 Journal of Cancer Therapy, 2012, 3, 718-730 http://dx.doi.org/10.4236/jct.2012.325091 Published Online October 2012 (http://www.SciRP.org/journal/jct) The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate Linda C. Enns, Warren C. Ladiges Department of Comparative Medicine, University of Washington, Seattle, USA. Email: wladiges@u.washington.edu Received August 30th, 2012; revised September 29th, 2012; accepted October 13th, 2012 ABSTRACT Obesity is associated with an increased risk of mortality from certain types of cancer, including cancer of the breast. Because obesity is associated with multiple risk factors, however, the exact reasons remain unclear. The objective of this study was to determine which of the risk factors associated with obesity are related to enhanced tumor development. The MMTV-PyMT mouse model develops mammary tumors which share numerous characteristics with those of hu- mans. We challenged these mice with a high fat/high carbohydrate, high caloric (HC) diet, and looked for relationships between enhanced primary tumor development and adiposity, various aspects of glucose homeostasis, and metabolic factors. The HC diet enhanced tumor progression in PyMT mice. While mice on the HC diet also developed increased adiposity, hyperglycemia and hyperinsulinemia, none of these risk factors was found to be associated with the observed increases in tumor growth. Rather, we found that while overall, tumor growth was enhanced in HC diet-fed mice com- pared to those maintained on a regular diet, it was attenuated in individuals by an HC diet-induced increase in metabolic rate and decrease in respiratory exchange ratio. Tumor size in HC diet-fed mice was directly related to p38 phosphory- lation and Bcl-2 inhibition, and the degree of vascularization of these tumors was closely and indirectly related to the rate of mouse oxygen consumption. The data suggest that an increase in metabolic rate and oxygen consumption, in- duced by the introduction of a high caloric diet, has a protective effect against tumor growth by increasing the activity levels of the tumor suppressor p38 and decreasing the activity of the antiapoptotic protein Bcl-2, as well as by reducing hypoxia-induced tumor vascularization. Keywords: Obesity; Breast Cancer; Metabolism; Oxygen; ROS; p38; Hypoxia 1. Introduction With numerous studies documenting an increase in obesity worldwide [1], obesity and its associated diseases are rapidly becoming a central health problem of this century. Abdominal obesity has been found to be associated with an enhanced risk of cancer mortality in humans [2,3]; in fact, a 2007 report by the World Cancer Research Fund and the American Institute of Cancer Research has suggested that the maintenance of a healthy body weight throughout life is one of the most important ways to protect against cancer [4]. In addition to being at increased risk of developing certain cancers compared to people who are lean, obese individuals have a higher likelihood of dying from those cancers once they occur [5]. One cancer in particular that shows a high cor- relation between obesity and mortality is cancer of the breast, with an estimated 30% to 50% of deaths caused by breast cancer thought to be associated with obesity [6]. While the epidemiology supporting the link between obesity and cancer-caused mortality is compelling, the mechanisms behind this association are not clear, in part because obesity is associated with multiple physio- logical risk factors. This study was designed to de- termine which of these different risk factors could be associated with tumor growth in a high fat/high carbo- hydrate, high caloric (HC) diet-challenged mammary cancer mouse model, in an effort to assess the im- portance of these individual factors in their contribution to enhanced cancer progression. Transgenic mice that carry the middle T oncogene under the transcriptional control of the mouse mammary tumor virus promoter/ enhancer (MMTV-PyMT) develop widespread transfor- mation of the mammary epithelium and the production of multi- focal mammary adenocarcinomas, with secondary metastasis to the lungs [7]. We developed a population of transgenic (MMTV-PyMT)634Mul mice on a C57BL6/J background, a strain known to be obesity and diabetes Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 719 sensitive when challenged with a high caloric diet [8]. Because we were interested in the role that obesity plays in tumor progression, rather than its contribution to in- creased cancer risk, we waited until tumors were pal- pable before switching mice to an HC diet. In addition to tumor burden, various physiological parameters of these mice were monitored over the course of their tumor progression, including adiposity, blood pressure, blood glucose and serum insulin, metabolic rate (rate of oxygen consumption) and respiratory exchange ratio. While the rate of tumor growth was increased in mice challenged with the HC diet, specific relationships between various physiological risk factors and increased tumor growth were not found; rather, a surprising picture emerged showing some protective effects of the diet, related to a nutrient overload-induced metabolic enhancement. 2. Methods 2.1. Mice FVB/N-Tg (MMTV-PyMT)634Mul/J transgenic males on a 100% FVB background [9] were obtained from Jackson Labs and crossed with transgenic females on a congenic C57/BL6(J) background for 9 generations. Experimental cohorts were then generated by crossing male PyMT heterozygotes of these mice with congenic C57/BL6(J) females. Experimental cohorts consisted of female WT and littermates heterozygous for the PyMT transgene. Mice were maintained in a 25˚C-specific pathogen-free barrier facility with 12-hour alternating light and dark cycles and were given free access to food and water. All procedures used in this study were approved by the Animal Care and Use Committee of the University of Washington. 2.2. Specialized Diet Cohorts The two diets used in our studies were standard rodent chow (5053; Picolab, Richmond, IN) containing 20% (wt/wt) protein, 4.5% fat (ether extract), and 55% carbo- hydrate (primarily starch), and a high-fat, high-sucrose, high caloric (HC) diet (S3282; Bio-Serv, Frenchtown, NJ) containing 20% protein, 36% fat (primarily lard), and 36% carbohydrate (primarily sucrose). Because we wanted to look at the effects of a high calorie diet on tumor growth, as opposed to cancer risk, we chose to eliminate the variable of tumor incidence by waiting until tumors were present before administering the dietary challenge. All mice were maintained on standard rodent chow until palpable tumors were just detectable (61 days of age), at which time they were either switched to the high-caloric diet or continued on the standard chow for either 79 or 109 days (140 and 170 days of age, respec- tively). Cohorts were designated as either “140 day” or “170 day”. At each of these time points, mice were euthanized and tumors were excised, measured and weighed. For each genotype, diet and time point, there were 15 - 20 mice per cohort, housed 5 to a cage with genotypes randomized. 2.3. Body Composition, Blood Glucose and Serum Insulin, and Blood Pressure Body composition, blood glucose, serum insulin and blood pressure are all measurable non-invasively and both 140 day and 170 day cohorts of mice were moni- tored for all of these parameters over the course of the study. Body composition measurements were measured using quantitative magnetic resonance imaging (QMR), with an EchoMRI-100 analyzer (EchoMRI, Houston, TX). Body composition measurements were performed on live, unsedated mice. For blood glucose measure- ments, food was removed from mice 6 hours before blood was drawn by tail pricking. Analyses were per- formed using a glucometer and Comfort Curve Test Strips (Advantage; Accu-Chek, Roche, Basel, Switzer- land). For serum insulin measurements, blood was col- lected from the retro-orbital sinus into serum separator tubes (365,956; Becton Dickinson); after separation, plasma was either used immediately or stored at –80˚C until analysis. Plasma insulin was measured using an ELISA kit (EZRMI-13K; LINCO, St Charles, MO) as per the manufacturer’s instructions. Systolic and diastolic blood pressures were measured on unsedated, restrained individuals using a volume pressure recording sensor and an occlusion tail-cuff (CODA non-invasive blood pres- sure system; Kent Scientific, Torrington, CT). Mice were kept warmed to a temperature of 30˚C on a warming platform (Kent Scientific), and 60 blood pressure meas- urements were performed over 20 minutes to ensure ac- climation, as determined by consistency of measure- ments. 2.4. Metastasis/Pathology At the time of necropsy, lungs were removed from the mice and each lobe was perfused directly with 10% neu- tral buffered formalin. Pulmonary metastatic foci and tumor burden were quantified from paraffin-embedded, H & E stained lung tissue sections that went through at least 4 of the 5 lobes. Slides were scanned to virtual digital files using Nanozoomer (Hamamatsu, Hamamatsu City, Japan). Images were magnified to 2.5×, captured and used for quantification. The number of metastatic foci was counted in the entire section and standardized to lung surface area. The surface area of all tumors ob- served in the section was combined and divided by the number of foci for average tumor surface area. Metastatic Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 720 incidence was defined as the percentage of mice present- ing any pulmonary metastatic foci, determined from the histological sections. Histopathologic reviews were conducted of hematoxy- lin and eosin-(H & E) stained primary tumors for a pre- liminary examination of qualitative differences between experimental groups [10]. H & E-stained sections of pri- mary tumors were evaluated for mitosis, necrosis, and inflammation at various (20 - 400×) magnifications. Nor- mal and aberrant mitotic figures, characterized by trira- dial or circular metaphase plates, were counted in three random fields at 400× magnification. Necrosis was esti- mated as a percent of necrotic area within the tumor sec- tion, that is, areas that were hypocellular and hypo- or hypereosinophilic with basophilic cellular and nuclear debris. Inflammation was graded as 0 (minimal), 1 (mild), 2 (moderate), or 3 (marked) at a 40× or 100× magnifica- tion. Vascularity was scored from 0 - 4; 0 = unremark- able; 1 = few small vessels at periphery; 2 = clusters of peripheral vessels; 3 = small vessels within tumor bulk; 4 = prominent (readily identifiable at 4× mag.) clusters within the bulk of the tumor. 2.5. Metabolic Measurements Indirect calorimetry is a non-invasive process and the metabolism of both the 140 and 170 day cohorts of mice was monitored over the course of the study. Metabolic parameters were measured using an open-circuit indirect calorimeter (Oxymax; Columbus Instruments, Columbus, OH). Measurements were taken of the rates of both oxy- gen consumption (VO2) and carbon dioxide production (VCO2), and the respiratory exchange ratio (RER), de- fined as the ratio of the amount of carbon dioxide pro- duced to the amount of oxygen consumed. Mice were measured individually and for a period of 48 hours (2 light and 2 dark cycles). Just prior to taking metabolic measurements, the lean tissue mass of each mouse was assessed using quantitative magnetic resonance imaging. VO2 for each mouse were standardized to its lean mass. 2.6. Immunoblotting Primary tumors were harvested from mice at 140 days of age. Tissue was ground in liquid nitrogen followed by homogenization in 1 mL of a cocktail of 20 mL ice-cold RIPA, 2 protease inhibitor mini-tabs (11836153001; Roche, Indianapolis, IN) and 400 ul each of the phosphatase inhibitor cocktails P2850 and P5726 (Sigma-Aldrich, St Louis, MO). Twenty micrograms of protein from the cytosolic fraction was loaded to each lane of a NuPAGE Novex 4% - 12% Bis-Tris Gel (NP0322BOX; Invitrogen, Grand Island, NY). Blots were probed with primary an- tibodies to AMPK-P (Abcam; ab72845; diluted 1/1000), GLUT 1 (Santa Cruz Biotechnology;sc-7903; diluted 1/200), HIF1α (Novus Biologicals; NB100-134; diluted 1/1000), VEGF (Abcam; ab46154; 0.5μl/mL), P-Bcl-2 (ser87) (Assay Biotechnology; #A0460;1/500), P-p38 (Thr180/Tyr182) (Cell Signaling; cs#9211; diluted 1/ 500), p38-total (Cell Signaling; cs#9212; 1/500) and β- actin (Abcam; ab8227; 1/1000). A goat anti-rabbit, hor- seradish peroxidase-conjugated secondary antibody (Ab- cam; ab6721) was used at a dilution of 1/3000, and de- tection was accomplished using ECL (95038-560; VWR Scientific, Brisbane, CA). 2.7. Statistics Error bars, where present, indicate ± standard deviation. The probability of differences between means was de- termined using the Student’s t test. Probabilities of indi- vidual data points being different are indicated where the P < 0.1. To determine the strength of relationships be- tween different factors, the coefficient of determination (R2) was calculated. 3. Results 3.1. PyMT Mice on a High Caloric Diet Accumulate Body Fat, and Become Hyperglycemic and Hyperinsulinemic Mice were put on an HC diet at the time when palpable tumors were detected (61 days). At 79 and 109 days after the introduction of the diet (when mice were 140 and 170 days old, respectively), body fat, blood glucose, serum insulin and systolic and diastolic blood pressure were measured. The HC diet caused both PyMT mice and WT controls to accumulate fat over the course of the dietary challenge, although the rate of fat accumulation was lower in the presence of tumors (Figure 1(a)). Before the introduction of the HC diet, both genotypes had an aver- age body fat weight of between 2 g and 3 g. After 79 and 109 days on the diet, WT mice showed 4 and 6-fold in- creases in fat mass, respectively. While PyMT mice also accumulated body fat, they lagged behind the WT mice, showing 3 and 4-fold increases in fat mass respectively at the two time points. Both PyMT and WT control mice experienced similar increases of about 50% in blood glucose by 79 days after the introduction of the HC diet (Figure 1(b)). PyMT mice displayed a steady increase in serum insulin over the course of the dietary challenge (Figure 1(c)). The HC diet had no effect on blood pres- sures (data not shown). 3.2. PyMT Mice on an HC Diet Show Enhanced Primary Tumor Growth PyMT mice maintained on an HC diet showed enhanced Copyright © 2012 SciRes. JCT 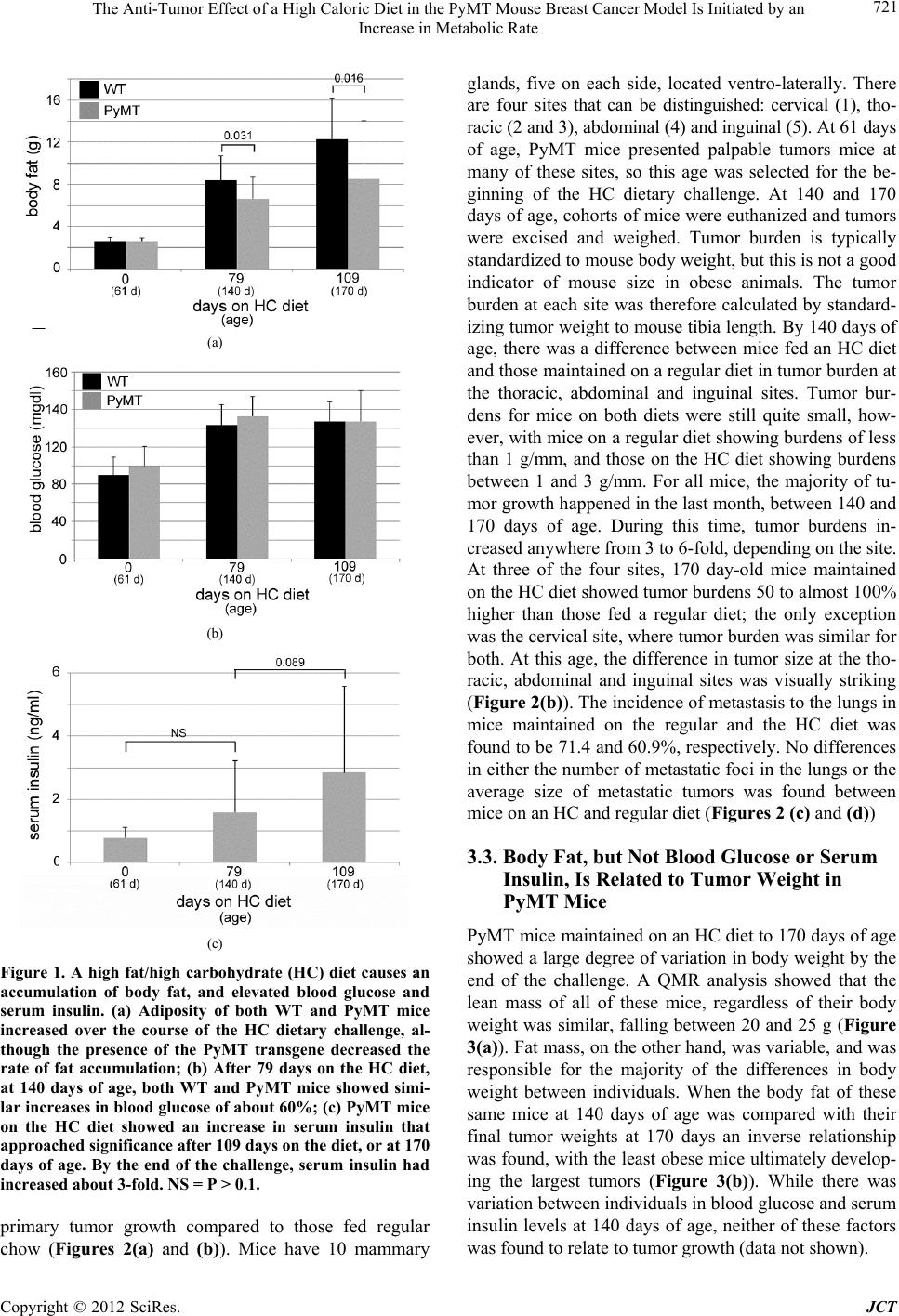 The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 721 (a) (b) (c) Figure 1. A high fat/high carbohydrate (HC) diet causes an accumulation of body fat, and elevated blood glucose and serum insulin. (a) Adiposity of both WT and PyMT mice increased over the course of the HC dietary challenge, al- though the presence of the PyMT transgene decreased the rate of fat accumulation; (b) After 79 days on the HC diet, at 140 days of age, both WT and PyMT mice showed simi- lar increases in blood glucose of about 60%; (c) PyMT mice on the HC diet showed an increase in serum insulin that approached significance after 109 days on the diet, or at 170 days of age. By the end of the challenge, serum insulin had increased about 3-fold. NS = P > 0.1. primary tumor growth compared to those fed regular chow (Figures 2(a) and (b)). Mice have 10 mammary glands, five on each side, located ventro-laterally. There are four sites that can be distinguished: cervical (1), tho- racic (2 and 3), abdominal (4) and inguinal (5). At 61 days of age, PyMT mice presented palpable tumors mice at many of these sites, so this age was selected for the be- ginning of the HC dietary challenge. At 140 and 170 days of age, cohorts of mice were euthanized and tumors were excised and weighed. Tumor burden is typically standardized to mouse body weight, but this is not a good indicator of mouse size in obese animals. The tumor burden at each site was therefore calculated by standard- izing tumor weight to mouse tibia length. By 140 days of age, there was a difference between mice fed an HC diet and those maintained on a regular diet in tumor burden at the thoracic, abdominal and inguinal sites. Tumor bur- dens for mice on both diets were still quite small, how- ever, with mice on a regular diet showing burdens of less than 1 g/mm, and those on the HC diet showing burdens between 1 and 3 g/mm. For all mice, the majority of tu- mor growth happened in the last month, between 140 and 170 days of age. During this time, tumor burdens in- creased anywhere from 3 to 6-fold, depending on the site. At three of the four sites, 170 day-old mice maintained on the HC diet showed tumor burdens 50 to almost 100% higher than those fed a regular diet; the only exception was the cervical site, where tumor burden was similar for both. At this age, the difference in tumor size at the tho- racic, abdominal and inguinal sites was visually striking (Figure 2(b)). The incidence of metastasis to the lungs in mice maintained on the regular and the HC diet was found to be 71.4 and 60.9%, respectively. No differences in either the number of metastatic foci in the lungs or the average size of metastatic tumors was found between mice on an HC and regular diet (Fi gur es 2 (c) and (d)) 3.3. Body Fat, but Not Blood Glucose or Serum Insulin, Is Related to Tumor Weight in PyMT Mice PyMT mice maintained on an HC diet to 170 days of age showed a large degree of variation in body weight by the end of the challenge. A QMR analysis showed that the lean mass of all of these mice, regardless of their body weight was similar, falling between 20 and 25 g (Figure 3(a)). Fat mass, on the other hand, was variable, and was responsible for the majority of the differences in body weight between individuals. When the body fat of these same mice at 140 days of age was compared with their final tumor weights at 170 days an inverse relationship was found, with the least obese mice ultimately develop- ing the largest tumors (Figure 3(b)). While there was variation between individuals in blood glucose and serum insulin levels at 140 days of age, neither of these factors was found to relate to tumor growth (data not shown). Copyright © 2012 SciRes. JCT 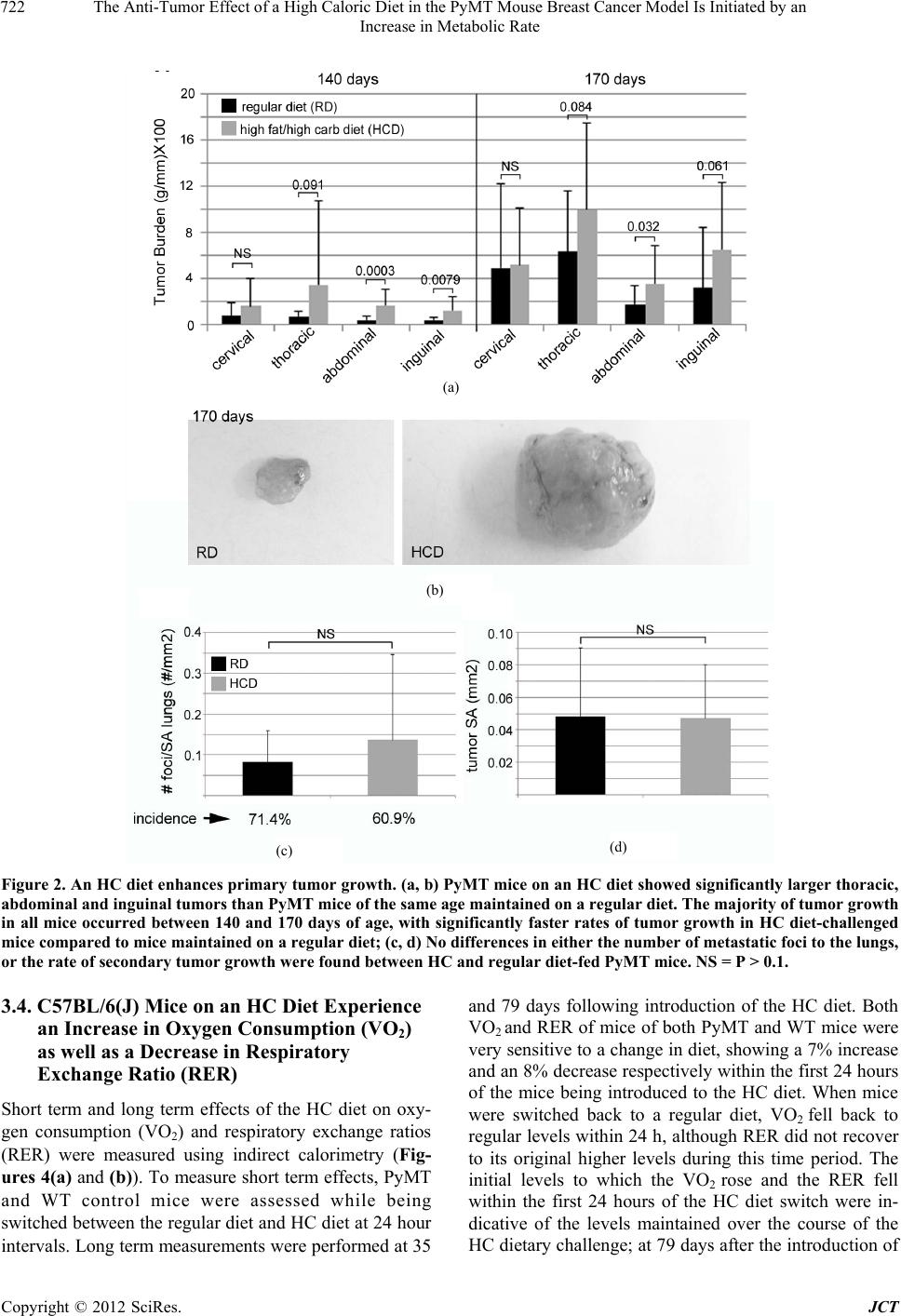 The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate Copyright © 2012 SciRes. JCT 722 (a) (d) (c) (b) Figure 2. An HC diet enhances pr imary tumor growth. (a, b) PyMT mice on an HC diet showed significantly larger thoracic, abdominal and inguinal tumors than PyMT mice of the same age maintained on a regular diet. The majority of tumor growth in all mice occurred between 140 and 170 days of age, with significantly faster rates of tumor growth in HC diet-challenged mice compared to mice maintained on a regular diet; (c, d) No differences in either the number of metastatic foci to the lungs, or the rate of secondary tumor growth were found between HC and regular diet-fed PyMT mice. NS = P > 0.1. 3.4. C57BL/6(J) Mice on an HC Diet Experience an Increase in Oxygen Consumption (VO2) as well as a Decrease in Respiratory Exchange Ratio (RER) and 79 days following introduction of the HC diet. Both VO2 and RER of mice of both PyMT and WT mice were very sensitive to a change in diet, showing a 7% increase and an 8% decrease respectively within the first 24 hours of the mice being introduced to the HC diet. When mice were switched back to a regular diet, VO2 fell back to regular levels within 24 h, although RER did not recover to its original higher levels during this time period. The initial levels to which the VO2 rose and the RER fell within the first 24 hours of the HC diet switch were in- dicative of the levels maintained over the course of the HC dietary challenge; at 79 days after the introduction of Short term and long term effects of the HC diet on oxy- gen consumption (VO2) and respiratory exchange ratios (RER) were measured using indirect calorimetry (Fig- ures 4(a) and (b)). To measure short term effects, PyMT and WT control mice were assessed while being switched between the regular diet and HC diet at 24 hour intervals. Long term measurements were performed at 35 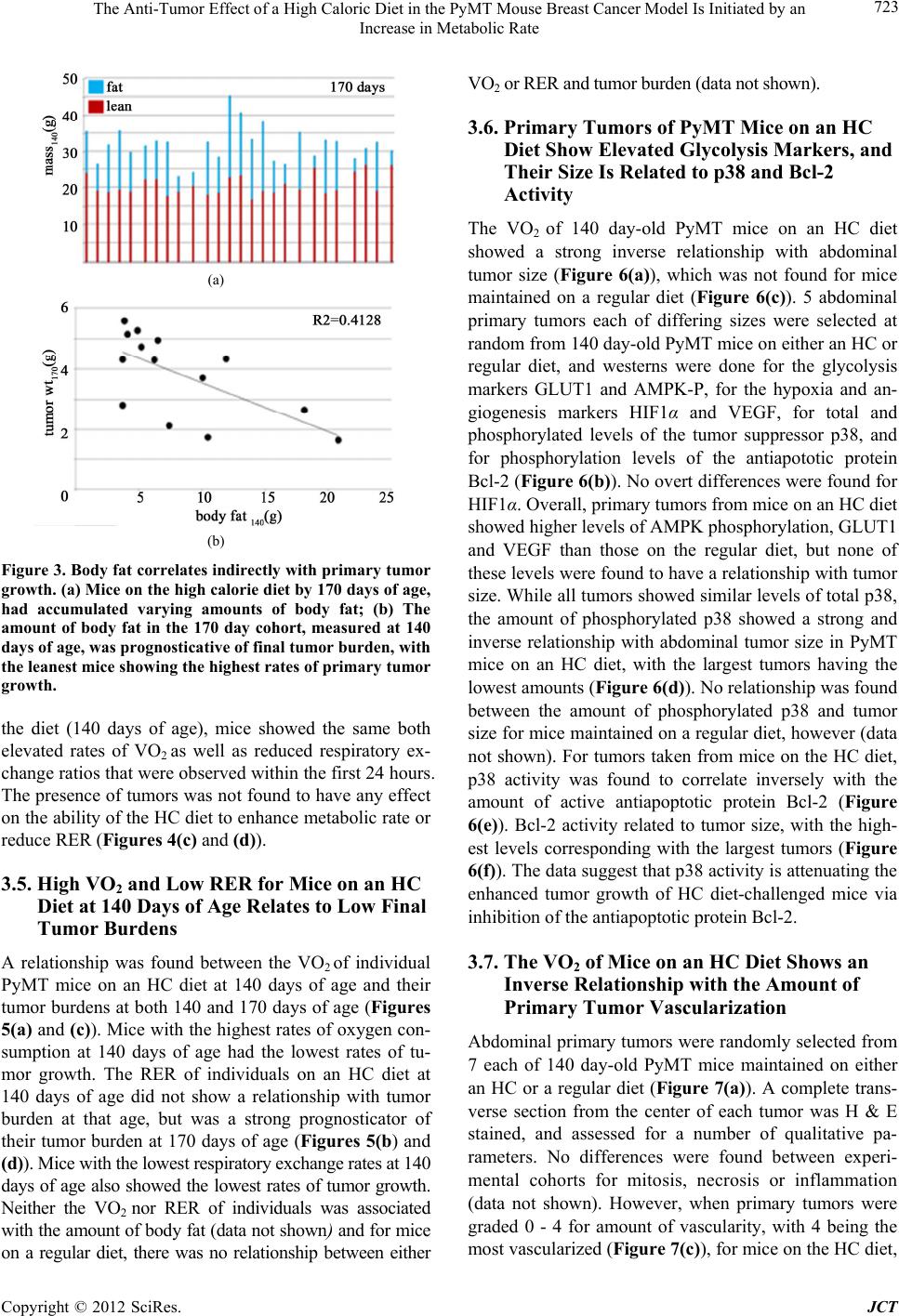 The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 723 (a) (b) Figure 3. Body fat correlates indirectly with primary tumor growth. (a) Mice on the high calorie diet by 170 days of age, had accumulated varying amounts of body fat; (b) The amount of body fat in the 170 day cohort, measured at 140 days of age, was prognosticative of final tumor burden, with the leanest mice showing the highest rates of primary tumor growth. the diet (140 days of age), mice showed the same both elevated rates of VO2 as well as reduced respiratory ex- change ratios that were observed within the first 24 hours. The presence of tumors was not found to have any effect on the ability of the HC diet to enhance metabolic rate or reduce RER (Figures 4(c) and (d)). 3.5. High VO2 and Low RER for Mice on an HC Diet at 140 Days of Age Relates to Low Final Tumor Burdens A relationship was found between the VO2 of individual PyMT mice on an HC diet at 140 days of age and their tumor burdens at both 140 and 170 days of age (Figures 5(a) and (c)). Mice with the highest rates of oxygen con- sumption at 140 days of age had the lowest rates of tu- mor growth. The RER of individuals on an HC diet at 140 days of age did not show a relationship with tumor burden at that age, but was a strong prognosticator of their tumor burden at 170 days of age (Figures 5(b) and (d)). Mice with the lowest respiratory exchange rates at 140 days of age also showed the lowest rates of tumor growth. Neither the VO2 nor RER of individuals was associated with the amount of body fat (data not shown) and for mice on a regular diet, there was no relationship between either VO2 or RER and tumor burden (data not shown). 3.6. Primary Tumors of PyMT Mice on an HC Diet Show Elevated Glycolysis Markers, and Their Size Is Related to p38 and Bcl-2 Activity The VO2 of 140 day-old PyMT mice on an HC diet showed a strong inverse relationship with abdominal tumor size (Figure 6(a)), which was not found for mice maintained on a regular diet (Figure 6(c)). 5 abdominal primary tumors each of differing sizes were selected at random from 140 day-old PyMT mice on either an HC or regular diet, and westerns were done for the glycolysis markers GLUT1 and AMPK-P, for the hypoxia and an- giogenesis markers HIF1α and VEGF, for total and phosphorylated levels of the tumor suppressor p38, and for phosphorylation levels of the antiapototic protein Bcl-2 (Figure 6(b)). No overt differences were found for HIF1α. Overall, primary tumors from mice on an HC diet showed higher levels of AMPK phosphorylation, GLUT1 and VEGF than those on the regular diet, but none of these levels were found to have a relationship with tumor size. While all tumors showed similar levels of total p38, the amount of phosphorylated p38 showed a strong and inverse relationship with abdominal tumor size in PyMT mice on an HC diet, with the largest tumors having the lowest amounts (Figure 6(d)). No relationship was found between the amount of phosphorylated p38 and tumor size for mice maintained on a regular diet, however (data not shown). For tumors taken from mice on the HC diet, p38 activity was found to correlate inversely with the amount of active antiapoptotic protein Bcl-2 (Figure 6(e)). Bcl-2 activity related to tumor size, with the high- est levels corresponding with the largest tumors (Figure 6(f)). The data suggest that p38 activity is attenuating the enhanced tumor growth of HC diet-challenged mice via inhibition of the antiapoptotic protein Bcl-2. 3.7. The VO2 of Mice on an HC Diet Shows an Inverse Relationship with the Amount of Primary Tumor Vascularization Abdominal primary tumors were randomly selected from 7 each of 140 day-old PyMT mice maintained on either an HC or a regular diet (Figure 7(a)). A complete trans- verse section from the center of each tumor was H & E stained, and assessed for a number of qualitative pa- rameters. No differences were found between experi- mental cohorts for mitosis, necrosis or inflammation (data not shown). However, when primary tumors were graded 0 - 4 for amount of vascularity, with 4 being the most vascularized (Figure 7(c)), for mice on the HC diet, Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate Copyright © 2012 SciRes. JCT 724 (a) (b) (c) (d) Figure 4. An HC diet causes an immediate and significant increase in VO2 as well as decrease in respiratory exchange ratio (RER), neither of which is affected by the presence of the PyMT transgene. (a) When mice on a regular diet were put on an HC diet, their rate of oxygen consumption, as measured by indirect calorimetry, increased about 7% over the course of 24 hours. When mice were switched back to a regular diet, their VO2 returned to original levels. Over the course of the dietary challenge, mice maintained high rates of VO2 that did not differ significantly from the original and immediate increases ob- served within the first 24 hours of consuming the diet; (b) Within 24 hours of being switched from a regular to an HC diet, mice experienced an 8% decrease in RER. Mice over the course of the HC dietary challenge maintained lower RER’s that did not differ significantly from the original drop that was observed following the first 24 hours of being introduced to the diet; (c, d) The presence of tumors did not influence either the increases in VO2 or the decreases in RER observed in HC diet-challenged mice. NS = P > 0.1. VO2 at 140 days of age showed a strong and inverse rela- tionship with the degree of primary tumor vascularization, with the tumors from the mice with the highest rates of oxygen consumption having the least degree of vascu- larization (Figure 7(b)). There was no relationship be- tween VO2 and tumor vascularization for mice main tained on a regular diet (data not shown). The data sug- gest that the enhanced rate of oxygen consumption by the HC diet is attenuating tumor vascularization. 4. Discussion Metabolic syndrome is characterized by abdominal obe- sity, high blood glucose and insulin resistance, and high blood pressure. It has been associated with increased mortality by a number of different types of cancer, but the relative importance of these different risk factors is unknown, and the mechanisms by which obesity en- hances cancer growth are poorly understood. One cancer in particular that has been associated with obesity is can- cer of the breast. To study the effects of obesity on mammary cancer in mice, we used the MMTV-PyMT mouse model. PyMT is a membrane bound polypeptide and active analogue of a receptor that harbors docking sites for a number of effector proteins that stimulate mi- togenesis. The MMTV-PyMT mouse model shares nu- merous characteristics with human breast tumors, in that tumors develop with high penetrance and show gradual loss of estrogen and progesterone receptors, the full mul- tistage progression from hyperplasia to full-blown ma- lignancy and metastasis is represented, and metastatic potential appears to be independent of hormonal fluctua- tions with a reproducible and measurable progression rate [11]. We developed a MMTV-PyMT transgenic line on a C57BL6/J background, known to be obesity sensi- tive to a high fat/high carbohydrate, high caloric (HC) diet. As expected, mice on this diet developed significant in- creases in a number of factors associated with metabolic 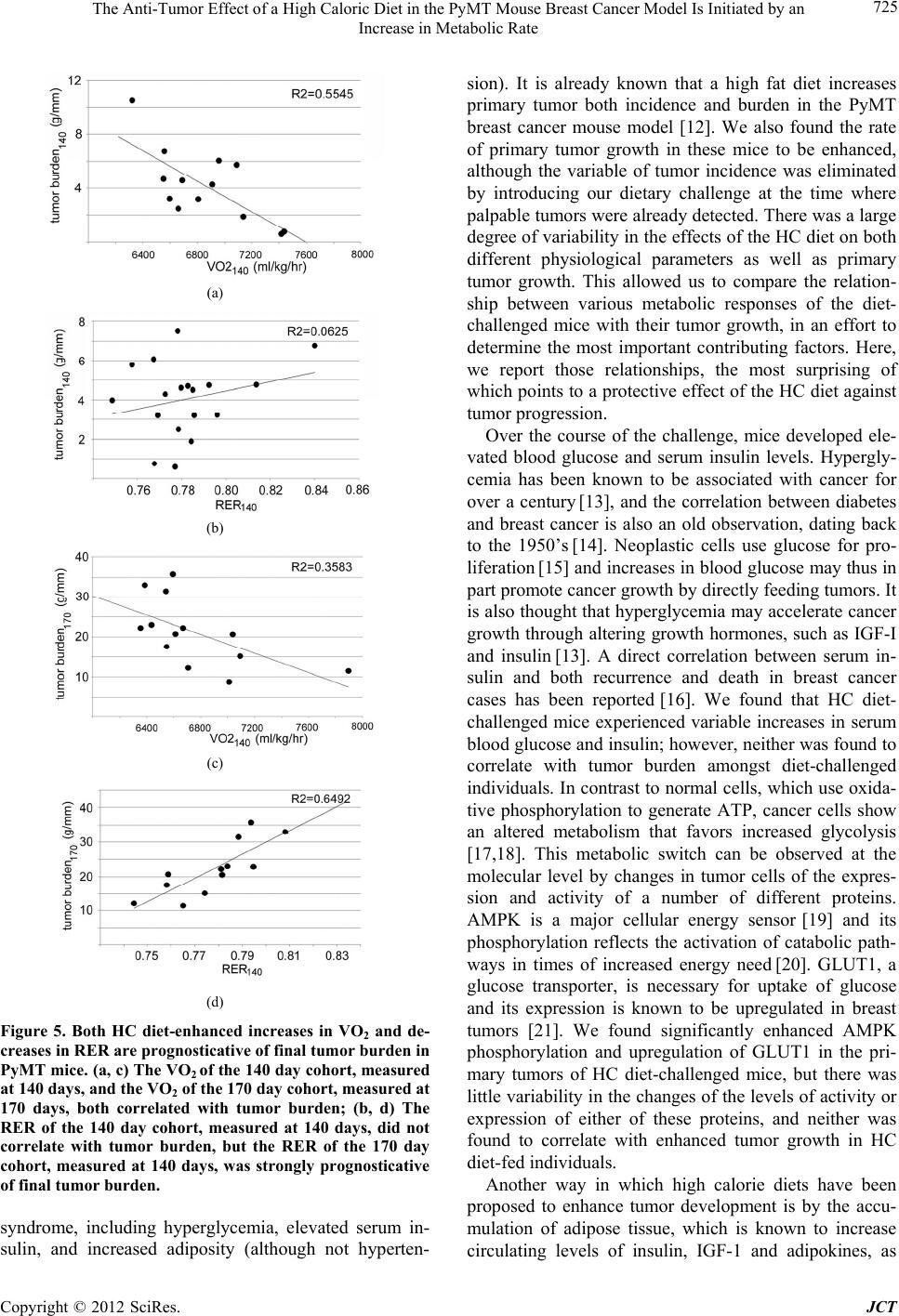 The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 725 (a) (b) (c) (d) Figure 5. Both HC diet-enhanced increases in VO2 and de- creases in RER are prognosticative of final tumor burden in PyMT mice. (a, c) The VO2 of the 140 day cohort, measured at 140 days, and the VO2 of the 170 day cohort, measured at 170 days, both correlated with tumor burden; (b, d) The RER of the 140 day cohort, measured at 140 days, did not correlate with tumor burden, but the RER of the 170 day cohort, measured at 140 days, was strongly prognosticative of final tumor burden. syndrome, including hyperglycemia, elevated serum in- sulin, and increased adiposity (although not hyperten- sion). It is already known that a high fat diet increases primary tumor both incidence and burden in the PyMT breast cancer mouse model [12]. We also found the rate of primary tumor growth in these mice to be enhanced, although the variable of tumor incidence was eliminated by introducing our dietary challenge at the time where palpable tumors were already detected. There was a large degree of variability in the effects of the HC diet on both different physiological parameters as well as primary tumor growth. This allowed us to compare the relation- ship between various metabolic responses of the diet- challenged mice with their tumor growth, in an effort to determine the most important contributing factors. Here, we report those relationships, the most surprising of which points to a protective effect of the HC diet against tumor progression. Over the course of the challenge, mice developed ele- vated blood glucose and serum insulin levels. Hypergly- cemia has been known to be associated with cancer for over a century [13], and the correlation between diabetes and breast cancer is also an old observation, dating back to the 1950’s [14]. Neoplastic cells use glucose for pro- liferation [15] and increases in blood glucose may thus in part promote cancer growth by directly feeding tumors. It is also thought that hyperglycemia may accelerate cancer growth through altering growth hormones, such as IGF-I and insulin [13]. A direct correlation between serum in- sulin and both recurrence and death in breast cancer cases has been reported [16]. We found that HC diet- challenged mice experienced variable increases in serum blood glucose and insulin; however, neither was found to correlate with tumor burden amongst diet-challenged individuals. In contrast to normal cells, which use oxida- tive phosphorylation to generate ATP, cancer cells show an altered metabolism that favors increased glycolysis [17,18]. This metabolic switch can be observed at the molecular level by changes in tumor cells of the expres- sion and activity of a number of different proteins. AMPK is a major cellular energy sensor [19] and its phosphorylation reflects the activation of catabolic path- ways in times of increased energy need [20]. GLUT1, a glucose transporter, is necessary for uptake of glucose and its expression is known to be upregulated in breast tumors [21]. We found significantly enhanced AMPK phosphorylation and upregulation of GLUT1 in the pri- mary tumors of HC diet-challenged mice, but there was little variability in the changes of the levels of activity or expression of either of these proteins, and neither was found to correlate with enhanced tumor growth in HC diet-fed individuals. Another way in which high calorie diets have been proposed to enhance tumor development is by the accu- mulation of adipose tissue, which is known to increase circulating levels of insulin, IGF-1 and adipokines, as Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate Copyright © 2012 SciRes. JCT 726 (c) (b) (d) (e) (f) a Figure 6. Primary tumor size in HC diet-fed individuals correlates with the amount of phosphorylation of tumor suppressor p38 and the antiapoptotic protein Bcl-2. (a) The VO2 of individuals from the 140 day, hf diet-fed cohort correlated with the weight of their abdominal tumors; mice with the highest VO2 presented with the smallest tumors. This correlation was not found for the 140 day cohort of regular diet-fed PyMT mice (c); (b) 5 primary tumors were selected from the HC diet-challenged mice shown in (a) (indicated in grey) and 5 from the regular diet-fed mice shown in (c); HC diet-challenged mice showed higher levels of phosphorylated AMPK, and of GLUT1 and VEGF than regular diet-fed mice, with the excep- tion of one tumor (tumor 6). No differences were seen between dietary cohorts for either HIF1α or p38 total. The amount of p38 phosphorylation correlated strongly and inversely with the size of the tumors for the HC diet-fed cohort, however, with the smallest tumors showed the highest levels of P-p38 (d); The amount of phosphorylated p38 correlated inversely with Bcl-2 phosphorylation levels (e); For mice on the HF diet, the amount of phosphorylated Bcl-2 correlated directly with tumor size, with the highest levels found in the largest tumors (f). well as to increase inflammatory conditions by attracting immune cells [22]. Surprisingly, we found that in our PyMT transgenic mouse model of breast cancer, while a high calorie diet leads to increases in both adiposity and tumor growth, it is the leanest of the HC diet-challenged mice that develop the highest tumor loads. There is the possibility that in addition to the anti-tumor effect pro- vided by the enhanced metabolism of nutrient-over- loaded mice, there is protection by the adipose tissue itself. An inverse association between body mass index (BMI) and the risk of breast cancer among premeno- pausal women has been observed in numerous studies, although the mechanisms behind this are not understood [23]. It is also possible that the reduced adiposity in mice with higher rates of tumor growth simply reflects an en- ergy balance that favors tumor development over fat ac- cumulation. This idea is supported by our observations that mice with PyMT-generated tumors accumulate fat slower than wild-type mice. The observation that increased metabolic rate in re- sponse to nutrient overload correlates with a reduced cancer load is novel. In fact, it has been hypothesized that metabolic stress is one way by which a high calorie diet might promote tumor growth, by increasing the gen- eration of reactive oxygen species (ROS) [22]. While this is possible, it is also known that the tumor suppressor  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 727 (a) (b) (c) Figure 7. The VO2 of HC diet-fed individuals correlates inversely with the degree of vascularization of their primary tumors. (a) Abdominal tumors were selected from 7 of the 140 day HC diet-fed cohort (indicated in green) and from 7 of the regular diet-fed cohort (not shown); (b) The degree of primary tumor vascularization, graded from 0 - 4 with 4 being the most vascu- larized, was found to correlate inversely with the HC diet-enhanced VO2 of the indi vid ual. This corr ela tio n was n ot found for regular diet-fed mice (data not shown); (c) Representative images of H & E-stained sections of primary mammary neoplasias, magnification 20×. Vascularity was scored on a scale of 0 - 4 as detailed in the methods. Vascularity scores are indicated in the bottom right corner of panels. Blood vessels are highlighted by white arrows. p38 is activated in response to increases in ROS [24], and this ROS-induced p38 phosphorylation has been linked to the down-regulation of several anti-apoptotic proteins including members of the Bcl-2 family [25-29]. Our findings that the level of p38 activity and Bcl-2 inhibition are highly correlative both with each other as well as with tumor size in HC diet-fed, but not regular diet-fed mice, indicates that the metabolic boost experienced by mice on the HC diet may be causing an anti-tumor effect by decreasing the inhibition of apoptosis through down- regulation of antiapototic proteins such as Bcl-2, via in- creased activity of p38. The presence of increased oxygen (O2) itself may play a role in suppressing tumor growth. In addition to the HC diet-enhanced rate of oxygen consumption, the degree of the drop in respiratory exchange rate of HC diet-fed mice was also found to be an early and excellent prognostica- tor of tumor growth. The respiratory exchange rate, or RER, is calculated as CO2 breathed out/O2 taken in; the lower the RER, the more oxygen being consumed. One potential way whereby enhanced oxygen consumption might attenuate tumor growth is by inhibition of tumor vascularization, or angiogenesis. We found that the rate of oxygen consumption in HC diet-fed mice correlated Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 728 strongly and inversely with the degree to which tumors were vascularized; mice with higher rates of oxygen consumption bore tumors with less prominent vasculari- zation. When tumors are microscopic, their cells are oxygenated by simple diffusion of oxygen from the tu- mor microenvironment. As they grow, lack of oxygen, or hypoxia, stimulates the development of vasculature which is in turn necessary for further tumor growth [30]. One way in which hypoxic regions in tumors can be re- duced is by increasing blood flow rate and blood oxygen content [31]. Higher oxygen consumption translates di- rectly to more oxygen in the blood and an increase in the oxygen being delivered to tumors. By reducing hypoxia, enhanced oxygen consumption may attenuate tumor growth by slowing the development of tumor vasculature. It is thought that one molecular pathway by which hy- poxia regulates angiogenesis is through increased ex- pression of the hypoxia-inducible factor HIF1α, and its downstream target vascular endothelial growth factor (VEGF) [32]. We did not find strong differences in HIF1α levels between regular and HC diet-fed mice. VEGF levels were clearly higher in the latter, but we did not find correlations between metabolic rate and either HIF1α or VEGF levels. It is, however, already known that the molecular evidence supporting the relationship between hypoxia and angiogenesis is contentious [33]. It is possible that there is a spatial aspect to the colocaliza- tion of hypoxia and angiogenesis; in other words, looking at protein extracts from whole tumors may obscure rela- tionships that could be found in select areas of the tumor. Overall, our study validates the ability of an HC diet to enhance tumor growth in a mouse cancer model. But our data clearly show HC diet-related beneficial effects that attenuate this effect. The beneficial effects of the high calorie diet, namely the metabolic enhancement that oc- curs in lean individuals, could be separated out from more harmful effects as a means of improving cancer therapies. Improving blood oxygen supply has long been recognized as having potential for sensitizing tumors to radiotherapy. Strategies that have been proposed include decreasing the oxygen affinity of hemoglobin in the bloodstream using synthetic agents [34]. Using metabolic enhancers such as carnitine [35] may also enhance the effects of different cancer therapies. Intense exercise is also known to increase VO2, and has been found to sig- nificantly increase phosphorylation of the tumor sup- pressor p38 [36]. The PyMT mouse model would be an excellent model for studying the effects of physical exer- cise on attenuating tumor growth. Also of extreme inter- est is the potential prognosticative application of this study. Within 24 hours of being put on the high calorie diet, the increases in VO2 and decreases in RER of lean mice with barely detectable tumors were highly prognos- ticative of cancer aggressiveness and final tumor burden. It has been found that overfeeding also causes the resting metabolic rate of lean humans to increase rapidly [37], so this finding may be translatable to humans. Our study isolated the effects of obesity on tumor pro- gression from its effects on cancer risk by introducing the dietary challenge when primary tumors were already palpable. The data show that there are clear anti-tumor effects of an HC diet on tumors already present in mice. These mice, however, were lean at the time of tumor incidence and HC diet administration. It is unknown how the metabolism of a long-term obese mouse or human would respond to an HC diet, and these findings may only be applicable to individuals who are metabolically healthy. This study shows that an increase in metabolic rate and oxygen consumption, induced by the introduction of a high caloric diet, has a protective effect against tumor growth in the PyMT murine breast cancer model. Ele- vated oxygen consumption was associated with an in- crease in the activity levels of the tumor suppressor p38, as well as a reduction in tumor vascularization. Overall, however, the effects of a high fat/high calorie diet still enhanced tumor progression. It is not our intention to recommend the use of an HC diet to treat human indi- viduals newly diagnosed with cancer. Rather, this study implicates metabolic stimulation as a potentially useful method for enhancing cancer therapies, and indicates that for lean individuals, administering a high calorie diet over the short term in combination with metabolic moni- toring may provide a highly prognosticative tool. 5. Acknowledgements WCL and LCE designed research; LCE conducted re- search and analyzed data; LCE performed statistical analysis and wrote paper; WCL and LCE had primary responsibility for final content. Histological preparations and staining were done by the Histology and Imaging Core (HIC), UW, Seattle, WA. Histological analyses of tumor sections were performed by Piper Treuting, Dept of Comparative Medicine, UW, Seattle, WA. Funding: R21 CA140916, NIH/NCI; P01 AG01751 NIH/NIA. All authors read and approved the final manuscript.The au- thors declare that they have no competing interests. REFERENCES [1] R. Samper-Ternent and S. Al Snih, “Obesity in Older Adults: Epidemiology and Implications for Disability and Disease,” Reviews in Clinical Gerontology, Vol. 22, No. 1, 2012, pp. 10-34. doi:10.1017/S0959259811000190 [2] J. R. Jaggers, X. Sui, S. P. Hooker, M. J. LaMonte, C. E. Matthews, G. A. Hand and S. N. Blair, “Metabolic Syn- Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate 729 drome and Risk of Cancer Mortality in Men,” European Journal of Cancer, Vol. 45, No. 10, 2009, pp. 1831-1838. doi:10.1016/j.ejca.2009.01.031 [3] P. Pothiwala, S. K. Jain and S. Yaturu, “Metabolic Syn- drome and Cancer,” Metabolic Syndrome and Related Disorders, Vol. 7, No. 4, 2009, pp. 279-288. doi:10.1089/met.2008.0065 [4] World Cancer Research Fund/American Institute for Cancer Research, “Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective,” Wash- ington DC, 2007. [5] G. Taubes, “Unraveling the Obesity-Cancer Connection,” Science, Vol. 335, No. 6066, 2012, pp. 28-32. doi:10.1126/science.335.6064.28 [6] J. M. Petrelli, E. E. Calle, C. Rodriguez and M. J. Thun, “Body Mass Index, Height, and Postmenopausal Breast Cancer Mortality in a Prospective Cohort of US Women,” Cancer Causes and Control, Vol. 13, No. 4, 2002, pp. 325- 332. doi:10.1023/A:1015288615472 [7] C. T. Guy, R. D. Cardiff and W. J. Muller, “Induction of Mammary Tumors by Expression of Polyomavirus Mid- dle T Oncogene: A Transgenic Mouse Model for Metas- tatic Disease,” Molecular and Cellular Biology, Vol. 12, No. 3, 1992, pp. 965-961. [8] L. C. Enns, J. F. Morton, R. S. Mangalindan, G. S. McKnight, M. W. Schwartz, M. R. Kaeberlein, B. K. Kennedy, P. S. Rabinovitch and W. C. Ladiges, “At- tenuation of Age-Related Metabolic Dysfunction in Mice with a Targeted Disruption of the Cbeta Subunit of Pro- tein Kinase A,” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, Vol. 64, No. 12, 2009, pp. 1221-1231. doi:10.1093/gerona/glp133 [9] E. Y. Lin, J. G. Jones, P. Li, L. Zhu, K. D. Whitney, W. J. Muller and J. W. Pollard, “Progression to Malignancy in the Polyoma Middle T Oncoprotein Mouse Breast Cancer Model Provides a Reliable Model for Human Diseases,” American Journal of Pathology, Vol. 163, No. 5, pp. 2113- 2126. doi:10.1016/S0002-9440(10)63568-7 [10] P. M. Treuting, L. I. Chen, B. S. Buetow, W. Zeng, T. A. Birkebak, V. L. Seewaldt, K. M. Sommer, M. Emond, M, L. Maggio-Price and K. Swisshelm, “Retinoic Acid Re- ceptor Beta2 Inhibition of Metastasis in Mouse Mammary Gland Xenografts,” Breast Cancer Research and Treat- ment, Vol. 72, No. 1, 2002, pp. 79-88. doi:10.1023/A:1014906529407 [11] J. Goh, L. Enns, S. Fatemie, H. Hopkins, J. Morton, C. Pettan-Brewer and W. Ladiges, “Mitochondrial Targeted Catalase Suppresses Invasive Breast Cancer in Mice,” BMC Cancer, Vol. 11, 2011, p. 191. doi:10.1186/1471-2407-11-191 [12] G. Llaverias, C. Danilo, I. Mercier, K. Daumer, F. Capo- zza, T. M. Williams, F. Sotgia, M. P. Lisanti and P. G. Frank, “Role of Cholesterol in the Development and Pro- gression of Breast Cancer,” American Journal of Pathol- ogy, Vol. 178, No. 1, 2011, pp. 402-412. doi:10.1016/j.ajpath.2010.11.005 [13] F. Xue and K. B. Michels, “Diabetes, Metabolic Syn- drome, and Breast Cancer: A Review of the Current Evi- dence,” American Journal of Clinical Nutrition, Vol. 86, No. 3, 2007, pp. s823-s835. [14] A. S. Glicksman and R. W. Rawson, “Diabetes and Al- tered Carbohydrate Metabolism in Patients with Cancer,” Cancer, Vol. 9, No. 6, 1956, pp. 1127-1134. doi:10.1002/1097-0142(195611/12)9:6<1127::AID-CNC R2820090610>3.0.CO;2-4 [15] O. Warburg, “On the Origin of Cancer Cells,” Science, Vol. 123, No. 3191, 1956, pp. 309-314. doi:10.1126/science.123.3191.309 [16] P. J. Goodwin, M. Ennis, K. I. Pritchard, M. E. Trudeau, J. Koo, Y. Madarnas, W. Hartwick, B. Hoffman and N. Hood, “Fasting Insulin and Outcome in Early-Stage Breast Cancer: Results of a Prospective Cohort Study,” Journal of Clinical Oncology, Vol. 20, No. 1, 2002, pp. 42-51. doi:10.1200/JCO.20.1.42 [17] M. Ristow, “Oxidative Metabolism in Cancer Growth,” Current Opinions in Clinical Nutrition and Metabolic Care, Vol. 9, No. 4, 2006, pp. 339-345. doi:10.1097/01.mco.0000232892.43921.98 [18] R. A. Gatenby and R. J. Gillies, “Why Do Cancers Have High Aerobic Glycolysis?” Nature Reviews Cancer, Vol. 4, No. 11, 2004, pp. 891-899. doi:10.1038/nrc1478 [19] D. G. Hardie, “AMP-Activated/SNF1 Protein Kinases: Conserved Guardians of Cellular Energy,” Nature Re- views Molecular Cell Biology, Vol. 8, No. 10, 2007, pp. 774-785. doi:10.1038/nrm2249 [20] K. Inoki, J. Kim and K.-L. Guan, “AMPK and mTOR in Cellular Energy Homeostasis and Drug Targets,” An- nual Review of Pharmacology and Toxicology, Vol. 52, 2011, pp. 381-400. [21] M. Schmidt, M. Kapp, M. Krockenberger, J. Dietl and U. Kammerer, “Glycolytic Phenotype in Breast Cancer: Ac- tivation of Akt, Up-Regulation of GLUT1, TKTL1 and Down-Regulation of M2PK,” Journal of Cancer Re- search and Clinical Oncology, Vol. 136, No. 2, 2010, pp. 219-225. doi:10.1007/s00432-009-0652-y [22] M. K. Sung, J. Y. Yeon, S. Y. Park, J. H. Park and M. S. Choi, “Obesity-Induced Metabolic Stresses in Breast and Colon Cancer,” Annals of the New York Academy of Sci- ences, Vol. 1229, 2011, pp. 61-68. doi:10.1111/j.1749-6632.2011.06094.x [23] K. B. Michels, K. L. Terry and W. C. Willett, “Longitu- dinal Study on the Role of Body Size in Premenopausal Breast Cancer,” Archives of Internal Medicine, Vol. 166, No. 21, 2006, pp. 2395-2402. doi:10.1001/archinte.166.21.2395 [24] B. M. Emerling, L. C. Platanias, E. Black, A. R. Nebreda, R. J. Davis and N. S. Chandel, “Mitochondrial Reactive Oxygen Species Activation of p38 Mitogen-Activated Protein Kinase Is Required for Hypoxia Signaling,” Mo- lecular and Cellular Biology, Vol. 25, No. 12, 2005, pp. 4853-4862. doi:10.1128/MCB.25.12.4853-4862.2005 [25] Y. Ramiro-Cortes, A. Guemez-Gamboa and J. M. Andrade, “Reactive Oxygen Species Participate in the p38-Mediated Apoptosis Induced by Potassium Depriva- tion and Staurosporine in Cerebellar Granule Neurons,” The International Journal of Biochemistry and Cell Biol- Copyright © 2012 SciRes. JCT  The Anti-Tumor Effect of a High Caloric Diet in the PyMT Mouse Breast Cancer Model Is Initiated by an Increase in Metabolic Rate Copyright © 2012 SciRes. JCT 730 ogy, Vol. 43, No. 9, 2011, pp. 1373-1382. doi:10.1016/j.biocel.2011.06.001 [26] J. Pan J, Q. Chang, X. Wang, Y. Son, Z. Zhang, G. Chen, J. Luo, Y. Bi, F. Chen and X. Shi, “Reactive Oxygen Spe- cies-Activated Akt/ASK1/p38 Signaling Pathway in Nickel Compound-Induced Apoptosis in BEAS 2B Cells,” Chemical Research in Toxicology, Vol. 23, No. 3, 2010, pp. 568-577. [27] M. Pearl-Yafe, D. Halperin, O. Scheuerman and I. Fabian, “The p38 Pathway Partially Mediates Caspase-3 Activa- tion Induced by Reactive Oxygen Species in Fanconi Anemia C cells,” Biochemical Pharmacology, Vol. 67, No. 3, 2004, pp. 539-546. doi:10.1016/j.bcp.2003.09.024 [28] A. Van Laethem, K. Nys, S. Van Kelst, S. Claerhout, H. Ichijo, J. Vandenheede, M. Garmyn and P. Agostinis, “Apoptosis Signal Regulating Kinase-1 Connects Reac- tive Oxygen Species to p38 MAPK-Induced Mitochon- drial Apoptosis in UVB-Irradiated Human Keratino- cytes,” Free Radical Biology and Medicine, Vol. 41, No. 9, 2006, pp. 1361-1371. doi:10.1016/j.freeradbiomed.2006.07.007 [29] W.-S. Choi, D.-S. Eom, B. S. Han, W. K. Kim, B. H. Han, E. J. Choi, T. H. Oh, G. J. Markelonis, J. W. Cho and Y. J. Oh, “Phosphorylation of p38 MAPK Induced by Oxida- tive Stress Is Linked to Activation of Both Caspase-8- and -9-Mediated Apoptotic Pathways in Dopaminergic Neurons,” Journal of Biological Chemistry, Vol. 279, No. 19, 2004, pp. 20451-20460. doi:10.1074/jbc.M311164200 [30] P. Carmeliet and R. K. Jain, “Angiogenesis in Cancer and Other Diseases,” Nature, Vol. 407, No. 6801, 2000, pp. 249-257. doi:10.1038/35025220 [31] T. W. Secomb, R. Hsu, E. T. Ong, J. F. Gross and M. W. Dewhirst, “Analysis of the Effects of Oxygen Supply and Demand on Hypoxic Fraction in Tumors,” Acta Onco- logica, Vol. 34, No. 3, 1995, pp. 313-316. doi:10.3109/02841869509093981 [32] G. L. Semenza, “Angiogenesis Ischemic and Neoplastic Disorders,” Annual Review of Medicine, Vol. 54, 2003, pp. 17-28. doi:10.1146/annurev.med.54.101601.152418 [33] B. J. Moeller, Y. Cao, Z. Vujaskovic, C. Y. Li, Z. A. Haroon and M. W. Dewhirst, “The Relationship between Hypoxia and Angiogenesis,” Seminars in Radiation On- cology, Vol. 14, No. 3, 2004, pp. 215-221. doi:10.1016/j.semradonc.2004.04.005 [34] B. D. Kavanagh, T. W. Secomb, R. Hsu, P.-S. Lin, J. Venitz and M. W. Dewhirst, “A Theoretical Model of the Effects of Reduced Hemoglobin-Oxygen Affinity on Tumor Oxygenation,” International Journal of Radiation Oncology Biology Physics, Vol. 53, No. 1, 2002, pp. 172- 179. doi:10.1016/S0360-3016(02)02740-2 [35] L. L. Spriet, C. G. Perry and J. L. Talanian, “Legal Pre-Event Nutritional Supplements to Assist Energy Me- tabolism,” Essays in Biochemistry, Vol. 44, 2008, pp. 27- 43. doi:10.1042/BSE0440027 [36] M. Yu, N. K. Stepto, A. V. Chibalin, L. G. D. Fryer, D. Carling, A. Krook, J. A. Hawley and J. R. Zierath, “Metabolic and Mitogenic Signal Transduction in Human Skeletal Muscle after Intense Cycling Exercise,” Journal of Physiology, Vol. 546, Pt. 2, 2003, pp. 327-335. doi:10.1113/jphysiol.2002.034223 [37] A. M. Harris, M. D. Jensen and J. A. Levine, “Weekly Changes in Basal Metabolic Rate with Eight Weeks of Overfeeding,” Obesity, Vol. 14, No. 4, 2006, pp. 690-695. doi:10.1038/oby.2006.78
|