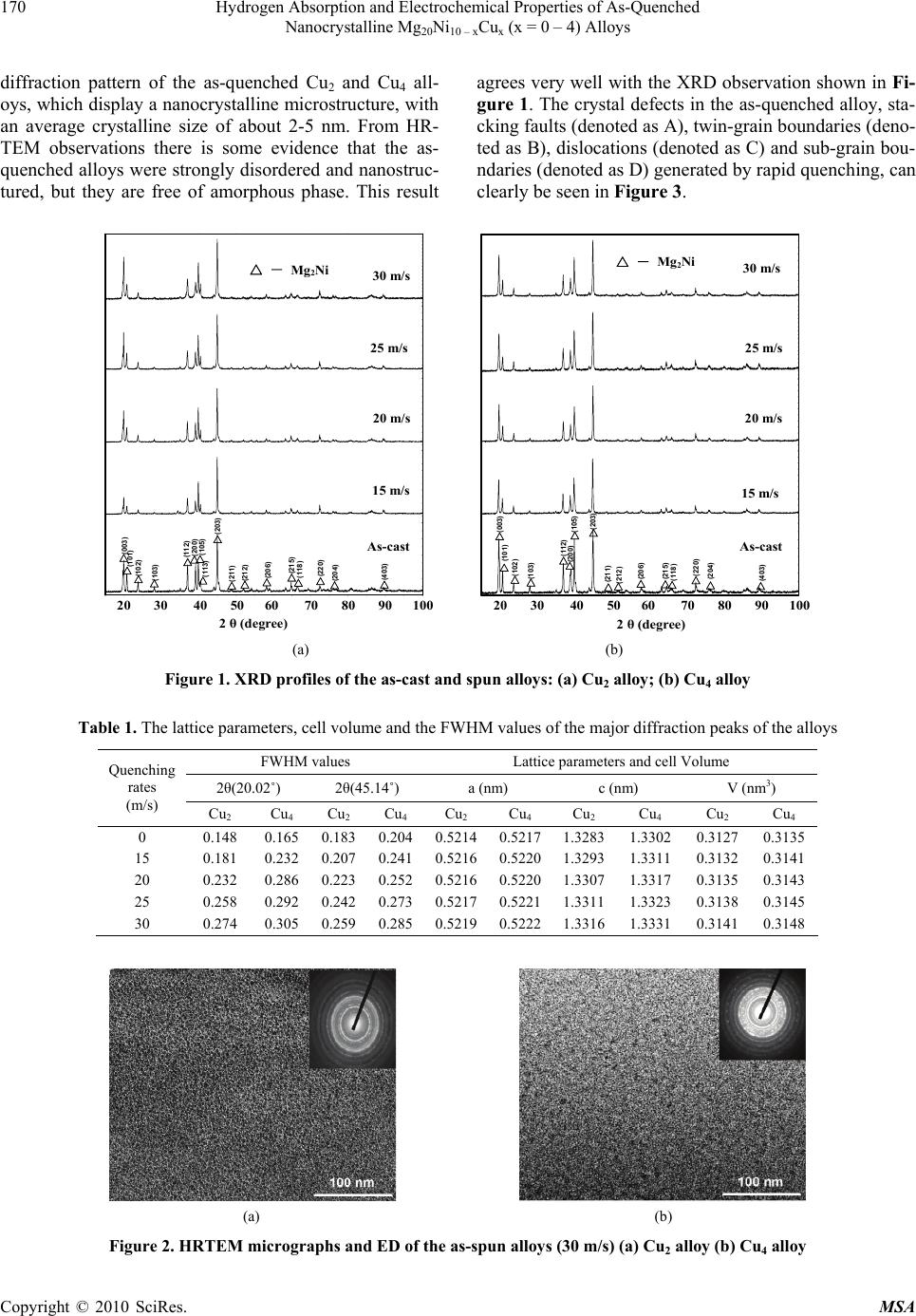 Hydrogen Absorption and Electrochemical Properties of As-Quenched Nanocrystalline Mg20Ni10 – xCux (x = 0 – 4) Alloys Copyright © 2010 SciRes. MSA 170 diffraction pattern of the as-quenched Cu2 and Cu4 all- oys, which display a nanocrystalline microstructure, with an average crystalline size of about 2-5 nm. From HR- TEM observations there is some evidence that the as- quenched alloys were strongly disordered and nanostruc- tured, but they are free of amorphous phase. This result agrees very well with the XRD observation shown in Fi- gure 1. The crystal defects in the as-quenched alloy, sta- cking faults (denoted as A), twin-grain boundaries (deno- ted as B), dislocations (denoted as C) and sub-grain bou- ndaries (denoted as D) generated by rapid quenching, can clearly be seen in Figure 3. (403) (204) (220) (118) (215) (206) (212) (211) (203) (113) (105) (200) (112) (103) (102) (101) (003) △ △ △ △ △ △ △ △ △ △ △ △ △ △ △ △ △As-cast 15 m/s 20 m/s 25 m/s (a) △-Mg2Ni 30 m/s (403) (204) (220) (118) (215) (206) (212) (211) (203) (105) (200) (112) (103) (102) (101) (003) △△ △ △ △ △△△ △ △ △ △ △ △ △ △As-cast 15 m/s 20 m/s 25 m/s (b) △-Mg2Ni 30 m/s 2 θ (degree) 20 30 40 50 60 70 80 90 10020 30 40 50 60 70 80 90 100 2 θ (degree) △ - Mg 2 Ni △ - Mg 2 Ni 30 m/s 25 m/s 30 m/s 25 m/s 20 m/s20 m/s 15 m/s15 m/s As-cast As-cast (a) (b) Figure 1. XRD profiles of the as-cast and spun alloys: (a) Cu2 alloy; (b) Cu4 alloy Table 1. The lattice parameters, cell volume and the FWHM values of the major diffraction peaks of the alloys FWHM values Lattice parameters and cell Volume 2θ(20.02˚) 2θ(45.14˚) a (nm) c (nm) V (nm3) Quenching rates (m/s) Cu2 Cu4 Cu2 Cu4 Cu2 Cu4 Cu2 Cu4 Cu2 Cu4 0 0.148 0.165 0.183 0.2040.52140.52171.32831.33020.3127 0.3135 15 0.181 0.232 0.207 0.2410.52160.52201.32931.33110.3132 0.3141 20 0.232 0.286 0.223 0.2520.52160.52201.33071.33170.3135 0.3143 25 0.258 0.292 0.242 0.2730.52170.52211.33111.33230.3138 0.3145 30 0.274 0.305 0.259 0.2850.52190.52221.33161.33310.3141 0.3148 (a) (b) Figure 2. HRTEM micrographs and ED of the as-spun alloys (30 m/s) (a) Cu2 alloy (b) Cu4 alloy 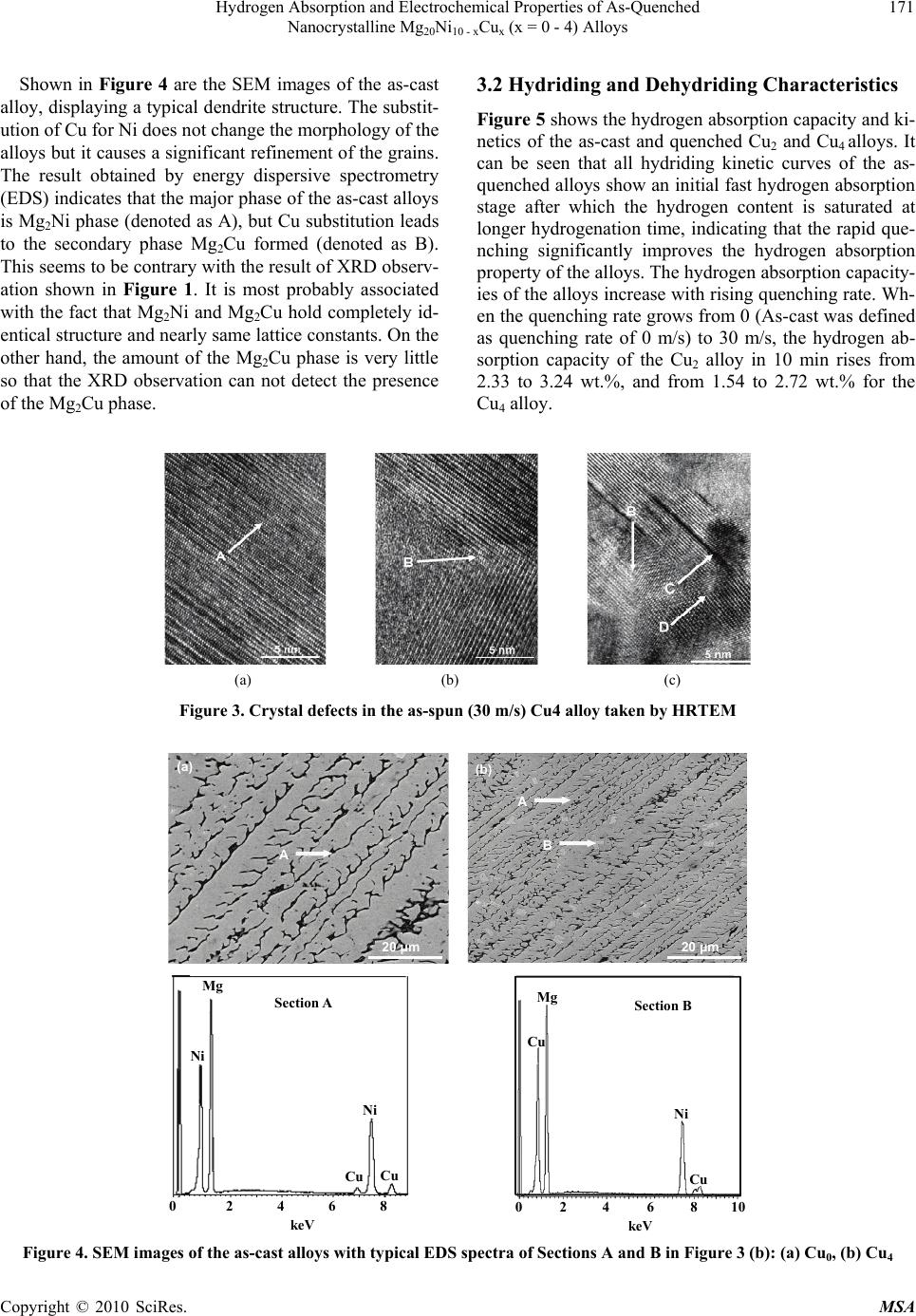 Hydrogen Absorption and Electrochemical Properties of As-Quenched 171 Nanocrystalline Mg20Ni10 - xCux (x = 0 - 4) Alloys Shown in Figure 4 are the SEM images of the as-cast alloy, displaying a typical dendrite structure. The substit- ution of Cu for Ni does not change the morphology of the alloys but it causes a significant refinement of the grains. The result obtained by energy dispersive spectrometry (EDS) indicates that the major phase of the as-cast alloys is Mg2Ni phase (denoted as A), but Cu substitution leads to the secondary phase Mg2Cu formed (denoted as B). This seems to be contrary with the result of XRD observ- ation shown in Figure 1. It is most probably associated with the fact that Mg2Ni and Mg2Cu hold completely id- entical structure and nearly same lattice constants. On the other hand, the amount of the Mg2Cu phase is very little so that the XRD observation can not detect the presence of the Mg2Cu phase. 3.2 Hydriding and Dehydriding Characteristics Figure 5 shows the hydrogen absorption capacity and ki- netics of the as-cast and quenched Cu2 and Cu4 alloys. It can be seen that all hydriding kinetic curves of the as- quenched alloys show an initial fast hydrogen absorption stage after which the hydrogen content is saturated at longer hydrogenation time, indicating that the rapid que- nching significantly improves the hydrogen absorption property of the alloys. The hydrogen absorption capacity- ies of the alloys increase with rising quenching rate. Wh- en the quenching rate grows from 0 (As-cast was defined as quenching rate of 0 m/s) to 30 m/s, the hydrogen ab- sorption capacity of the Cu2 alloy in 10 min rises from 2.33 to 3.24 wt.%, and from 1.54 to 2.72 wt.% for the Cu4 alloy. (a) (b) (c) Figure 3. Crystal defects in the as-spun (30 m/s) Cu4 alloy taken by HRTEM 20 μm A (a) (b) B A 20 μm Section A 0 Mg Ni keV Ni Cu Cu 2 46 8 Section B M g Cu Ni Cu 02 468 10 k eV Figure 4. SEM images of the as-cast alloys with typical EDS spectra of Sections A and B in Figure 3 (b): (a) Cu0, (b) Cu4 Copyright © 2010 SciRes. MSA 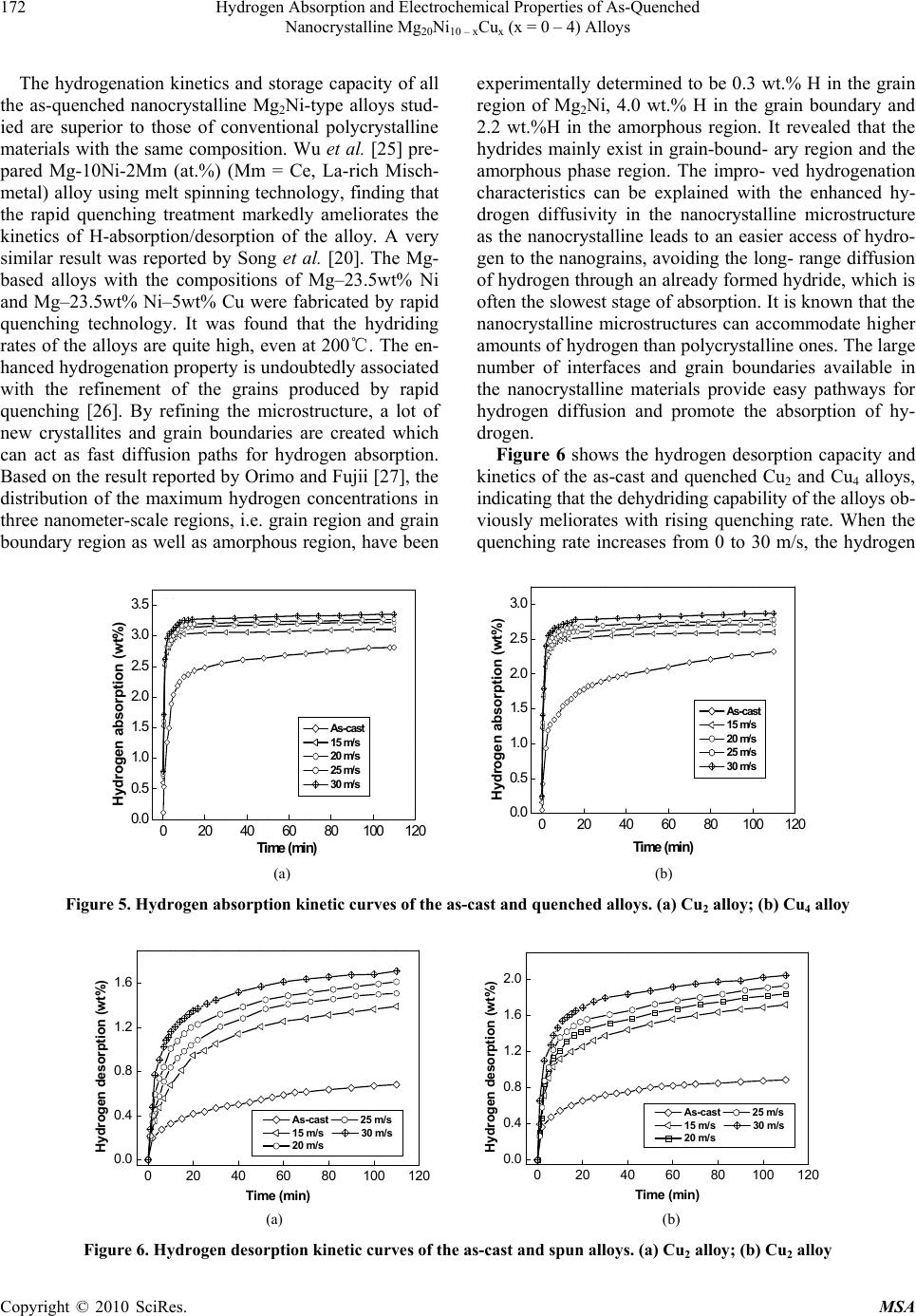 Hydrogen Absorption and Electrochemical Properties of As-Quenched 172 Nanocrystalline Mg20Ni10 – xCux (x = 0 – 4) Alloys 0 The hydrogenation kinetics and storage capacity of all the as-quenched nanocrystalline Mg2Ni-type alloys stud- ied are superior to those of conventional polycrystalline materials with the same composition. Wu et al. [25] pre- pared Mg-10Ni-2Mm (at.%) (Mm = Ce, La-rich Misch- metal) alloy using melt spinning technology, finding that the rapid quenching treatment markedly ameliorates the kinetics of H-absorption/desorption of the alloy. A very similar result was reported by Song et al. [20]. The Mg- based alloys with the compositions of Mg–23.5wt% Ni and Mg–23.5wt% Ni–5wt% Cu were fabricated by rapid quenching technology. It was found that the hydriding rates of the alloys are quite high, even at 200℃. The en- hanced hydrogenation property is undoubtedly associated with the refinement of the grains produced by rapid quenching [26]. By refining the microstructure, a lot of new crystallites and grain boundaries are created which can act as fast diffusion paths for hydrogen absorption. Based on the result reported by Orimo and Fujii [27], the distribution of the maximum hydrogen concentrations in three nanometer-scale regions, i.e. grain region and grain boundary region as well as amorphous region, have been experimentally determined to be 0.3 wt.% H in the grain region of Mg2Ni, 4.0 wt.% H in the grain boundary and 2.2 wt.%H in the amorphous region. It revealed that the hydrides mainly exist in grain-bound- ary region and the amorphous phase region. The impro- ved hydrogenation characteristics can be explained with the enhanced hy- drogen diffusivity in the nanocrystalline microstructure as the nanocrystalline leads to an easier access of hydro- gen to the nanograins, avoiding the long- range diffusion of hydrogen through an already formed hydride, which is often the slowest stage of absorption. It is known that the nanocrystalline microstructures can accommodate higher amounts of hydrogen than polycrystalline ones. The large number of interfaces and grain boundaries available in the nanocrystalline materials provide easy pathways for hydrogen diffusion and promote the absorption of hy- drogen. Figure 6 shows the hydrogen desorption capacity and kinetics of the as-cast and quenched Cu2 and Cu4 alloys, indicating that the dehydriding capability of the alloys ob- viously meliorates with rising quenching rate. When the quenching rate increases from 0 to 30 m/s, the hydrogen 0 2040608010012 0. 0 0. 5 1. 0 1. 5 2. 0 2. 5 3. 0 3.5 (a) Hydrogen absorption (wt%) Time (min) As-cast 15 m/s 20 m/s 25 m/s 30 m/s 020 40 60 80100120 0.0 0.5 1.0 1.5 2.0 2.5 3.0 (b) Hydrogen absorption (wt%) Time (min) As-cast 15 m/s 20 m/s 25 m/s 30 m/s (a) (b) Figure 5. Hydrogen absorption kinetic curves of the as-cast and quenched alloys. (a) Cu2 alloy; (b) Cu4 alloy 020 40 60 80100120 0.0 0.4 0.8 1.2 1.6 (a) Hydrogen desorption (wt%) Time (min) As-cast 25 m/s 15 m/s 30 m/s 20 m/s 020 40 60 80100120 0.0 0.4 0.8 1.2 1.6 2.0 (b) Hydrogen desorption (wt%) Time (min) As-cast 25 m/s 15 m/s 30 m/s 20 m/s (a) (b) Figure 6. Hydrogen desorption kinetic curves of the as-cast and spun alloys. (a) Cu2 alloy; (b) Cu2 alloy Copyright © 2010 SciRes. MSA  Hydrogen Absorption and Electrochemical Properties of As-Quenched 173 Nanocrystalline Mg20Ni10 - xCux (x = 0 - 4) Alloys desorption capacity of the Cu2 alloy in 20 min rises from 0.42 to 1.35 wt.%, and from 0.65 to 1.68 wt.% for Cu4 alloy, respectively. The nanocrystalline Mg2Ni-type all- oys produced by rapid quenching exhibit higher H-abso- rption capacity and faster kinetics of hydriding/dehyd- riding than crystalline Mg2Ni. A similar result was repo- rted by Spassov et al [28,29]. The specific capacity and hydriding/dehydriding kinetics of hydride materials de- pend on their chemical composition and crystalline stru- cture [30]. The observed essential differences in the hyd- riding/dehydriding kinetics of the as-quenched nanocry- stalline Mg2Ni type alloys studied most probably have to be associated with the composition of the alloys as well as with the differences in their microstructure due to the different quenching rates. It was reported that the high surface to volume ratios, i.e. high specific surface area, and the presence of large number of grain boundaries in nanocrystalline alloys enhance the kinetics of hydrogen absorption/desorption [28]. Zaluski et al. [31] and Orimo et al. [32] confirmed that the hydriding/dehydriding char- acteristics at low temperatures (lower than 200˚C) of na- nocrystalline Mg2Ni alloys prepared by mechanical al- loying can be improved by reducing the grain size (20- 30 nm), due to hydrogen occupation in the disordered interface phase. 3.3 Electrochemical Hydrogen Storage Characteristics 3.3.1 Activation Capability and Discharge Capacity Electrochemical galvanostatic charge/discharge is a more effective and less time-consuming method for determin- ing the absorbing hydrogen capacity than a gaseous tech- nique. The influences of rapid quenching on the activa- tion capability of the alloys were shown in Figure 7, and the charging-discharging current density being 20 mA/g. The figures show that all the alloys have excellent acti- vation capabilities and attain their maximum discharge capacities at first charging-discharging cycle. The rapid quenching does not affect the activation performances of the alloys. The evolution of the maximum discharge ca- pacities of the alloys with the quenching rate is shown in Figure 8. It can be derived in Figure 8 that the discharge capacity of the alloys increases with rising quenching rate. When quenching rate increases from 0 to 30 m/s, the discharge capacity of the Cu2 alloy enhances from 65.9 to 189.3 mAh/g, and from 53.3 to 140.4 mAh/g for Cu4 alloy. A similar result was reported by Simičić et al [2]. It must be mentioned that the discharge capacity of the alloys containing Cu are higher than that of Cu-free alloy, suggesting that the substitution of Cu for Ni en- hances the discharge capacity of the Mg2Ni-type alloy. Two reasons are mainly responsible for this result. Firstly, the partial substitution of Cu for Ni in Mg2Ni compound 0246810121416182022 40 80 120 160 200 (a) Discharge capactiy (mAh/g) Cycle number, n As- cas t 15 m/s 20 m/s 25 m/s 30 m/s (a) 0246810121416182022 40 60 80 100 120 140(b) Discharge capactiy (mAh/g) Cycle number, n As-cast 15 m/s 20 m/s 25 m/s 30 m/s (b) Figure 7. Evolution of the discharge capacity of the alloys with the cycle number: (a) Cu2 alloy; (b) Cu4 alloy 0510 15 20 25 30 25 50 75 100 125 150 175 200 Discharge capacity (mAh/g) Quenching rate (m/s) Cu0 Cu1 Cu2 Cu3 Cu4 Figure 8. Evolution of the discharge capacity of the alloys with the quenching rate may help to destabilize the hydride and activate the Mg2Ni phase to absorb/desorb reversibly hydrogen in the alkaline electrolyte [2]. On the other hand, the secondary phase Mg2Cu probably act as an efficient catalyst for dissociating H2 molecules and transferring the H atoms to the surrounding Mg2Ni matrix [20]. The observed es- sential differences in the discharge capacity of the alloys caused by rapid quenching most probably have to be as- sociated with the differences in their microstructures. Copyright © 2010 SciRes. MSA  Hydrogen Absorption and Electrochemical Properties of As-Quenched 174 Nanocrystalline Mg20Ni10 – xCux (x = 0 – 4) Alloys The crystalline material, when rapidly quenched, beco- mes at least partially disordered and its structure changes to nanocrystalline. Consequently, high densities of crys- tal defects such as dislocations, stacking faults and grain boundaries are introduced. The densities of the crystal defects mainly depend on the quenching rate. The higher the quenching rate, the larger the densities of the crystal defects are. The large number of interfaces and grain boundaries available in the nanocrystalline materials provide easy pathways for hydrogen diffusion and accel- erates the hydrogen absorbing/desorbing process. Addi- tionally, as a result of the defects introducing distortion of crystal lattice, the stored sufficient energy as chemical disorder and the introduced defects (including both stac- king faults as well as grain boundaries) will produce in- ternal strain. It was concluded by Northwood et al. [33] that the exchange current density and H-diffusion coeffic ient are directly proportional to the internal strain. The- refore, it is understandable that the introduction of de- fects, disordering and internal strain leads to an increas- ing hydriding/dehydriding rates and capacity. 3.3.2 Charging and Discharging Cycle Stability The cycle stability of the electrode alloy is a decisive fac- tor of the life of the Ni-MH battery. The capacity retain- ing rate (Sn), which was introduced to evaluate accurately the cycle stability of the alloy, is defined as Sn= Cn/Cmax × 100%, where Cmax is the maximum discharge capacity and Cn is the discharge capacity of the nth charge-discharge cycle, respectively. According to the above menti- oned definition, it can be known that the larger the cap- acity retaining rate (Sn), the better the cycle stability of the alloy. The evolution of the capacity retaining rate (S20) of the alloys with quenching rate is illustrated in Figure 9. The figure show that the capacity retaining rate (S20) of the alloys clearly declines with rising quenching rate. When quenching rate increases from 0 to 30 m/s, the capacity retaining rate of the Cu2 alloy after 20 cycles falls from 58.6 to 42.3%, and from 74.4 to 51.1% for the Cu4 alloy. It can also be seen in Figure 9 that, for a fixed quenching rate, the capacity retaining rate of the alloys mounts up with rising Cu content, reflecting that the sub- stitution of Cu for Ni enhances the cycle stability of the alloys. In order to clearly see the process of the capacity degradation of the alloy electrode, the evolution of the capacity retaining rate of the as-cast and quenched Cu2 and Cu4 alloys with the cycle number is shown in Figure 10. A rough tendency can be seen in the Figure 10 that the rapid quenching causes an increase of the decay rates of the discharge capacities of the alloys, suggesting that the rapid quenching impairs the cycle stability of the alloys. It was well known that the essential reason of which leads to the capacity degradation of the Mg-based alloy electrodes is the severe corrosion of Mg in the alkaline KOH solution. Especially, during the discharging process, the alloys are anodically polarized so that corrosion wo- uld be faster [2]. On the other hand, the metastable str- uctures formed by rapid quenching or ball milling tended to vanish during multiple charging/discharging cycles, which is an important factor for the capacity decay of the alloys. Two reasons are responsible for the enhanced cy- cle stability of the Mg2Ni-type alloy by Cu substitution. 0510 15 20 25 30 20 30 40 50 60 70 80 C20/Cmax (%) Quenching rate (m/s) Cu0 Cu3 Cu1 Cu4 Cu2 Figure 9. Evolution of the capacity retaining rate (S20) of the alloys with the quenching rate 246810121416182022 40 50 60 70 80 90 100 (a) Cn/Cmax (%) Cycle number, n As-cast 15 m/s 20 m/s 25 m/s 30 m/s (a) 246810 12 1416 18 2022 50 60 70 80 90 100 (b) Cn/Cmax (%) Cycle number, n As-cast 15 m/s 20 m/s 25 m/s 30 m/s (b) Figure 10. Evolution of the capacity retaining rate of the as-cast and quenched alloys with cycle number (a) Cu2 alloy; (b) Cu4 alloy Copyright © 2010 SciRes. MSA  Hydrogen Absorption and Electrochemical Properties of As-Quenched 175 Nanocrystalline Mg20Ni10 - xCux (x = 0 - 4) Alloys 02. Firstly, the improved performance in the cycle life of substituted alloy electrodes is presumably attributed to preferential oxidation of Cu on the alloy surface and the prevention of the formation of the Mg(OH)2 passive layer. Secondly, the additive of a third element significa- ntly stabilizes the nanostructure of Mg-Ni-based alloy [28], reflecting an increase of the cycle stability of the alloy. The nanostructure of the alloys formed by rapid quenching was detrimental for corrosion in the electro- lyte during cycling due to the fact that intercrystalline corrosion is inevitable. Therefore, it is comprehensible why rapid quenching leads to a decline of the cycle sta- bility of the Mg-Ni-Cu system alloy. 4. Conclusions The structures and hydrogen storage characteristics of the nanocrystalline Mg20Ni10 – xCux (x = 0 – 4) alloys were investigated, and the conclusions obtained are summa- rized as follows: 1) All the as-quenched Mg20Ni10 – xCux (x = 0 – 4) al- loys hold nanocrystalline structures and are free of amorphous phase. The rapid quenching does not change the major phase of Mg2Ni-type in the alloy, but it leads to an increment of the lattice parameters and cell volume as well as the FWHM values of the major diffraction peaks of the alloys. 2) Rapid quenching significantly improves the hydrid- ing and dehydriding properties of the alloys. Hydriding and dehydriding capacities and rates of the alloy mark- edly rise with increasing quenching rate. 3) Additionally, rapid quenching considerably enha- nces the electrochemical discharge capacity of the alloys, whereas it slightly weakens the charging/discharging cy- cle stability of the alloys, for which the nanocrystalline structure formed by rapid quenching is basically respon- sible. 5. Acknowledgements This work is supported by Hi-Tech Research and Devel- opment Program of China (2007AA03Z227), National Natural Science Foundations of China (50871050 and 50701011), Natural Science Foundation of Inner Mongo- lia, China (200711020703) and Higher Education Scien- ce Research Project of Inner Mongolia, China (NJzy 08071). REFERENCES [1] L. Schlapbach and A. Züttel, “Hydrogen-Storage Materi- als for Mobile Applications,” Journal of Nature, Vol. 414, 2001, pp. 353-358. [2] M. V. Simičić, M. Zdujić, R. Dimitrijević, L. Nikolić- Bujanović and N. H. Popović, “Hydrogen Absorption and Electrochemical Properties of Mg2Ni-Type Alloys Syn- thesized by Mechanical Alloying,” Journal of Power Sources, Vol. 158, No. 1, 2006, pp. 730-734. [3] A. Ebrahimi-Purkani and S. F. Kashani-Bozorg, “Nan- ocrystalline Mg2Ni-Based Powders Produced by High- Energy Ball Milling and Subsequent Annealing,” Journal of Alloys and Compounds, Vol. 456, No. 1-2, 2008, pp. 211-215. [4] D. Kyoi, T. Sakai, N. Kitamura, A. Ueda and S. Tanase, “Synthesis of FCC Mg-Ta Hydrides Using GPa Hydrogen Pressure Method and their Hydrogen-Desorption Proper- ties,” Journal of Alloys and Compounds, Vol. 463, No. 1-2, 2008, pp. 306-310. [5] P. Palade, S. Sartori, A. Maddalena, G. Principi, S. Lorusso, M. Lazarescu, G. Schinteie, V. Kuncser and G. Filoti, “Hydrogen Storage in Mg-Ni-Fe Compounds Pre- pared by Melt Spinning and Ball Milling,” Journal of Al- loys and Compounds, Vol. 415, No. 1-2, 2006, pp. 170- 176. [6] M. Y. Song, C. D. Yim, J. S. Bae, D. R. Mummd and S. H. Hong, “Preparation by Gravity Casting and Hydro- gen-Storage Properties of Mg—23.5 wt.% Ni—(5, 10 and 15 wt.%) La,” Journal of Alloys and Compounds, Vol. 463, No. 1-2, 2008, pp. 143-147. [7] L. H. Kumar, B. Viswanathan and S. S. Murthy, “Hydro- gen Absorption by Mg2Ni Prepared by polyol Reduc- tion,” Journal of Alloys and Compounds, Vol. 461, No. 1-2, 2008, pp. 72-76. [8] X. F. Liu, Y. F. Zhu and L. Q. Li, “Structure and Hydro- genation Properties of Nanocrystalline Mg2Ni Prepared by Hydriding Combustion Synthesis and Mechanical Milling,” Journal of Alloys and Compounds, Vol. 455, No. 1-2, 2008, pp. 197-2 [9] F. J. Liu and S. Suda, “A Method for Improving the Long-Term Storability of Hydriding Alloys by Air Water Exposure,” Journal of Alloys Compounds, Vol. 231, No. 1-2, 1995, pp. 742-750. [10] T. Czujko, R. A. Varin, C. Chiu and Z. Wronski, “Inves- tigation of the Hydrogen Desorption Properties of Mg + 10 wt.% X (X = V, Y, Zr) Submicrocrystalline Compos- ites,” Journal of Alloys Compounds, Vol. 414, No. 1-2, 2006, pp. 240- 247. [11] C. X. Shang, M. Bououdina, Y. Song and Z. X. Guo, “Mechanical Alloying and Electronic Simulations of (MgH2 + M) Systems (M = Al, Ti, Fe, Ni, Cu and Nb) for Hydrogen Storage,” International Journal of Hydrogen Energy, Vol. 29, No. 1, 2004, pp. 73-80. [12] B. Sakintuna, F. Lamari-Darkrim and M. Hirscher, ”Metal Hydride Materials for Solid Hydrogen Storage: A Re- view,” International Journal of Hydrogen Energy, Vol. 32, No. 9, 2007, pp. 1121-1140. [13] A. Zaluska, L. Zaluski and J. O. Stroem-Olsen, “Synergy of Hydrogen Sorption in Ball-Milled Hydrides of Mg and Mg2Ni,” Journal of Alloys Compounds, Vol. 289, No. 1-2, 1999, pp. 197-206. [14] N. Hanada, T. Ichikawa and H. Fujii, “Catalytic Effect of Nanoparticle 3d-Transition Metals on Hydrogen Storage Copyright © 2010 SciRes. MSA  Hydrogen Absorption and Electrochemical Properties of As-Quenched Nanocrystalline Mg20Ni10 – xCux (x = 0 – 4) Alloys Copyright © 2010 SciRes. MSA 176 Properties in Magnesium Hydride MgH2 Prepared by Mechanical Milling,” Journal of Physical Chemistry B, Vol. 109, No. 15, 2005, pp. 7188-7194. [15] N. Recham, V. V. Bhat, M. Kandavel, L. Aymard, J. M. Tarascon and A. Rougier, “Reduction of Hydrogen De- sorption Temperature of Ball-Milled MgH2 by NbF5 Ad- dition,” Journal of Alloys Compounds, Vol. 464, No. 1-2, 2008, pp. 377-382. [16] V. D. Dobrovolsky, O. G. Ershova, Y. M. Solonin, O. Y. Khyzhuna and V. Paul-Boncour, “Influence of TiB2 Ad- dition upon Thermal Stability and Decomposition Tem- perature of The Mgh2 Hydride of a Mg-Based Mechanical Alloy,” Journal of Alloys Compounds, Vol. 465, No. 1-2, 2008, pp. 177-182. [17] N. Cui, B. Luan, H. J. Zhao, H. K. Liu and S. X. Dou, “Effects of Yttrium Additions on the Electrode Perform- ance of Magnesium-Based Hydrogen Storage Alloys,” Journal of Alloys Compounds, Vol. 233, No. 1-2, 1996, pp. 236- 240. [18] T. Kohno and M. Kanda, “Effect of Partial Substitition on Hydrogen Storage Properties of Mg2Ni Alloy,” Journal of Electrochemical Society, Vol. 144, 1997, pp. 2384- 2388. [19] G. Y. Liang, “Synthesis and Hydrogen Storage Properties of Mg-Based Alloys,” Journal of Alloys Compounds, Vol. 370, No. 1-2, 2004, pp. 123-128. [20] M. Y. Song, S. N. Kwon, J. S. Bae and S. H. Hong, “Hy- drogen-Storage Properties of Mg–23.5Ni–(0 and 5)Cu Prepared by Melt Spinning and Crystallization Heat Treatment,” International Journal of Hydrogen Energy, Vol. 33, No. 6, 2008, pp. 1711-1718. [21] M. Savyak, S. Hirnyj, H. D. Bauer, M. Uhlemann, J. Eckert, L. Schultz and A. Gebert, “Electrochemical Hy- drogenation of Mg65Cu25Y10 Metallic Glass,” Journal of Alloys Compounds, Vol. 364, No. 1-2, 2004, pp. 229-237. [22] T. Spassov and U. Köster, “Electrode Properties of Melt-Spun Mg–Ni–Nd Amorphous Alloys,” Journal of Power Sources, Vol. 160, No. 1, 2006, pp. 684-687. [23] G. Friedlmeier, M. Arakawa, T. Hiraia and E. Akiba, “Preparation and Structural, Thermal and Hydriding Characteristics of Melt-Spun Mg–Ni Alloys,” Journal of Alloys Compounds, Vol. 292, No. 1-2, 1999, pp. 107-117. [24] Y. Wu, W. Han, S. X. Zhou, M. V. Lototsky, J. K. Sol- berg and V. A. Yartys, “Microstructure and Hydrogena- tion Behavior of Ball-Milled and Melt-Spun Mg– 10Ni–2Mm Alloys,” Journal of Alloys and Compounds, Vol. 466, No. 1-2, 2008, pp. 176-181. [25] K. Tanaka, Y. Kanda, M. Furuhashi, K. Saito, K. Kuroda, H. Saka, “Improvement of Hydrogen Storage Properties of Melt-Spun Mg–Ni–RE Alloys by Nancrystallization,” Journal of Alloys Compounds, Vol. 295, 1999, pp. 521- 525. [26] S. Orimo and H. Fujii, “Materials Science of Mg-Ni- Based New Hydrides” Journal Applied Physics A, Vol. 72, No. 2, 2001, pp. 167-186. [27] T. Spassov and U. Köster, “Thermal Stability and Hydriding Properties of Nanocrystalline Melt-Spun Mg63Ni30 Y7 Al- loy,” Journal of Alloys Compounds, Vol. 279, 1998, pp. 279-286. [28] T. Spassov, L. Lyubenova, U. Köster and M. D. Baró, “Mg–Ni–RE Nanocrystalline Alloys for Hydrogen Storage,” Journal of Materials Science and Engineering A, Vol. 375-377, 2004, pp. 794-799. [29] G. Mulas, L. Schiffini and G. Cocco, “Mechanochemical Study of the Hydriding Properties of Nanostructured Mg2Ni–Ni Composites,” Journal of Materials Research, Vol. 19, 2004, pp. 3279-3289. [30] L. Zaluski, A. Zaluska and J. O. Ström-Olsen, “Nanocrys- talline Metal Hydrides,” Journal of Alloys Compounds, Vol. 2532, No. 54, 1997, pp. 70-79. [31] S. Orimo, H. Fujii and K. Ikeda, “Notable Hydriding Properties of a Nanostructured Composite Material of the Mg2Ni-H System Synthesized by Reactive Mechanical Grinding,” Acta Materialia, Vol. 45, No. 1, 1997, pp. 331-341. [32] H. Niu and D. O. Northwood, “Enhanced Electrochemical Properties of Ball-Milled Mg2Ni Electrodes,” Interna- tional Journal of Hydrogen Energy, Vol. 27, No. 1, 2002, pp. 69-77. |

