Paper Menu >>
Journal Menu >>
 Materials Sciences and Applications, 2010, 1, 162-167 doi:10.4236/msa.2010.13026 Published Online August 2010 (http://www.SciRP.org/journal/msa) Copyright © 2010 SciRes. MSA Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions Issa Katime1, Eduardo Mendizábal2 1Grupo de Nuevos Materialesy Espectroscopia Supramolecular, Departamento de Química Física, Facultad de Ciencia y Tecnología (Campus de Leioa), Universidad del País Vasco, Bilbao, España; 2CUCEI, Universidad de Guadalajara, Guadalajara, México. Email: issa.katime@ehu.es, lalomendizabal@gmail.com Received May 22nd, 2010; revised June 17th, 2010; accepted July 21st, 2010. ABSTRACT Hydrogels of dimethylaminoethyl acrylate methyl chloride quaternary salt (Q9) have been synthesized with different monomer ratio by copolymerization of this poorly studied monomer either with acrylic acid or with 2-methylene bu- tane-1,4-dioic acid. Hydrogel swelling was measured as a function of the composition of the hydrogel and of the crosslinking agent ratio. High values of swelling have been obtained at very high crosslinking values (< 14 wt %) and the equilibrium swelling was reached at very low time (less than 15 minutes). The swelling isotherms consisted of a steep initial portion and then levelled off as asymptotically to the equilibrium swelling limit. The experimental data suggest clearly that the swelling process obeys second-order kinetics. According to this, the kinetics rate constant and the equilibrium water content were determined at different comonomer composition and crosslinker concentration. The calculated kinetic constants ranged from 0.48 to 3.76 × 10-2 min-1 for poly (acrylic acid-co-Q9) hydrogels and from 0.68 to 4.0 × 10-2 min-1 for poly (2-methylene butane-1,4-dioic acid-co-Q9) hydrogels depending on the hydrogels composi- tion. The diffusion process was evaluated for each hydrogel showing a non-Fickian type diffusion. In all cases was ob- served a considerable increase in diffusion coefficient as Q9 content increases. Keywords: Dimethyl Amino Ethyl Acrylate Methyl Chloride Quaternary Salt, 2-methylene butane-1,4-Dioic Acid, Acrylic Acid, Swelling, Diffusion Coefficients, Kinetic Order 1. Introduction A gel is a polymer network which swell when immersed in solvent, but which is prevented from dissolving by the presence of crosslinks which hold the structure intact. Ge- ls which swell in aqueous solvents are called hydrogels [1,2]. Equilibrium water content in hydrogels is one of their basic properties. A hydrogel with high water con- tent is generally more advantageous for medical applica- tions because of its permeability and biocompability [3]. Usually a large swelling is accompanied by poor mech- anical properties. There are several alternatives to find a compromise between “large swelling” and “good mech- anical behavior”. Increasing the crosslinking agent dens- ity is a way to improve the mechanical properties but this affects adversely the swelling. Copolymerization of hydr- ophilic monomer (which favors swelling) with a less hy- drophilic monomer results in a hydrogel with good water absorbance and improved mechanical properties in the resulting hydrogel [4]. It is now well established that some gels can reversibly swell and shrink by as much as several hundred times in response to small changes in environmental conditions su- ch as temperature, solvent composition, pH, ionic streng- th, electric field, and light. Knowledge of swelling kinet- ics are important for designing controlled-released devic- es for drugs and agriculture pesticides based on swellable polymer matrices and for predicting the release rates of the active ingredients [5]. In this paper, different hydrogels have been synthesi- 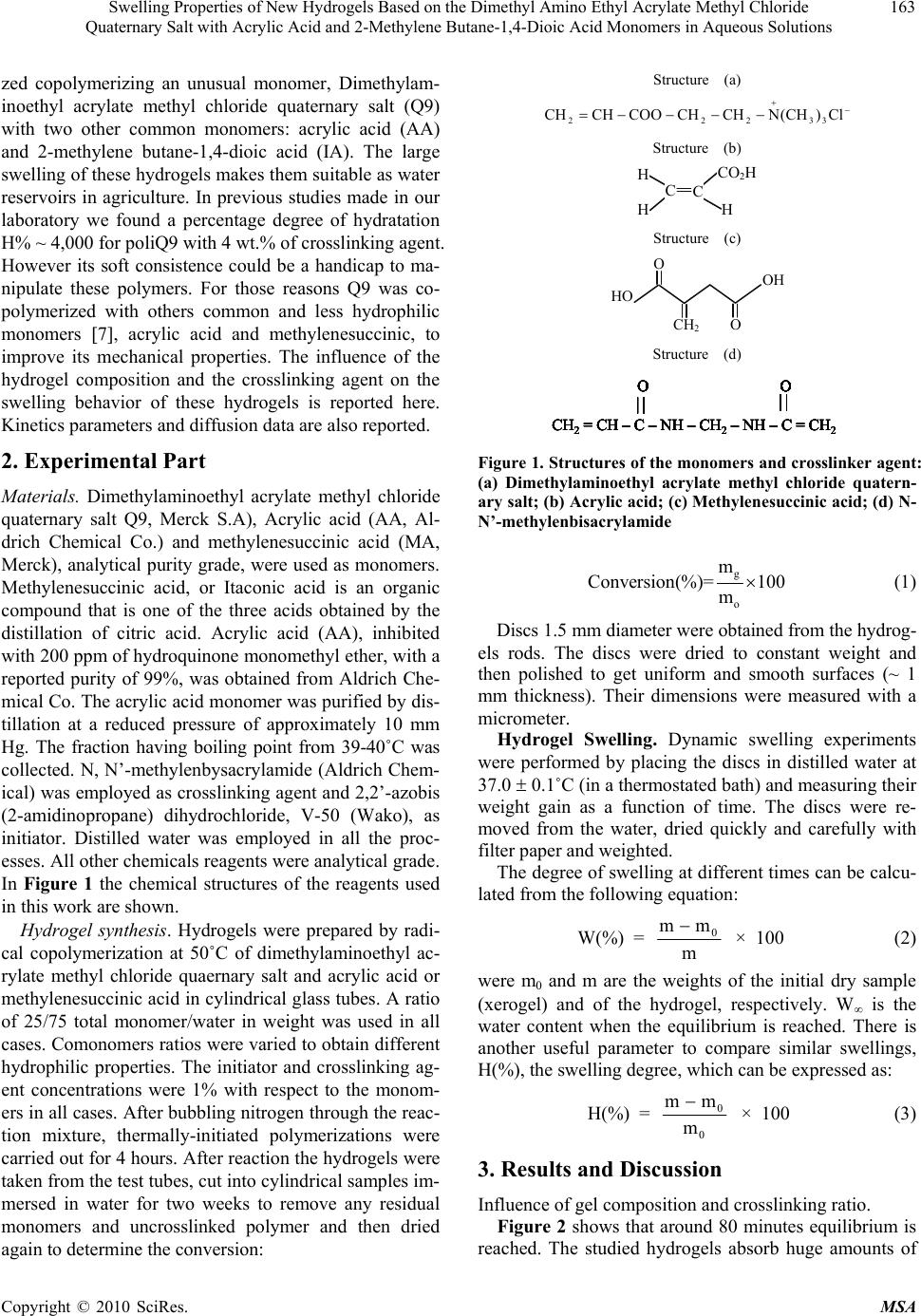 Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride 163 Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions zed copolymerizing an unusual monomer, Dimethylam- inoethyl acrylate methyl chloride quaternary salt (Q9) with two other common monomers: acrylic acid (AA) and 2-methylene butane-1,4-dioic acid (IA). The large swelling of these hydrogels makes them suitable as water reservoirs in agriculture. In previous studies made in our laboratory we found a percentage degree of hydratation H% ~ 4,000 for poliQ9 with 4 wt.% of crosslinking agent. However its soft consistence could be a handicap to ma- nipulate these polymers. For those reasons Q9 was co- polymerized with others common and less hydrophilic monomers 7], acrylic acid and methylenesuccinic, to improve its mechanical properties. The influence of the hydrogel composition and the crosslinking agent on the swelling behavior of these hydrogels is reported here. Kinetics parameters and diffusion data are also reported. 2. Experimental Part Materials. Dimethylaminoethyl acrylate methyl chloride quaternary salt Q9, Merck S.A), Acrylic acid (AA, Al- drich Chemical Co.) and methylenesuccinic acid (MA, Merck), analytical purity grade, were used as monomers. Methylenesuccinic acid, or Itaconic acid is an organic compound that is one of the three acids obtained by the distillation of citric acid. Acrylic acid (AA), inhibited with 200 ppm of hydroquinone monomethyl ether, with a reported purity of 99%, was obtained from Aldrich Che- mical Co. The acrylic acid monomer was purified by dis- tillation at a reduced pressure of approximately 10 mm Hg. The fraction having boiling point from 39-40˚C was collected. N, N’-methylenbysacrylamide (Aldrich Chem- ical) was employed as crosslinking agent and 2,2’-azobis (2-amidinopropane) dihydrochloride, V-50 (Wako), as initiator. Distilled water was employed in all the proc- esses. All other chemicals reagents were analytical grade. In Figure 1 the chemical structures of the reagents used in this work are shown. Hydrogel synthesis. Hydrogels were prepared by radi- cal copolymerization at 50˚C of dimethylaminoethyl ac- rylate methyl chloride quaernary salt and acrylic acid or methylenesuccinic acid in cylindrical glass tubes. A ratio of 25/75 total monomer/water in weight was used in all cases. Comonomers ratios were varied to obtain different hydrophilic properties. The initiator and crosslinking ag- ent concentrations were 1% with respect to the monom- ers in all cases. After bubbling nitrogen through the reac- tion mixture, thermally-initiated polymerizations were carried out for 4 hours. After reaction the hydrogels were taken from the test tubes, cut into cylindrical samples im- mersed in water for two weeks to remove any residual monomers and uncrosslinked polymer and then dried again to determine the conversion: Structure (a) Cl)CH(NCHCHCOOCHCH 33222 Structure (b) C H H C H CO 2 H Structure (c) O HO CH 2 O H O Structure (d) CH2= CH – C – NH – CH2– NH –C = CH2 O O CH2= CH – C – NH – CH2– NH –C = CH2 O O O O CH2= CH – C – NH – CH2– NH –C = CH2 O O CH2= CH – C – NH – CH2– NH –C = CH2 O O O O CH2= CH – C – NH – CH2– NH –C = CH2 O O O O CH2= CH – C – NH – CH2– NH –C = CH2 O O O O O O O O O O CH2= CH – C – NH – CH2– NH –C = CH2 O O O O CH2= CH – C – NH – CH2– NH –C = CH2 O O O O O O O O O O Figure 1. Structures of the monomers and crosslinker agent: (a) Dimethylaminoethyl acrylate methyl chloride quatern- ary salt; (b) Acrylic acid; (c) Methylenesuccinic acid; (d) N- N’-methylenbisacrylamide g o m Conversion(%)= 100 m (1) Discs 1.5 mm diameter were obtained from the hydrog- els rods. The discs were dried to constant weight and then polished to get uniform and smooth surfaces (~ 1 mm thickness). Their dimensions were measured with a micrometer. Hydrogel Swelling. Dynamic swelling experiments were performed by placing the discs in distilled water at 37.0 0.1˚C (in a thermostated bath) and measuring their weight gain as a function of time. The discs were re- moved from the water, dried quickly and carefully with filter paper and weighted. The degree of swelling at different times can be calcu- lated from the following equation: W(%) = m mm 0 × 100 (2) were m0 and m are the weights of the initial dry sample (xerogel) and of the hydrogel, respectively. W is the water content when the equilibrium is reached. There is another useful parameter to compare similar swellings, H(%), the swelling degree, which can be expressed as: H(%) = 0 0 m mm × 100 (3) 3. Results and Discussion Influence of gel composition and crosslinking ratio. Figure 2 shows that around 80 minutes equilibrium is reached. The studied hydrogels absorb huge amounts of Copyright © 2010 SciRes. MSA 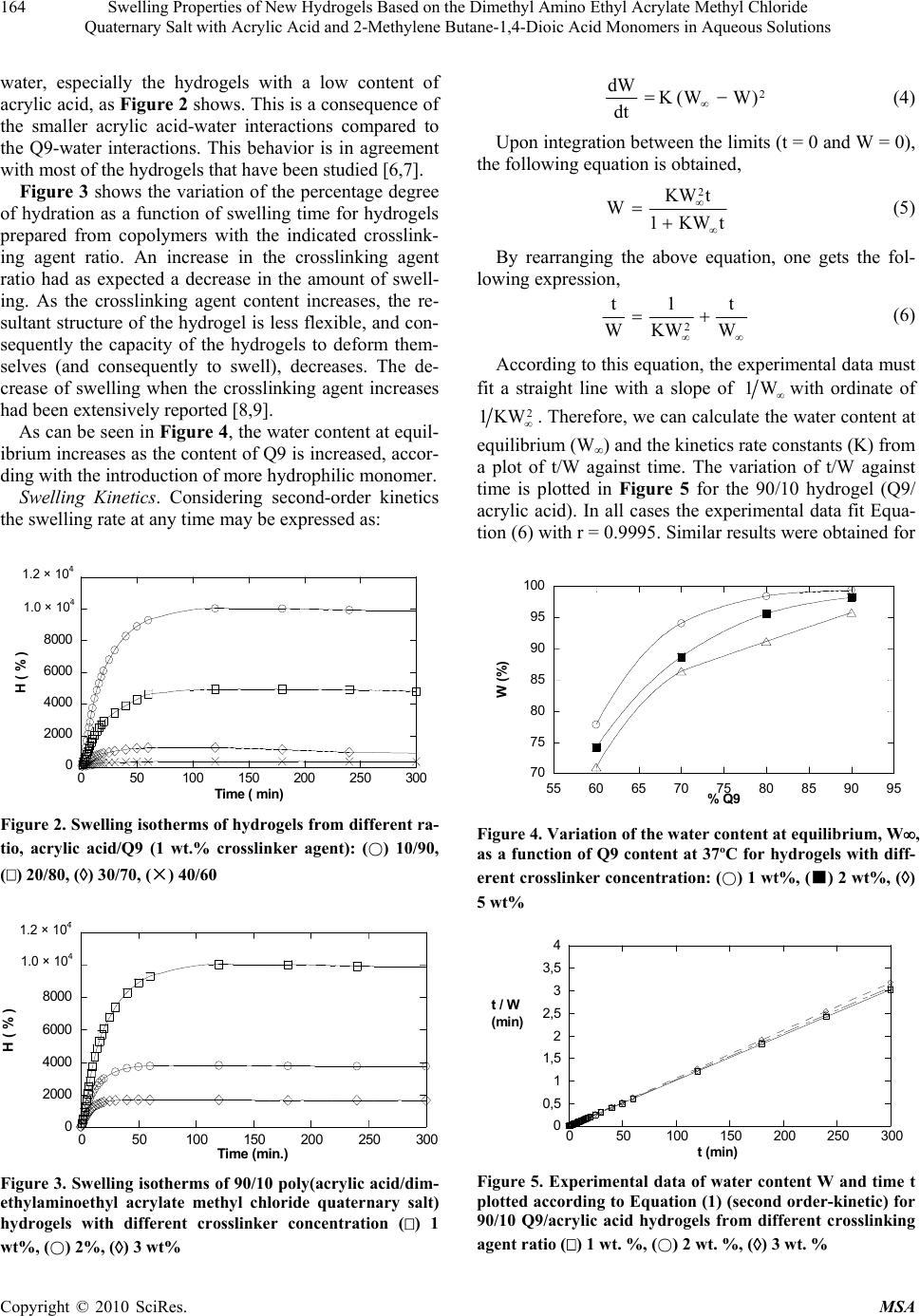 Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride 164 Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions water, especially the hydrogels with a low content of acrylic acid, as Figure 2 shows. This is a consequence of the smaller acrylic acid-water interactions compared to the Q9-water interactions. This behavior is in agreement with most of the hydrogels that have been studied [6,7]. Figure 3 shows the variation of the percentage degree of hydration as a function of swelling time for hydrogels prepared from copolymers with the indicated crosslink- ing agent ratio. An increase in the crosslinking agent ratio had as expected a decrease in the amount of swell- ing. As the crosslinking agent content increases, the re- sultant structure of the hydrogel is less flexible, and con- sequently the capacity of the hydrogels to deform them- selves (and consequently to swell), decreases. The de- crease of swelling when the crosslinking agent increases had been extensively reported [8,9]. As can be seen in Figure 4, the water content at equil- ibrium increases as the content of Q9 is increased, accor- ding with the introduction of more hydrophilic monomer. Swelling Kinetics. Considering second-order kinetics the swelling rate at any time may be expressed as: 0 2000 4000 6000 8000 1 104 1,2 104 050100 150 200 250 300 H ( % ) Time ( min) 1.2 × 10 4 1.0 × 10 4 Figure 2. Swelling isotherms of hydrogels from different ra- tio, acrylic acid/Q9 (1 wt.% crosslinker agent): () 10/90, () 20/80, () 30/70, (×) 40/60 0 2000 4000 6000 8000 1 104 1,2 104 050100 150 200 250 300 H ( % ) Time ( min. ) 1.2 × 10 4 1.0 × 10 4 Figure 3. Swelling isotherms of 90/10 poly(acrylic acid/dim- ethylaminoethyl acrylate methyl chloride quaternary salt) hydrogels with different crosslinker concentration () 1 wt%, () 2%, () 3 wt% 2 )WW(K dt dW (4) Upon integration between the limits (t = 0 and W = 0), the following equation is obtained, tKW1 tKW W 2 (5) By rearranging the above equation, one gets the fol- lowing expression, W t KW 1 W t 2 (6) According to this equation, the experimental data must fit a straight line with a slope of W1 with ordinate of 2 KW1 . Therefore, we can calculate the water content at equilibrium (W) and the kinetics rate constants (K) from a plot of t/W against time. The variation of t/W against time is plotted in Figure 5 for the 90/10 hydrogel (Q9/ acrylic acid). In all cases the experimental data fit Equa- tion (6) with r = 0.9995. Similar results were obtained for 70 75 80 85 90 95 100 55 60 65 70 75 80 85 90 95 W (%) % Q9 Figure 4. Variation of the water content at equilibrium, W, as a function of Q9 content at 37ºC for hydrogels with diff- erent crosslinker concentration: () 1 wt%, (■) 2 wt%, () 5 wt% 0 0,5 1 1,5 2 2,5 3 3,5 4 050 100 150 200 250 300 t / W (min) t (min) Figure 5. Experimental data of water content W and time t plotted according to Equation (1) (second order-kinetic) for 90/10 Q9/acrylic acid hydrogels from different crosslinking agent ratio () 1 wt. %, () 2 wt. %, () 3 wt. % Copyright © 2010 SciRes. MSA  Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride 165 Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions the other hydrogels. Therefore, we could calculate the wa ter content at equilibrium (W) and the kinetics rate con- stants (K). In Table 1 are summarized the experimental values of swelling kinetic rate constants (K) as a function of the Q9 and crosslinking percentage. An increase in the cro- sslinking and a decrease in the more hydrophilic mono- mer content, drive to a higher physical restriction in the network, which causes that the swelling rate decreases. Diffusion. When a glassy hydrogel is brought into con- tact with water, water diffuses into the hydrogel and the hydrogel swells [10]. Diffusion involves migration of wa- ter into pre-existing or dynamically formed spaces bet- ween hydrogel chains whereas swelling of the hydrogel involves a larger scale segmental motion resulting in a bigger separation between hydrogel chains [11]. The foll- owing useful equation was used to determinate the nature of diffusion of water into hydrogels: n tKt M M (7) where Mt and M denote the amount of solvent diffused into the gel at time t and at infinite time respectively, K is a constant related to the structure of the network and n is a characteristic exponent of the transport mode of the solvent 12. Depending on the relative rates of diffusion and polymer relaxation three classes of diffusion mecha- nisms are distinguished [13]: 1) Case I or Fickian diffu- sion in which the rate of diffusion is much less than that of relaxation (n = 0.50), 2) Case II diffusion, in which diffusion is very rapid compared with the relaxation processes (n = 1), and 3) Non-Fickian or anomalous dif- fusion which occurs when the diffusion and relaxation rates are comparables (0.50 < n < 1). To elucidate the transport mechanisms, the swelling curves were fitted to the following equation: log tlognklog M Mt (8) The exponents n were calculated from the slopes and values ranging from 0.66 to 1.24 were obtained (Table 2). This indicates that diffusion of water to the interior of the discs follows an anomalous diffusion mechanism, revea- ling the existence of certain coupling between molecular Table 1. Experimental values of kinetics rate constant (K) as function of Q9 concentration and crosslinker concentra- tion for poly(acrylic acid-co-Q9) hydrogels K × 102 (min-1) (%) Q9 (%) NMBA 60 70 80 90 1 0.70 2.00 2.37 3.76 2 0.56 1.67 2.19 3.40 5 0.48 1.43 1.65 2.47 diffusion and tension relaxation which are developed du- ring swelling of the xerogels. As Table 2 shows the hy- drogel which swells the most is an unusual system with a characteristic n exponent higher than 1. This table also shows that as the Q9 percentage and crosslinker concent- ration decreases the system deviates more from a Fickian behavior. For an anomalous mechanism the coupling bet- ween molecular transport and stress relaxation during sw- elling causes deviations with respect to Fickian mechan- ism. This becomes more important when Q9 content inc- reases because it is a more hydrophilic monomer, and when the crosslinker increases due to the decrease in free volume. The diffusion coefficients for non-Fickian sorption pro- cesses can be obtained by mean of the following equation 14: 2 0.049 (4 ) Dt (9) where t is the time at which the swelling is one half the equilibrium value and is the radius of the cylindrical hydrogel at this time. Figure 6 shows the variation of the water diffusion coefficient of water i, D, for different hydrogel compositions. Experimental values show that when the Q9 content increases, the diffusion coefficient of hydrogels, D, increases. The reason, is the higher sw- elling degree at equilibrium when the more hydrophilic component increases. Hydrogels of methylenesuccinic acid/Q9. Swelling of hydrogels of methylenesuccinic acid and Q9 were also Table 2. Values of n (diffusion exponent), for poly(acrylic acid-co-Q9) hydrogels as a function of Q9 composition and crosslinker concentration n % Q9 % NMBA 60 70 80 90 1 0.87 0.92 1.00 1.24 2 0.78 0.80 0.93 0.97 5 0.66 0.71 0.77 0.94 % Q9 55 60 65 70 75 80 85 90 95 D × 10 6 ( cm 2 /s ) 10 9 8 7 6 5 4 3 2 Figure 6. Variation of water diffusion coefficient at 37ºC , D, of poly(acrylic acid-co-Q9) hydrogels Copyright © 2010 SciRes. MSA  Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride 166 Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions studied. Figures 7 and 8 show the variation of %H as a function of swelling time for hydrogels prepared with different Q9 concentration and crosslinker agent, respec- tively. These hydrogels show a similar swelling depend- ence on the content of the more hydrophilic monomer (Q9) and on crosslinker agent, than the poly(acrylic acid/Q9) hydrogels, that is a larger swelling degree is obtained by using a larger concentration of Q9 and a lower concentration of crosslinker. 0 200 400 600 800 1000 1200 1400 0 102030405 H ( % ) Time ( min ) 0 Figure 7. Swelling isotherms of methylenesuccinic acid/Q9 hydrogels (6% crosslinker agent) for different Q9 concen- tration: () 80%, (■) 85%, () 90 wt%, (●) 95 wt% and () 100 wt% 0 500 1000 1500 2000 2500 3000 0 10203040 H ( % ) Time ( min ) 50 Figure 8. Swelling isotherms of 5/95 methylenesuccinic ac- id/Q9 hydrogels with different crosslinker concentration: () 4 %wt, (●) 6 wt%, () 8 wt%, (■) 10 wt%, ()12 wt% and (×) 14 wt% Table 3. Kinetics rate constant of poly(methylenesuccinic acid-co-Q9) hydrogels K × 102 min-1 % NMBA % Q9 4 6 8 10 12 14 80 0.68 2.83 2.30 3.26 2.79 4.00 85 2.14 2.06 1.92 3.72 3.65 3.46 90 2.75 2.56 2.32 2.26 2.21 2.17 95 2.61 2.46 2.33 2.25 2.06 2.12 100 2.90 2.89 2.38 2.62 2.17 2.46 Table 4. Values of n (diffusion exponent) for poly (methyl- enesuccinic acid-co-Q9) hydrogels as a function of Q9 com- position and crosslinker concentration % NMBA % Q9 4 6 8 10 12 14 80 0.97 0.73 0.82 0.63 0.56 0.54 85 0.90 0.82 0.66 0.68 0.63 0.59 90 0.88 0.74 0.84 0.66 0.67 0.68 95 0.87 0.86 0.71 0.77 0.58 0.84 100 0.97 0.86 0.67 0.73 0.83 0.93 By graphing the experimental data (not shown) using Equation (3), it was found that the swelling process of these hydrogels also obeys second-order kinetics. In Ta- ble 3 are summarized the experimental values of kinetics rate constants (K) as a function of Q9 and crosslinker agent content. An increase in the crosslinking ratio and a decrease in the more hydrophilic monomer content. The values of n exponent are listed in Table 4. In all cases n exponent is higher than 0.50. Therefore the water diffu- sion into hydrogels displays also a non-Fickian character. 3. Conclusions In this paper we have described the swelling kinetics of poly(acrylic acid/dimethylaminoethyl acrylate methyl ch- loride quaternary salt) and poly(methylenesuccinic acid/ dimethylaminoethyl acrylate methyl chloride quaternary salt) hydrogels. These hydrogels absorb huge amounts of water. The experimental data indicate that the swelling process follows second–order kinetics for all the studied systems. Hydrogels show large swelling properties as the Q9 content increases and crosslinking agent ratio de- creases. Water diffusion into hydrogels is a non-Fickian type diffusion. Diffusion coefficients increase with increasing Q9 content . The diffusion coefficients were determined by using equation (9). In these copolymers is observed that the crosslinking ratio has not an important effect on the wa- ter transport, as we concluded with poly(acrylic acid-co- dimethylaminoethyl acrylate methyl chloride quaternary) hydrogels. For lower Q9 content hydrogels, the diffusion coefficient remains constant with the composition, and in these cases the determined value was 6.5 × 10-6 cm2/s. It was only observed a considerable effect on diffusion coefficient due to Q9 content on 0/100 hydrogel (itaconic acid/Q9). The diffusion coefficient determined in this case was 1.4 × 10-5 cm2/s. 4. Acknowledgements The authors thanks the financial support for this work was provided by Ministerio de Ciencia e Innovación (Pr- Copyright © 2010 SciRes. MSA  Swelling Properties of New Hydrogels Based on the Dimethyl Amino Ethyl Acrylate Methyl Chloride Quaternary Salt with Acrylic Acid and 2-Methylene Butane-1,4-Dioic Acid Monomers in Aqueous Solutions Copyright © 2010 SciRes. MSA 167 oject Number: EUI2008-00178) and the Gobierno Vasco for their financial support REFERENCES [1] L. W. FU and S. Chich-Hsuan, “PH-Thermoreversible Hydrogels. II. Synthesis and Swelling Behaviors of N- Isopropylacrylamide-Co-Acrylic Acid-Co-Sodium Acry- late Hydrogels,” Journal of Applied Polymer Science, Vol. 73, No. 10, September 1999, pp. 1955-1967. [2] I. P. Lee, “Kinetics of Drug Release from Hydrogel Ma- trices,” Journal of Controlled Release, Vol. 2, November 1985, pp. 277-288. [3] I. Katime, R. Novoa and F. Zuluaga, “Swelling Kinetics and Release Studies of Theophylline and Aminophylline from Acrylic Acid/N-Alkyl Methacrylate Hydrogels,” European Polymer Journal, Vol. 37, No. 7, July 2001, pp. 1465-1471. [4] A. Nagaoka, “Mechanical Properties of Composite Hy- drogels,” Polymer Journal, Vol. 21, No. 10, April 2005, pp. 847- 850. [5] H. Schott, “Swelling Kinetics of Polymers,” Journal of Macromolecular Science, Vol. 31, No. 1, March 1992, pp. 1-9. [6] N. M. Franson and N. A. Pepas, “Influence of Copolymer Composition on Non-Fickian Water Transport through Glassy Copolymers,” Journal of Applied Polymer Science, Vol. 28, No. 4, April 1983, pp. 1299-1310. [7] I. Katime and E. Rodríguez, “Synthesis and Swelling Kinetics of Poly(Acrylic Acid-Co-Itaconic Acid) Hy- drogels,” Recent Research Developments in Polymer Science, Vol. 5, 2001, pp. 139-152. [8] P. E. M. Allen, D. J. Bennette and D. R. G. Williams, “Wa- ter in Methacrylates—I. Sorption and Desorption Properties of Poly(2-Hydroxyethyl Methacrylate-Co-Glycol Di- methacrylate) Networks,” European Polymer Journal, Vol. 28, No. 4, April 1992, pp. 347-352. [9] C. S. Brazel and N. A. Pepas, “Mechanisms of solute and Drug Transport in Relaxing, Swellable, Hydrophilic Glassy Polymers,” Polymer, Vol. 40, No. 12, June 1999, pp. 3383-3398 [10] I. Katime, N. Valderruten and J. R. Quintana, “Controlled Release of Aminophylline from Poly (N-Isopropylacrylamide- Co-Itaconic Acid) Hydrogels,” Polymer International, Vol. 50, No. 8, August 2001, pp. 869-879. [11] A. R. Berens and H. B. Hopfenberg, “Diffusion and Re- laxation in Glassy Polymer Powders: 2. Separation of Diffusion and Relaxation Parameters,” Polymer, Vol. 19, No. 5, May 1978, pp. 489-496. [12] H. L. Frisch, “Sorption and Transport in Glassy Poly- mers—A Review,” Polymer Engineering & Science, Vol. 20, No. 1, January 1980, pp. 2-13. [13] J. Crank “The Mathematics of Diffusion,” Clarendon Press, Oxford, 1975. [14] I. Katime, R. Novoa, E. Díaz de Apodaca, E. Mendizábal and J. E. Puig, “Theophylline Release from Poly(Acrylic Acid-Co-Acrylamide) Hydrogels,” Polymer Testing, Vol. 18, No. 7, October 1999, pp. 559-566. |

