 Journal of Cancer Therapy, 2012, 3, 741-748 http://dx.doi.org/10.4236/jct.2012.325093 Published Online October 2012 (http://www.SciRP.org/journal/jct) 741 CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India Durgadatta Tosh1, Bineet Panda1, Tipirisetti Nageswar Rao2, Arvind Babu1, Vishnupriya Satti2, Digumarti Raghnadharao3, Lalji Singh1, Lakshmi Rao1* 1Centre for Cellular and Molecular Biology, Hyderabad, India; 2Department of Genetics, Osmania University, Hyderabad, India; 3Department of Medical Oncology, Nizams Institute of Medical Sciences, Hyderabad, India. Email: *lakshmi@ccmb.res.in Received April 17th, 2012; revised May 22th, 2012; accepted June 13th, 2012 ABSTRACT Aim: The Androgen Receptor (AR) is a ligand-dependent transcriptional activator and the AR gene contains a highly polymorphic trinucleotide repeat CAG and GGN in the first exon. Given the lack of information AR-CAG and GGN repeat polymorphism and its potential correlation with breast cancer in South Indian women, we conducted a case-con- trol study to observe the effects of CAG & GGN repeat length polymorphism and risk of breast cancer. Methods: Polymorphisms for AR-CAG and GGN repeat length was detected by Gene Scan analysis in the genomic DNA from cases with breast cancer and controls. Results: Association between AR genotype was calculated by categorising alleles as short (S) and long (L) and taking median value as the cut-off. LL genotype of CAG repeat was found to be associated with breast cancer (OR, 4.58; 95% CI, 10.61 - 1.98; p—0.0004). GGN repeat having ≥21 was found in most of the cases and none of the cases showed 20 repeats thus indicate that alleles having homozygous repeat 20 may be protective towards breast cancer. Also, SS genotype was observed in 56.84% of cases and in 73.03% of controls (OR, 0.48; 95% CI, 0.26 - 0.89; p value, 0.02). Conclusion: Our results indicate that longer CAG and GGN repeat may be associated with breast cancer whereas, the shorter GGN repeat length genotype of AR are protective. Keywords: Breast Cancer; Androgen Receptor; CAG; GGN 1. Introduction Breast cancer is the most common cancer and the leading cause of the cancer deaths in women today. The inci- dence of breast cancer varies greatly around the world: it is lowest in less-developed countries, greatest in the more-developed countries and is on the rise especially in developing countries such as India. A recent data showed that India has one of the highest cancer rates in the world [1]. The incidence of breast cancer in India is on the rise and is rapidly becoming the number one cancer in women, pushing the cervical cancer to the second spot. The aetiopathogenesis of breast cancer is largely un- known, but most of the available evidences suggest that it is a multifactorial disease [2]. Exposures to endoge- nous and exogenous hormones are known to influence breast cancer risk. Androgens, the predominant sex ster- oid hormones in postmenopausal women, act through the androgen receptor (AR), a member of the steroid receptor subfamily. Among postmenopausal women, androgen lev- els appear to be positively associated with breast cancer risk [3], but it is not known whether AR has a role in the mediation of these effects. Androgen action is mediated at the transcriptional level by the AR in breast cancer cells [4,5]. The AR and androgens induce proteins, like prostate-specific antigen (PSA) (98%) and the gross cystic disease fluid protein-15 (GCDFP-15) (92%), in the breast cancer specimens, con- firming that AR is functionally active in human breast cancer cells [6]. Also, the AR is found to be expressed in >70% of breast cancers and has been implicated in breast cancer pathogenesis [7-10]. However, the role of andro- gens in breast cancer development and carcinogenesis remains unclear [11,12]. The AR, which is genetically polymorphic, is codified by the AR gene which is located on the X chromosome (q 11.2 - q 12). The AR gene spans 90 kb, contains eight exons and encodes for a protein of about 917 amino acids [13,14]. The exon 1 of AR gene contains two (microsa- tellite) trinucleotide repeat polymorphisms, the CAG (en- coding for polyglutamine) and the GGN (encoding for *Corresponding author Copyright © 2012 SciRes. JCT  CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India 742 polyglycine) repeat sequences. These polymorphisms flank the N-terminal domain of AR protein, where the transactivation activity of the protein resides [4]. Several studies have shown the effect of CAG poly- morphism on the transactivation capacity of the receptor; the longer the repeat the less efficient the transactivation [15,16], which typically is in the range of 6-39 repeat units. The role of (CAG)n polymorphism of AR in cancer predisposition is supported by association studies of breast and prostate cancer risk. Variation of the CAG- AR length polymorphism shows remarkable differences among diverse ethnic groups [17]. African populations have the shortest CAG repeats, whereas the Asian popu- lations possess the longest, while Caucasians and Ameri- can Indians have medium number of repeat sequences [18,19]. Hence, the predisposition of these populations to BC is also known to vary accordingly. The polyglycine tract in the AR protein is encoded by (CGT)3 GGG (GGT)2 (GGC)n, an invariant six-glycine tract (GGT/GGG) followed by a polymorphic GGC re- peat, that is less polymorphic than CAG repeat [14-20]. About 90% of normal AR contains 16-18 CGG repeats. Most of the experimental studies conducted so far re- port on the effects of lengthening either of the CAG or the GGN repeats, while the other repeat is maintained as a constant. However, recent observations on diverse hu- man tumors [21,22] suggest that combination of different repeat length in the normal range could increase the risk of developing cancer [6]. Hence, it remains to be deter- mined whether the combined effects of CAG and GGN repeat polymorphism on androgen action are independent or not. Till date, data supporting the relationship between AR polymorphism and BC came exclusively from Amer- ica and European countries. Though there are a few re- ports from Asia on AR polymorphism in BC, there is no data been reported from South India. Therefore, we con- ducted a case-control study to observe the effects of CAG & GGN repeat length polymorphism and risk of BC, which could ultimately prove to be useful for the early prognosis of the high risk patients and further management of such individuals. 2. Materials and Methods 2.1. Patient and Control Recruitment Ninety-six women with breast cancer of age group 25 - 79 years were recruited from Department of Medical Oncology, Nizams Institute of Medical Sciences, Hy- derabad, India. All the patients were assessed clinically, with complete medical history. Respective consent forms from the patients recruited for the study were collected by the concerned clinic. And eighty nine controls of age group 30 - 70 years were randomly selected from the healthy women of general population with no familial history of breast cancer. Recruitment of the controls was entirely ethnically population-based to support the study. The Institutional Review Board of Centre for Cellular and Molecular Biology (CCMB), Hyderabad, approved the study. 2.2. DNA Extraction A 5-ml aliquot of peripheral blood was collected in EDTA vacutainers for genomic DNA isolation. DNA was extracted using the Nucleon BACC2 DNA extraction kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA) according to the manufacturer’s protocol. 2.3. Molecular Analysis and Assessment of the CAG and GGN Repeats The CAG repeat region of the AR gene was amplified with a pair of primers, forward: 5’FAM-TCCGAATCTGTTCCAGAGCGTGC-3’, reverse: 5’-GCTGTGAAGGTTGCTGTTCCTC-3’ flanking the re- peat region. PCR reaction mixture consisted of 1.0 µl PCR buffer (10X), 1.0 µl MgCl2 (25mM), 1.0 µl dNTPs (10mM), 1.0 pM of each primer, 0.5 units AmpliTaq GoldTM DNA polymerase and 20 ng genomic DNA. PCR was done under the following conditions; initial denatu- ration at 94˚C for 12 minutes followed by 30 cycles of 94˚C for 1 minute, 60.5˚C for 1 minute and 72˚C for 1 minute with a final extension at 72˚C for 30 minutes. GGN repeat was amplified with a pair of primers: forward 5’FAM-CCGCTTCCTCATCCTGGCACAC 3’ and re- verse 5’GCCGCCAGGGTACCACACATC 3’ flanking the repeat region. PCR reaction mixture included 1.0 µl PCR buffer (10×), 1.0 µl MgCl (25 mM), 1.0 µl dNTPs (10 mM), 1.0 µl DMSO (100%), 1.0 µl glycerol (100%), 1.0 pM of each primer, 0.5 units Amplitaq Gold DNA polymerase and 20 ng genomic DNA. PCR conditions consisted of denaturation at 96˚C for 15 minutes, fol- lowed by 40 cycles of 96˚C for 1.5 minutes, 55.5˚C for 1 minute and 72˚C for 3 minutes and a final extension at 72˚C for 20 minutes. For GeneScan, 3.0 µl of PCR product was mixed with 0.2 µl of LIZ500 and 6.8 µl Hi-Di formamide. Upon denaturation for 5 minutes at 96˚C and cooling for 5 minutes on ice, samples were run on 3730 DNA analyzer (Applied Biosystems, USA). PCR and genotyping were repeated for all samples to confirm the number of repeats. The raw data was further analyzed using GeneMapper software (Applied Biosystems, USA). 2.4. Statistical Analysis of Results Each variable was summarized according to its scale and distribution. The genescan results were analyzed on Gene- Copyright © 2012 SciRes. JCT 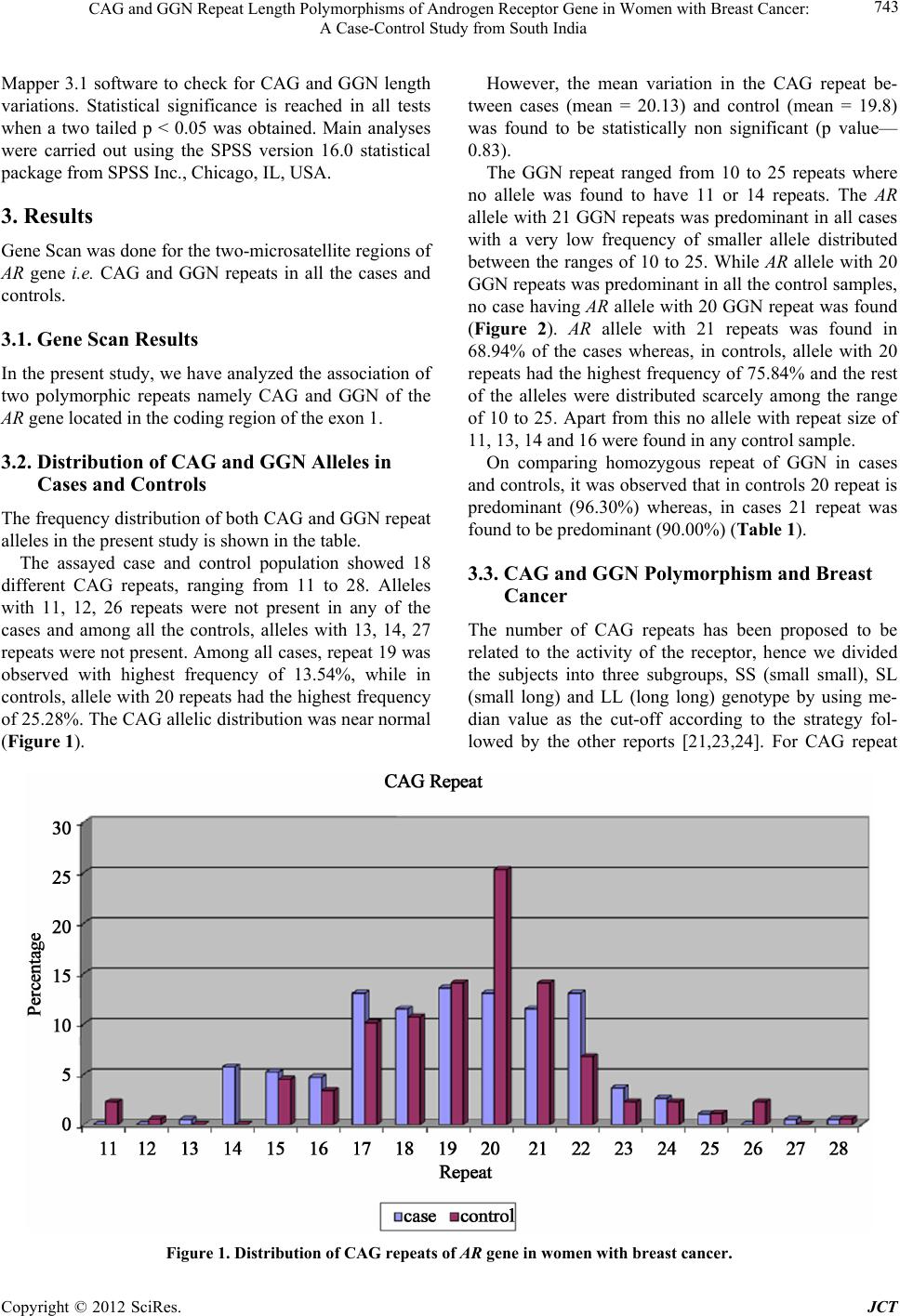 CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India Copyright © 2012 SciRes. JCT 743 However, the mean variation in the CAG repeat be- tween cases (mean = 20.13) and control (mean = 19.8) was found to be statistically non significant (p value— 0.83). Mapper 3.1 software to check for CAG and GGN length variations. Statistical significance is reached in all tests when a two tailed p < 0.05 was obtained. Main analyses were carried out using the SPSS version 16.0 statistical package from SPSS Inc., Chicago, IL, USA. The GGN repeat ranged from 10 to 25 repeats where no allele was found to have 11 or 14 repeats. The AR allele with 21 GGN repeats was predominant in all cases with a very low frequency of smaller allele distributed between the ranges of 10 to 25. While AR allele with 20 GGN repeats was predominant in all the control samples, no case having AR allele with 20 GGN repeat was found (Figure 2). AR allele with 21 repeats was found in 68.94% of the cases whereas, in controls, allele with 20 repeats had the highest frequency of 75.84% and the rest of the alleles were distributed scarcely among the range of 10 to 25. Apart from this no allele with repeat size of 11, 13, 14 and 16 were found in any control sample. 3. Results Gene Scan was done for the two-microsatellite regions of AR gene i.e. CAG and GGN repeats in all the cases and controls. 3.1. Gene Scan Results In the present study, we have analyzed the association of two polymorphic repeats namely CAG and GGN of the AR gene located in the coding region of the exon 1. 3.2. Distribution of CAG and GGN Alleles in Cases and Controls On comparing homozygous repeat of GGN in cases and controls, it was observed that in controls 20 repeat is predominant (96.30%) whereas, in cases 21 repeat was found to be predominant (90.00%) (Table 1). The frequency distribution of both CAG and GGN repeat alleles in the present study is shown in the table. The assayed case and control population showed 18 different CAG repeats, ranging from 11 to 28. Alleles with 11, 12, 26 repeats were not present in any of the cases and among all the controls, alleles with 13, 14, 27 repeats were not present. Among all cases, repeat 19 was observed with highest frequency of 13.54%, while in controls, allele with 20 repeats had the highest frequency of 25.28%. The CAG allelic distribution was near normal (Figure 1). 3.3. CAG and GGN Polymorphism and Breast Cancer The number of CAG repeats has been proposed to be related to the activity of the receptor, hence we divided the subjects into three subgroups, SS (small small), SL (small long) and LL (long long) genotype by using me- dian value as the cut-off according to the strategy fol- lowed by the other reports [21,23,24]. For CAG repeat Figure 1. Distribution of CAG repeats of AR gene in women with breast cancer. 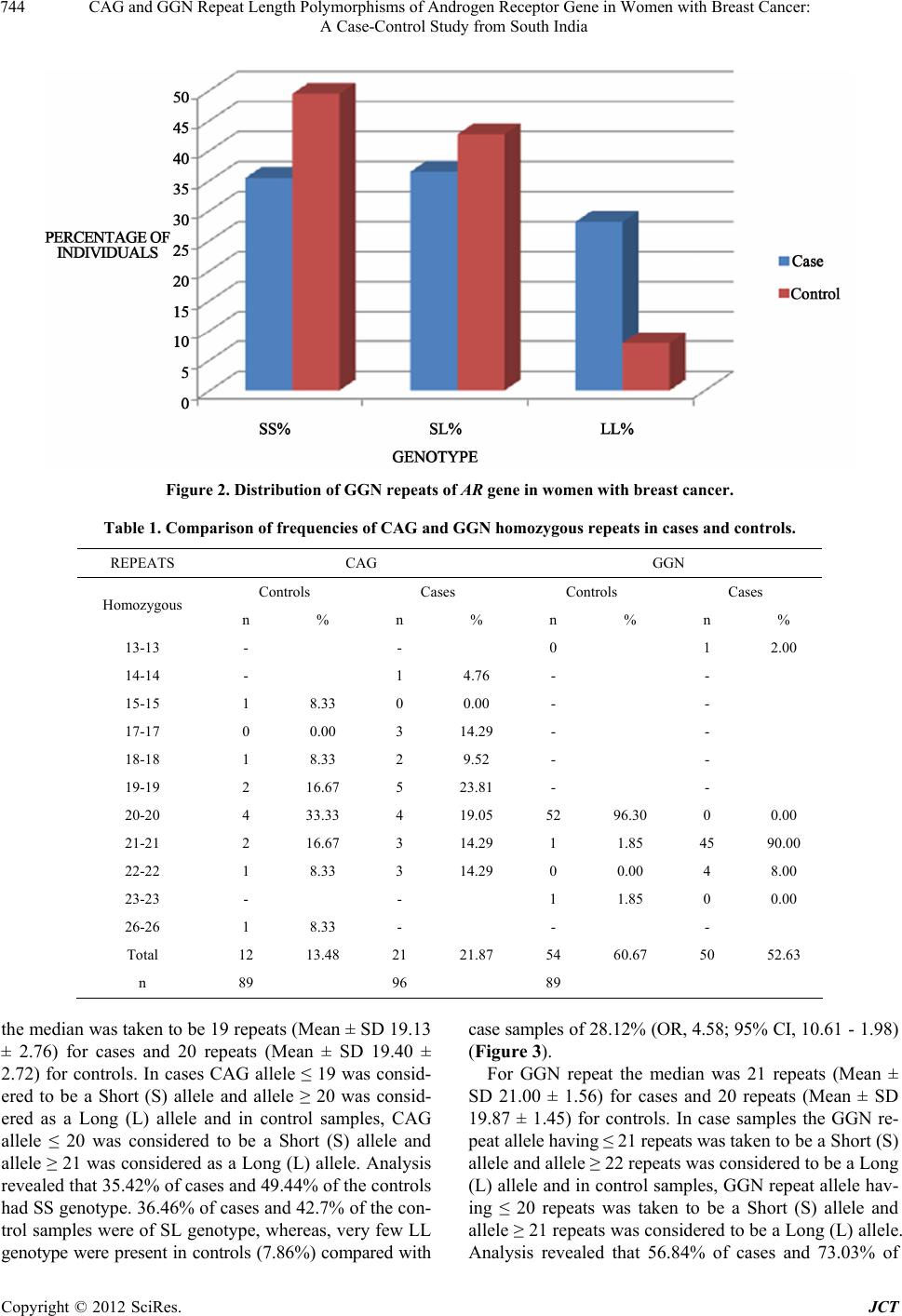 CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India 744 Figure 2. Distribution of GGN repeats of AR gene in women with br east cancer. Table 1. Comparison of frequencies of CAG and GGN homozygous repeats in cases and controls. REPEATS CAG GGN Controls Cases Controls Cases Homozygous n % n % n % n % 13-13 - - 0 1 2.00 14-14 - 1 4.76 - - 15-15 1 8.33 0 0.00 - - 17-17 0 0.00 3 14.29 - - 18-18 1 8.33 2 9.52 - - 19-19 2 16.67 5 23.81 - - 20-20 4 33.33 4 19.05 52 96.30 0 0.00 21-21 2 16.67 3 14.29 1 1.85 45 90.00 22-22 1 8.33 3 14.29 0 0.00 4 8.00 23-23 - - 1 1.85 0 0.00 26-26 1 8.33 - - - Total 12 13.48 21 21.87 54 60.67 50 52.63 n 89 96 89 the median was taken to be 19 repeats (Mean ± SD 19.13 ± 2.76) for cases and 20 repeats (Mean ± SD 19.40 ± 2.72) for controls. In cases CAG allele ≤ 19 was consid- ered to be a Short (S) allele and allele ≥ 20 was consid- ered as a Long (L) allele and in control samples, CAG allele ≤ 20 was considered to be a Short (S) allele and allele ≥ 21 was considered as a Long (L) allele. Analysis revealed that 35.42% of cases and 49.44% of the controls had SS genotype. 36.46% of cases and 42.7% of the con- trol samples were of SL genotype, whereas, very few LL genotype were present in controls (7.86%) compared with case samples of 28.12% (OR, 4.58; 95% CI, 10.61 - 1.98) (Figure 3). For GGN repeat the median was 21 repeats (Mean ± SD 21.00 ± 1.56) for cases and 20 repeats (Mean ± SD 19.87 ± 1.45) for controls. In case samples the GGN re- peat allele having ≤ 21 repeats was taken to be a Short (S) allele and allele ≥ 22 repeats was considered to be a Long (L) allele and in control samples, GGN repeat allele hav- ing ≤ 20 repeats was taken to be a Short (S) allele and allele ≥ 21 repeats was considered to be a Long (L) allele. Analysis revealed that 56.84% of cases and 73.03% of Copyright © 2012 SciRes. JCT  CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India 745 the controls had a SS genotype. 38.95% of cases and 24.72% of the control samples were of SL genotype, while LL genotype showed a very scarce distribution (Figure 4). 4. Discussion We have investigated a potential link between the CAG and GGN short tandem repeats of the AR gene in breast cancer in the South Indian women. The allelic frequen- cies of CAG in our control samples resemble those re- ported for Caucasian and Japanese populations [5,25,26]. In case of GGN repeat, alleles with 20, 21 and 22 repeats were found most frequently in our study. In CAG repeat, alleles with 20 repeats were observed in 13.02% of patients and 25.28% of controls (OR, 0.44; 95% CI, 0.26 - 0.75) which shows a significant correla- tion between case and control samples (p-value, 0.002). Taking median as the cut-off we have categorised CAG allele into 2 types: S (short) and L (long), and hence the possible genotypes are—SS (Short-Short), SL (Short-Long) and LL (Long-Long). SS and SL genotypes do not show any significant correlation with respect to their p value, whereas, the p value of LL genotype (0.0004) was highly significant (OR, 4.58; 95% CI, 10.61 - 1.98). This clearly indicates that long CAG alleles are highly associated with BC. Figure 3. Frequency (%) of CAG repeats in case-control and their subgroups having the SS (short/short), SL (short/long) or LL (long/long) alleles. Figure 4. Frequency (%) of GGN repeats in case-control and their subgroups having the SS (short/short), SL (short/long) or LL (long/long) alleles. Copyright © 2012 SciRes. JCT  CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India 746 In our study, controls showed a very high frequency of GGN alleles having 20 repeats (96.3%), whereas, in case samples, 21 (90%) and 22 (8%) repeats had very high frequencies. Having categorised GGN genotypes into three, i.e. SS, SL and LL, it was observed that SS geno- type was observed in 56.84% of cases and in 73.03% of controls (OR, 0.48; 95% CI, 0.26 - 0.89; p value, 0.02). Our result shows that 20 homozygous repeats are protec- tive whereas 21 homozygous repeat is affective in caus- ing breast cancer. It is difficult to say if this one or two repeat shift can be a plausible risk factor or not. It re- quires a large population study to validate. But in our study none of the cases showed 20 repeats which clearly indicate that alleles having homozygous repeat 20 may be protective towards breast cancer in south Indian women. Several case-control studies have investigated the rela- tionship between CAG repeat length in the AR and breast cancer risk but till date there have been only a few stud- ies which have taken both CAG and GGN repeat length into consideration. A population based study of young women from USA found larger CAG repeat (≥43) to increase the risk of breast cancer, whereas women with long GGN alleles and those with a ≥33 cumulative repeat size had a decreased risk of breast cancer [27]. A recent case-control study from Spain population showed CAG repeat ≥ 22 and GGN ≥ 24 to be associated with in- creased risk of breast cancer [5]. Our result in CAG repeat showed that patient having 20 repeat number and LL genotypes may have a risk of getting breast cancer. And GGN repeat ≥ 21 showed a high risk of getting breast cancer. Our study is in accord with the previous two studies with respect to the CAG repeat, whereas, our result found no evidence to support the above mentioned study from USA in the case of GGN repeats which had described long GGN repeats to be protective [27]. The magnitude of the increased risk we observed with the (CAG)n genotype was broadly similar to the findings of the population based study of breast cancer in USA and Spain, whereas it showed contradictory results with the data published by YouJin Hao et al. where they ana- lyzed seven detailed genotyping case-control studies and found that a long CAG sequence has a protective effect on breast cancer [28]. Our results suggest that breast cancer risk might be likely related to androgenic activity, and longer CAG and GGN repeat may be associated with disease whereas, the shorter GGN repeat length genotype of AR are protective. We observed a stronger and more consistent association between GGN repeat polymorphisms of the AR gene and breast cancer. Androgens are known to increase the risk of breast cancer by either acting as estrogen precursors or by direct action on breast cancer cells by binding to AR. Andro- gens have also been reported to act as antiproliferative agents in the presence of estrogens in some breast cancer cell lines. Both these effects of androgens are expressed through its binding to the AR [6]. Hence, the effects of androgens are regulated by the androgen receptor. There- fore, any polymorphism, like that of the CAG and GGN tracts, might result in a variation in the AR activity. Presence of long (L) CAG allele reduces the transcrip- tional activity of the AR. Tertiary structure of AR poly- glutamine repeats has not been characterized [6]. Repeat length probably modifies the conformation of the protein affecting either the homodimer structure, which is re- quired for the active form of AR, or the recognition and binding capability of AR homodimer to the Hormone responsive elements (HREs). Hence affecting the tran- scription of the genes that are androgen dependent and therefore increasing the BC risk. Similarly, the amount of the AR protein is important. Because, the transcription machinery requires the con- tinuous presence of androgen and androgen receptor and therefore the homodimers they form for the production of co-activators of transcription. Every time the gene is ex- pressed, the transactivators (co-activators) are degraded by a ubiquitin pathway [29], that is, transcription is a cyclic process. Presence of long (L) GGN allele reduces the AR protein yield. And, if the AR protein yield is low, transcription machinery is again affected, increasing the risk of breast cancer. Though it has to be ascertained whether the effects of CAG and GGN repeats on androgen action are inde- pendent or not, certainly the risk of BC increases if both together show any variation in repeat length since the presence of both long CAG and long GGN will result in both low levels of AR and low transcriptional activity, multiplying the risk of BC. Also, the SS genotype has been observed in aggressive forms of cancer [30]. This further goes to show that the androgens and hence the AR play a dual role—of pro- tecting against cancer and also being one of the causes of cancer. Hence, there seems to be a balance between the dual roles of the androgen and its receptor, which in a way seems to be related to the repeat lengths of CAG and GGN tracts. 5. Conclusion This is the first study addressing the association of CAG and GGN repeat length polymorphism in AR gene in breast cancer in South Indian population. This finding may help explain the higher rate of breast cancer among South Indians, as well as their tendency to be diagnosed with more extensive disease risk. Our results showed a Copyright © 2012 SciRes. JCT  CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India 747 strong correlation between CAG and GGN repeat poly- morphism and breast cancer. It requires a further study with a large population and different ethnic groups for the early prognosis of high risk patients of various ethnic populations and their respective management. 6. Acknowledgements We are thankful to SVM, USA, for critically going through the manuscript. The financial assistance from Council of Scientific and Industrial Research, New Delhi, Government of India, is greatly acknowledged. REFERENCES [1] M. Ganapati, “India Has Some of the Highest Cancer Rates in the World,” British Medical Journal, Vol. 330, No. 7485, 2005, p. 215. doi:10.1136/bmj.330.7485.215-c [2] E. Deutsch, L. Maggiorella, P. Eschwege, J. Bourhis, J. C. Soria and B. Abdulkarim, “Environmental, Genetic and Molecular Features of Prostate Cancer,” The Lancet On- cology, Vol. 5, No. 5, 2004, pp. 303-313. doi:10.1016/S1470-2045(04)01468-8 [3] J. F. Dorgan, C. Longcope, H. E. Stephenson Jr., R. T. Falk, R. Miller, C. Franz, L. Kahle, W. S. Campbell, J. A. Tangrea and A. Schatzkin, “Serum Sex Hormone Levels Are Related to Breast Cancer Risk in Postmenopausal Women,” Environmental Health Perspectives, Vol. 105, Suppl. 3, 1997, pp. 583-585. [4] T. S. Gao, M. Marcelli and M. J. McPhaul, “Transcrip- tional Activation and Transient Expression of the Human Androgen Receptor,” The Journal of Steroid Biochemis- try and Molecular Biology, Vol. 59, No. 1, 1996, pp. 9-20. doi:10.1016/S0960-0760(96)00097-0 [5] A. González, D. F. Javier, G. Rodriguez, B. Brito, M. A. Rodríguez, A. Cabrera, J. C. Díaz-hico, R. Reyes, A. Aguirre-Jaime and B. N. Díaz-Chico, “Increased Risk of Breast Cancer in Women Bearing a Combination of Large CAG and GGN Repeats in the Exon 1 of the Androgen Receptor Gene,” European Journal of Cancer, Vol. 43, No. 16, 2007, pp. 2373-2380. doi:10.1016/j.ejca.2007.07.001 [6] D.-C. B. Nicolás, R. F. Germán, A. González, R. Ramírez, C. Bilbao, A. C. de León, J. A. Aguirre, R. Chirino, D. Navarro and J. C. Díaz-Chico, “Androgens and Androgen Receptors in Breast Cancer,” Jou rna l of Ste roid Biochem- istry & Molecular Biology, Vol. 105, No. 1-5, 2007, pp. 1-15. [7] S. N. Birrell, R. E. Hall and W. D. Tilley, “Role of the Androgen Receptor in Human Breast Cancer,” Journal of Mammary Gland Biology and Neoplasia, Vol. 3, No. 1, 1998, pp. 95-103. doi:10.1023/A:1018730519839 [8] M. Brys, “Androgens and Androgen Receptor: Do They Play a Role in Breast Cancer?” Medical Science Monitor, Vol. 6, No. 2, 2000, pp. 433-438. [9] D. J. Liao and R. B. Dickson, “Roles of Androgens in the Development, Growth, and Carcinogenesis of the Mam- mary Gland,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 80, No. 2, 2002, pp. 175-189. doi:10.1016/S0960-0760(01)00185-6 [10] M. Langer, E. Kubista, M. Schemper and J. Spona, “An- drogen Receptors, Serum Androgen Levels and Survival of Breast Cancer Patients,” Archives of Gynecology and Obstetrics, Vol. 247, No. 4, 1990, pp. 203-209. doi:10.1007/BF02389545 [11] J. B. Adams, “Adrenal Androgens and Human Breast Cancer: A New Appraisal,” Treat Breast Cancer Re- search and Treatment, Vol. 51, No. 2, 1998, pp. 183-188. doi:10.1023/A:1006050720900 [12] L. Bernstein and R. K. Ross, “Endogenous Hormones and Breast Cancer Risk,” Epidemiologic Reviews, Vol. 15, No. 1, 1993, pp. 48-65. [13] H. C. Shen and G. A. Coetzee, “The Androgen Receptor: Unlocking the Secrets of Its Unique Transactivation Do- main,” Vitamins & Hormones, Vol. 71, 2005, pp. 301-319. doi:10.1016/S0083-6729(05)71010-4 [14] D. Navarro, O. P. Luzardo, L. Fernandez, N. Chesa and B. N. Diaz-Chico, “Transition to Androgen-Independence in Prostate Cancer,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 81, No. 3, 2002, pp. 191- 201. doi:10.1016/S0960-0760(02)00064-X [15] J. Beilin, E. M. Ball, J. M. Favaloro and J. D. Zajac, “Ef- fect of the Androgen Receptor CAG Repeat Polymor- phism on Transcriptional Activity: Specificity in Prostate and Non-Prostate Cell Lines,” Journal of Molecular En- docrinology, Vol. 25, No. 1, 2000, pp. 85-96. doi:10.1677/jme.0.0250085 [16] I. Knoke, A. Allera and P. Wieacker, “Significance of the CAG Repeat Length in the Androgen Receptor Gene (AR) for the Transactivation Function of an M780I Mutant AR,” Human Genetics, Vol. 104, No. 3, 1999, pp. 257- 261. doi:10.1007/s004390050945 [17] S. P. Huang, Y. H. Chou, W. S. Chang, et al., “Androgen Receptor Gene Polymorphism and Prostate Cancer in Tai- wan,” Journal of the Formosan Medical Association, Vol. 102, No. 10, 2003, pp. 680-686. [18] E. Esteban, N. Rodon, M. Via, E. Gonzalez-Perez, J. Santamaria, J. M. Dugoujon, F. E. Chennawi, M. Mel- haoui, M. Cherkaoui, G. Vona, N. Harich and P. Moral, “Androgen Receptor CAG and GGC Polymorphisms in Mediterraneans: Repeat Dynamics and Population Rela- tionships,” Journal of Human Genetics, Vol. 51, No. 2, 2006, pp. 129-136. doi:10.1007/s10038-005-0336-7 [19] S. M. Gilbert, M. C. Benson and J. M. McKiernan, “Link- age Disequilibrium between the Androgen Receptor Gene CAG and GGC Repeats in the African-American Popula- tion,” Current Urology Reports, Vol. 3, No. 3, 2002, 189-193. doi:10.1007/s11934-002-0063-y [20] H. F. Sleddens, B. A. Oostra, A. O. Brinkmann and J. Trapman, “Trinucleotide (GGN) Repeat Polymorphism in the Human Androgen Receptor (AR) Gene,” Human Mo- lecular Genetics, Vol. 2, No. 4, 1993, p. 493. doi:10.1093/hmg/2.4.493 Copyright © 2012 SciRes. JCT  CAG and GGN Repeat Length Polymorphisms of Androgen Receptor Gene in Women with Breast Cancer: A Case-Control Study from South India Copyright © 2012 SciRes. JCT 748 [21] G. Rodriguez, C. Bilbao, R. Ramirez, O. Falcon, L. Leon, R. Chirino, O. Falcon Jr., B. P. Diaz, J. F. Rivero, M. Pe- rucho, B. N. Diaz-Chico and J. C. Diaz-Chico, “Alleles with Short CAG and GGN Repeats in the Androgen Re- ceptor Gene Are Associated with Benign Endometrial Cancer,” International Journal of Cancer, Vol. 118, No. 6, 2006, pp. 1420-1425. doi:10.1002/ijc.21516 [22] M. P. Zeegers, L. A. Kiemeney, A. M. Nieder and H. R. Ostrer, “How Strong Is the Association between CAG and GGN Repeat Length Polymorphisms in the Androgen Receptor Gene and Prostate Cancer Risk?” Cancer Epi- demiology, Biomarkers and Prevention, Vol. 13, No. 11, 2004, pp. 1765-1771. [23] A. W. Hsing, I. Y.-T. Gao, G. Wu, X. Wang, J. Deng, Y.-L. Chen, I. A. Sesterhenn, F. K. Mostofi, J. Benichou and C. Chang, “Polymorphic CAG and GGN Repeat Lengths in the Androgen Receptor Gene and Prostate Cancer Risk: A Population-Based Case-Control Study in China,” Cance r Research, Vol. 60, No. 18, 2000, pp. 5111- 5116. [24] A. Khattri, R. K. Pandey, N. J. Gupta, B. Chakravarty, M. Deendayal, L. Singh and K. Thangaraj, “CA Repeat and RsaI Polymorphisms in ERb Gene Are Not Associated with Infertility in Indian Men,” International Journal of Andrology, Vol. 32, No. 1, 2007, pp. 1365-2605. [25] R. A. Kittles, D. Young, S. Weinrich, et al., “Extent of Linkage Disequilibrium between the Androgen Receptor Gene CAG and GGC Repeats in Human Populations: Im- plications for Prostate Cancer Risk,” Human Genetics, Vol. 109, No. 3, 2001, pp. 253-261. doi:10.1007/s004390100576 [26] M. Sasaki, M. Kaneuchi, N. Sakuragi, S. Fujimoto, P. R. Carroll and R. Dahiya, “The Polyglycine and Polygluta- mine Repeats in the Androgen Receptor Gene in Japanese and Caucasian Populations,” Biochemical and Biophysi- cal Research Communications, Vol. 312, No. 4, 2003, pp. 1244-1247. doi:10.1016/j.bbrc.2003.11.075 [27] N. M. Suter, K. E. Malone, J. R. Darling, D. R. Doody and E. A. Ostrander, “Androgen Receptor (CAG)n and (GGC)n Polymorphisms and BC Risk in a Popula- tion-Based Case-Control Study of Young Women,” Can- cer Epidemiology, Biomarkers & Prevention, Vol. 12, No. 2, 2003, pp. 127-135. [28] Y. J. Hao, R. Montiel, B. H. Li, E. Y. Huang, L. Zeng and Y. S. Huang, “Association between Androgen Receptor Gene CAG Repeat Polymorphism and Breast Cancer Risk: A Meta-Analysis,” Breast Cancer Research and Treat- ment, Vol. 124, No. 3, 2010, pp. 815-820. [29] Z. Nawaz and B. W. O’Malley, “Urban Renewal in the Nucleus: Is Protein Turnover by Proteasomes Absolutely Required for Nuclear Receptor Regulated Transcription?” Molecular Endocrinology, Vol. 18, No. 3, 2004, pp. 493- 499. doi:10.1210/me.2003-0388 [30] H. Yu, B. Bharaj, E. J. Vassilikos, M. Giai and E. P. Dia- mandis, “Shorter CAG Repeat Length in the Androgen Receptor Gene Is Associated with More Aggressive Forms of Breast Cancer,” Breast Cancer Research and Treatment, Vol. 59, No. 2, 2000, pp. 153-161. doi:10.1023/A:1006356502820
|