 Advances in Chemical Engineering and Science, 2012, 2, 444-452 http://dx.doi.org/10.4236/aces.2012.24054 Published Online October 2012 (http://www.SciRP.org/journal/aces) A Laboratory Study of the Effect of Temperatur e on Densities and Viscosities of Binary and Ternary Blends of Soybean Oil, Soy Biodiesel and Petroleum Diesel Oil Oluwafunmilayo A. Aworanti, Samuel E. Agarry*, Ayobami O. Ajani Biochemical Engineering and Biotechnology Research Laboratory, Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria Email: *sam_agarry@yahoo.com Received April 20, 2012; revised May 25, 2012; accepted June 6, 2012 ABSTRACT The depletion of world petroleum reserves and the increased environmental concerns have stimulated the search for alternative sources for petroleum based fuel. The possibility of using vegetable oils as fuel has been recognized, how- ever, due to its high viscosities and low volatilities makes it inefficient for most combustion en gines and thus th e need to get them chemically altered or transesterified to obtain fatty alkyl esters of the oil (biodiesel). In this study, binary and ternary blends of biodiesel were produced and the effect of temperature on their viscosity and density was investi- gated. Biodiesel was produced from soybean oil by transesterification of the oil with methanol using potassium hy- droxide as a catalyst at a temperature of 60˚C in a batch reactor. Binary and ternary blends of the soy-biodiesel were prepared with soy bean oil and petroleum diesel fuel, respectively. Viscosities and densities of the binary and ternary blends were measured at different temperatures of 20˚C to 90˚C as to determine the effect of temperature on viscosities and densities of the blends. The properties of the soy-biodiesel produced were compared with ASTM standard and found to be within the limits. The results show that the viscosities and densities of both the binary and ternary blends are temperature dependent. The viscosities of binary and ternary blends decreased nonlinearly with temperature, while their densities decreased linearly with temperature. Th e variation of temperature with viscosity and density of the blends was correlated and the polynomial equation offered th e best correlation between temperature and viscosity, while lin ear equation gave the best correlation between temperature and density. In conclusion, the efficiency of binary and ternary blends of biodiesel in combustion engines is dependent on the viscosity and density of the blends which are invariably temperature dependent. Keywords: Densities; Viscosities; Batch Reactor; Diesel Fuel; Soy-Biodiesel; Vegetable Oil 1. Introduction The depletion of world petroleum reserves and the in- creased environmental concerns have stimulated the search for alternative sources for petroleum based fuel, including diesel fuels. With increasing demand on the use of fossil fuels, stronger threat to clean environment is being posed as burning of fossil fuels is associated with emissions like CO2, CO, SO2, NO2 and particulate matter which are currently the dominant global source of emis- sions. The harmful exhaust emissions from the engines, rapid increase in the prices of petroleum products, the increasing fuel prices and uncertainties of their supply have jointly created renewed interest among researchers to search for suitable alternative fuels. There is therefore a growing substitution of fossil fuels with fuel derived from renewable resources. This substitution requires in- creased efforts in the research and development of pro- ducing these fuels from different renewable resources. This is the case for biodiesel (alkyl esters) production from vegetable oils [1]. The use of vegetable oil as an alternative fuel had been under study as far back as 1979 [2]. Vegetable oil based fuels are sustainable sources of fuel because as long as they are produced in an ecologi- cally sustainable way, they will not run out. Depending upon the climate and soil conditions, different countries are looking for different types of vegetable oils as sub- stitutes for diesel fuels. For example, soybean oil in the US, rapeseed and sunflower oils in Europe and palm oil in Southeast Asia are being con sidered [3]. The possibil- ity of using vegetable oils as fuel has been recognized since the beginning of diesel engines. In 1911, Rudolph Diesel presented an engine based on compression ignition; the diesel engine. At that time, there was no specific fuel to feed this engine and *Corresponding a uthor. C opyright © 2012 SciRes. ACES  O. A. AWORANTI ET AL. 445 groundnut oil was used [4]. There have been many prob- lems associated with using vegetable oils directly in die- sel engines; coking and trumpet formation on the injec- tors to such an extent that fuel atomization does not oc- cur properly or is even prevented, decrease in power output and thermal efficiency of the engines, carbon de- posits, oil ring sticking; thickening or gelling of the lu- bricating oil as a result of contamination by vegetable oils [2,5]. Vegetable oils also have high viscosity (11 - 17 times higher than diesel fuel) and lower volatility that results in carbon deposits in engines due to incomplete combustion. However, the high viscosities and low vola- tilities of this oil have been reported to make them ineffi- cient for most combustion engines [6] and thus the need to get them chemically altered or transesterified to obtain alkyl esters of the oil (biodiesel). Besides all the above, vegetable oils con tain polyunsaturated compounds. Transesterification has been tested to be one of the chemical modifications to overcome these problems caused by the use of vegetable oils. The transesterifica- tion reduces the molecular weight of vegetable oils and also reduces the viscosity and improves the volatility. The product of the reaction is biodiesel, glycerol, alcohol and catalyst. Biodiesel is produced through a transesteri- fication reaction [7]. In this reaction, with the presence of a catalyst, triglycerides react with an alcohol producing a mixture of fatty acid alkyl esters (FAAE) and glycerol [8]. The stoichiometric reaction requires 1 mole of tri- glycerides and 3 mole of alcohol. However, excess alco- hol is required to drive the reaction close to completion [9,10]. Biodiesel extracted from vegetable oil is one of such renewable alternative under consideration, and be- cause of its closer properties to diesel fuel, biodiesel fuel (fatty acid methyl ester) from vegetable oil is considered as the best candidate for diesel fuel substitute in diesel engine [11]. Biodiesel can therefore be technically de- fined as the alkyl ester of fatty acids, made by the trans- esterification of oils or fats, from plants or animals, with short ch ain alcohols such a s methanol and ethanol. The catalysts used for transesterification could be classified as homogeneous and heterogeneous catalysts Homogeneous catalysts are alkalis such as hydroxides (NaOH, KOH, carbonates and corresponding sodium and potassium alkoxides) [12] and acids like sulphuric acid and hydrochloric acid; while heterogeneous catalysts include among others tungsten oxides, resins and sul- phonated saccharides [7,13]. However, in the last few years, the studies of the enzymatic catalyzed production of biodiesel have shown significant progress [14-16]. The main problem of the enzyme catalyzed process is the high cost of the lipases (enzyme) used as catalyst [16]. At industrial scale, alkaline catalysis is usually used in bio- diesel production from edible oil. The properties of biodiesel are close to conventional diesel and hence become a strong candidate to replace the diesel fuel [11]. Its advantages over conventional diesel fuels are its lower toxicity, high biodegradability, substantial reduction in SOx emissions, considerable re- duction in carbon monoxide (CO), polyaromatic hydro- carbons, smoke and particulate matter [12]. In addition, biodiesel has a high heat value, high oxygen content (10% - 11%) [17] and does not contribute to global warming due to its carbon closed cycle [1]. Biodiesel has substantially different properties than vegetable oils and result in bet- ter engine performance. However, there are some draw- backs of biodiesel like higher cost and cold flow proper- ties. Among the possible raw materials for the production of biodiesel, the use of rapeseed oil, canola oil, soybean oil, palm kernel oil, coconut oil, cotton seed oil and citrus seeds oil has been investigated [5,18-22]. Several studies on binary blends of biodiesel and diesel fuel or vegetable oils have been investigated, but not much has been done on ternary blends of biodiesel, diesel fuels and vegetable oils. Viscosity is one of the most important physical prop- erties of a fluid system [23]. Viscosity changes with shear rate, temperature, pressure, moisture, and concen- tration; all these changes can be modelled by equations [24,25]. Studies on viscosity have been performed on pineapple juice, vegetable oil, etc. [26]. However, there is a dearth of information on the effect of temperature on the viscosity and density of binary and ternary blends of biodiesel oil. Modelling of the temperature effect on the dynamic viscosity of oils is important and has been investigated by some researchers [25,27-29]. However, modelling of the effect of temperature on vis- cosity and density of biodiesel is rarely reported. There- fore, the main objective of this study was to produce soy-biodiesel from soybeans oil and the effect of tem- perature on the viscosities and densities of binary and ternary blends of this biodiesel. Furthermore, polynomial and/or linear dependence of dynamic viscosity and den- sity of soy biodiesel and its blends on temperature was determined. 2. Materials and Methods 2.1. Materials Refined soybeans oil (being a product of UAC Foods, Nigeria) was purchased from a local market in Lagos, Nigeria. Methanol (99% purity) and potassium hydroxide being products of Merck Dam were purchased from a chemical store in Lagos, Nigeria. The equipments used for the experiments are: VT 550 rotating viscometer, flash point analyzer, 50ml density bottle, mass balance, mechanical stirrer (overhead stirrer fixed with a stainless steel propeller) and electromagnetic stirrer, water bath, thermometer, 1000 ml jacketed glass, electric burner, Copyright © 2012 SciRes. ACES  O. A. AWORANTI ET AL. 446 reflux condenser and oven. 2.2. Methods 2.2.1. Methanol y si s o f Soybean Oil : Productio n of Biodiesel The production of biodiesel was carried out in a batch reactor. The reactor consisted of a 1 L jacketed glass, electrical stirrer fitted with a stainless steel propeller(for thorough mixing/agitation), thermometer and a reflux condenser(for preventing the methanol from escaping out of the reactor) since methanol boils at 65˚C in a hot water bath to control the reaction temperature. Transesterifica- tion reaction of soybean oil was carried out with potas- sium hydroxide (1%). Potassium hydroxide (mass frac- tion 1%) was dissolved in 46 ml of methanol using the magnetic stirrer to make a homogenous mixture. Soy- beans oil was measured (167.04 g), pre-heated to a tem- perature of 60˚C and poured into the reactor. The ho- mogenous mixture of methanol and potassium hydroxide was then poured into the reactor containing the soybean oil. The electrical stirrer was set into motion and the re- action was carried out at a temperature of 60˚C fo r 1 hour. The mixing mole ratio of methanol to oil used was 4:1. At the end of 1 hour, the oil has been effectively trans- esterified; two layers were formed, an upper layer of amber yellow colour [0.5 ASTM] was suspected to be biodiesel while the lower layer of a wine//oxbow lake colour [2.5 ASTM] was suspected to be glycerol. The dark colour of the glycerol was due to the presence of excess catalyst in the lower layer. The suspected bio- diesel was separated, washed with warm water and then heated above 65˚C (boiling point of methanol) to remove any excess methanol in it. The suspected biodiesel was then analysed for its fuel properties. 2.2.2. Binary and Ternary Blending of Soy Biodiesel The biodiesel produced from soybean oil was blended with petroleum diesel oil and soybean oil, respectively, using direct blending method. The blends were prepared according to the stated measured percentages (Table 1) using a beaker, electrical stirrer with a stainless steel propeller and mixed at room temperature for 1 hour. For example, the blend with a mixture of 30% biodiesel and 70% petroleum diesel is referred to as B30 blend. 2.2.3. Anal y ses The suspected biodiesel was then analysed for some properties such as dynamic viscosity, density, cloud point, pour point, flash point and colour, respectively. Density and viscosity measurements were made according to ASTM standard D1298 and D445, respectively. Dynamic viscosity 14˚C was measured using a Bookfield vis- cometer. Flash point was measured using the flash point analyzer, colour was measured using the colour meter, and pour point and cloud point were measured using ice packs, test tubes and transparent cooling chamber. The pour and flash points were determined following ASTM standard D297, D25100-8 and D56, respectively. 1) Determination of Viscosity The viscosities of the blends were measured using VT 550 rotating viscometer by putting the blend in a beaker while the beaker was put in a water bath (to control the temperature of the mixture) on an electric burner. Tem- peratures were increased at 10˚C interval from 20˚C to 90˚C and readings of viscosities were recorded. Ther- mometer was placed in both the water bath and blend mixture to ensure accuracy in temperature readings. The readings of viscosities were done in triplicates and the average value s were used. 2) Determination of Density The densities of the blends were taken from a tem- perature range of 20˚C to 90˚C. The blends were put in a 50 ml density bottle and weighed on a mass balance. Th e densities were then obtained as given in Equation (1). The densities were measured in triplicates and the aver- age values were used. LB L MM DV (1) where D is density of liquid (g/cm3); L , the mass of bottle and liquid (g); , the mass of bottle only and V is the volume of the liquid (cm3). 3. Results and Discussion 3.1. Properties of the Soy-Biodiesel The physical properties of the suspected biodiesel (vis- cosity, density, cloud point, pour point, flash point and colour) was determined and compared with the interna- tional standard for biodiesel as shown in Table 2. It could be seen from Table 2 that the fuel properties of the soy-biodiesel are within the value range limits specified by international standard for biodiesel. 3.2. Dynamic Viscosities and Densities of Blends Viscosity is a measure of the internal flow resistance of a liquid (i.e. the thickness of the oil) and this constitutes an intrinsic property of vegetab le oils. This is determined by measuring the amount of time taken for a given measure of oil to pass through an orifice of a specific size. Vis- cosity affects injection lubrication and fuel atomization [30]. The higher is the viscosity, the greater is the ten- dency for the fuel to form engine deposits [31]. The variations in viscosity of different binary blends of soybean oil and petroleum diesel with temperature are shown in Figure 1. The result shows that for each of the Copyright © 2012 SciRes. ACES 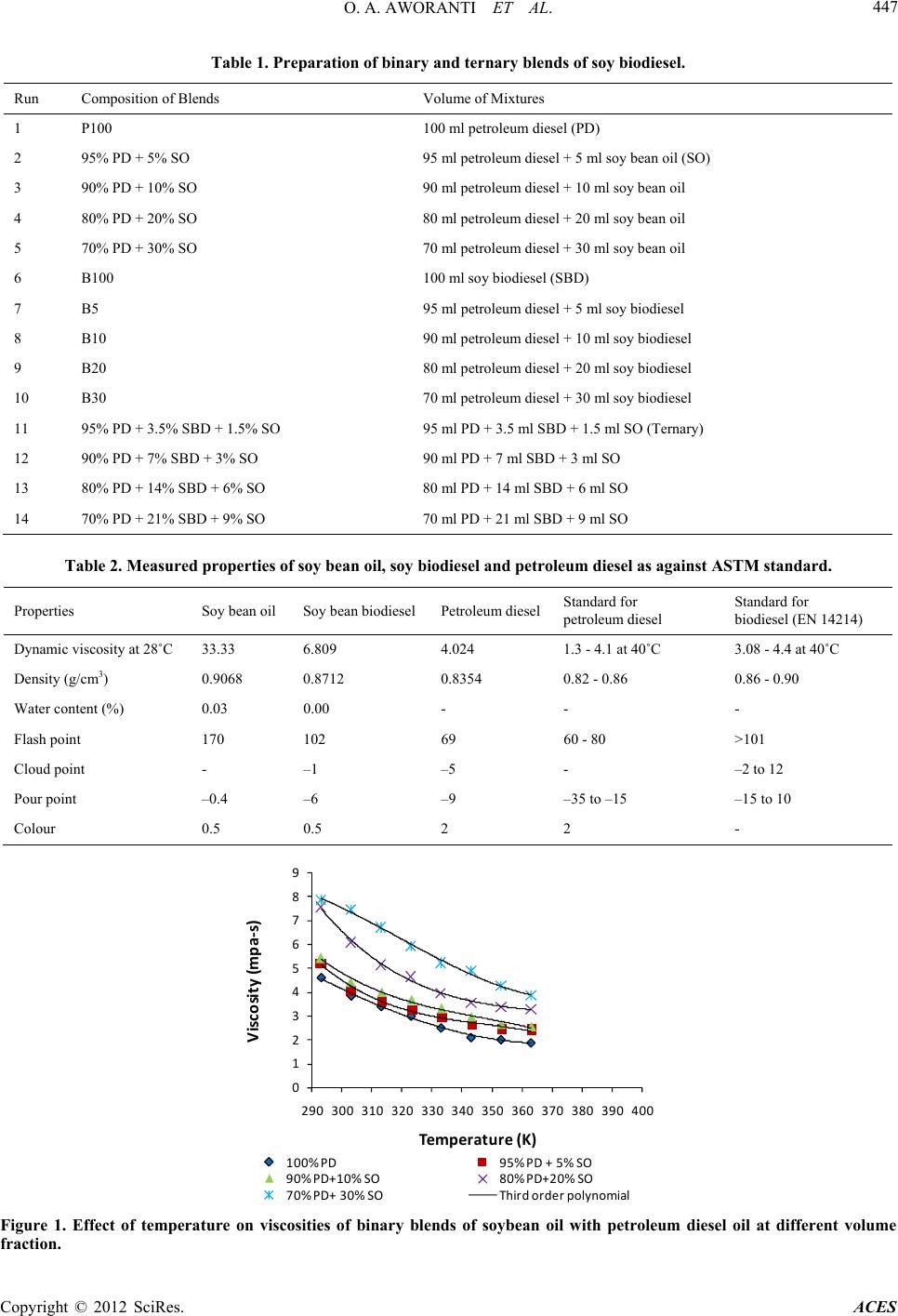 O. A. AWORANTI ET AL. Copyright © 2012 SciRes. ACES 447 Table 1. Preparation of binary and ternary blends of soy biodiesel. Run Composition of Blends Volume of Mixtu res 1 P100 100 ml petroleum diesel (PD) 2 95% PD + 5% SO 95 ml petroleum diesel + 5 ml soy bean oil (SO) 3 90% PD + 10% SO 90 ml petroleum diesel + 10 ml soy bean oil 4 80% PD + 20% SO 80 ml petroleum diesel + 20 ml soy bean oil 5 70% PD + 30% SO 70 ml petroleum diesel + 30 ml soy bean oil 6 B100 100 ml soy biodiesel (SBD) 7 B5 95 ml petroleum diesel + 5 ml soy biodiesel 8 B10 90 ml petrole um diesel + 10 ml soy biodiesel 9 B20 80 ml petrole um diesel + 20 ml soy biodiesel 10 B30 70 ml petroleum diesel + 30 ml soy biodiesel 11 95% PD + 3.5% SBD + 1.5% SO 95 ml PD + 3.5 ml SBD + 1.5 ml SO (Ternary) 12 90% PD + 7% SBD + 3% SO 90 ml PD + 7 ml SBD + 3 ml SO 13 80% PD + 14% SBD + 6% SO 80 ml PD + 14 ml SBD + 6 ml SO 14 70% PD + 21% SBD + 9% SO 70 ml PD + 21 ml SBD + 9 ml SO Table 2. Measured properties of soy bean oil, soy biodiesel and petroleum diesel as against ASTM standard. Properties Soy bean oil Soy bean biodiesel Petroleum dieselStandard for petroleum diesel Standard for biodiesel (EN 14214) Dynamic viscosity at 28˚C 33.33 6.809 4.024 1.3 - 4.1 at 40˚C 3.08 - 4.4 at 40˚C Density (g/cm3) 0.9068 0.8712 0.8354 0.82 - 0.86 0.86 - 0.90 Water content (%) 0.03 0.00 - - - Flash point 170 102 69 60 - 80 >101 Cloud point - –1 –5 - –2 to 12 Pour point –0.4 –6 –9 –35 to –15 –15 to 10 Colour 0.5 0.5 2 2 - 0 1 2 3 4 5 6 7 8 9 290 300310 320330 340350 360370 Viscosity(mpa‐s) Temperature(K) 380 390400 100%PD95%PD+5%SO 90%PD+10%SO 80%PD+2 0%SO 70%PD+30%SO Th i rdorderpolynomial Figure 1. Effect of temperature on viscosities of binary blends of soybean oil with petroleum diesel oil at different volume fraction. 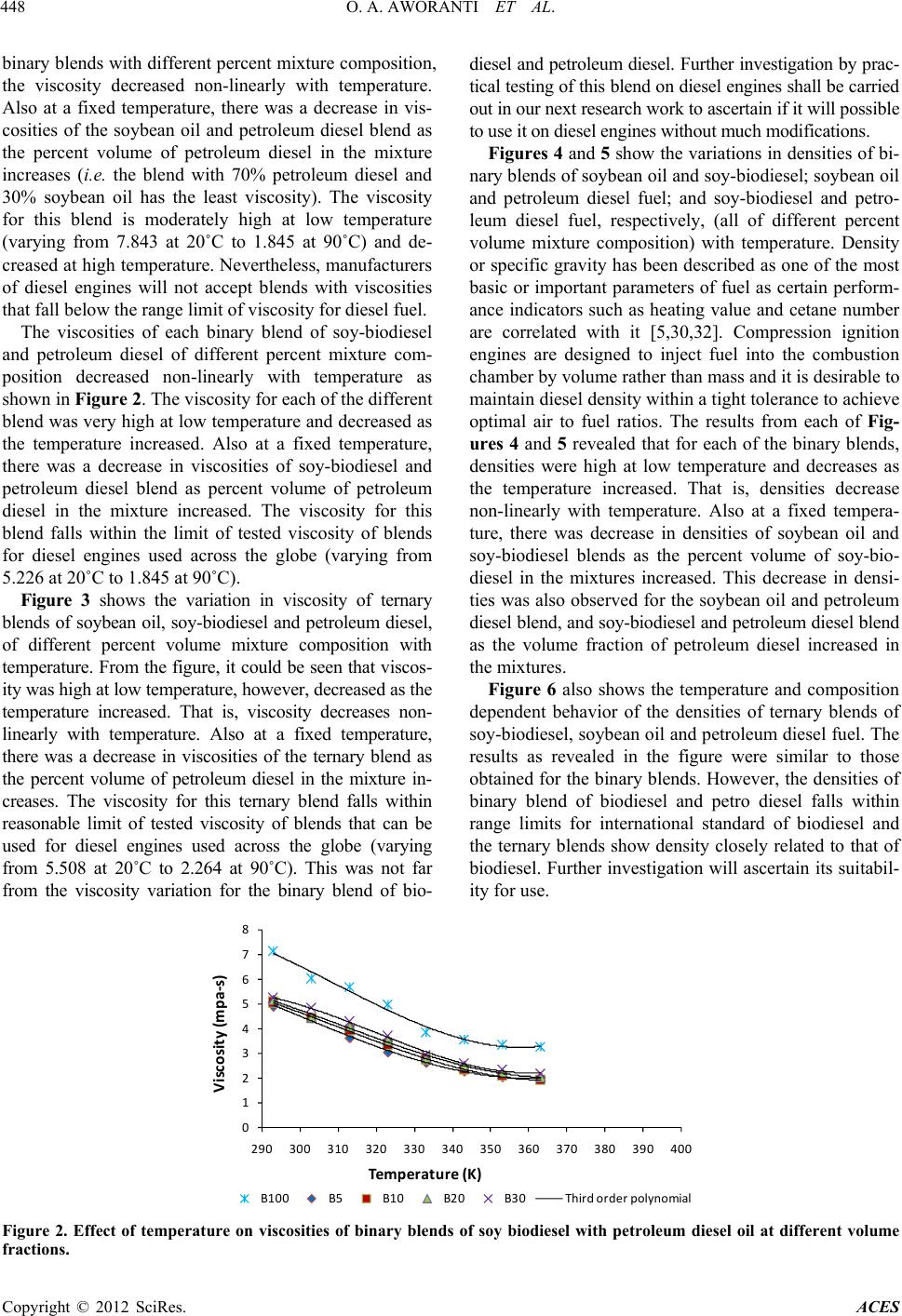 O. A. AWORANTI ET AL. 448 binary blends with differen t percent mixture compos ition, the viscosity decreased non-linearly with temperature. Also at a fixed temperature, there was a decrease in vis- cosities of the soybean oil and petroleum diesel blend as the percent volume of petroleum diesel in the mixture increases (i.e. the blend with 70% petroleum diesel and 30% soybean oil has the least viscosity). The viscosity for this blend is moderately high at low temperature (varying from 7.843 at 20˚C to 1.845 at 90˚C) and de- creased at high temperature. Nevertheless, manufacturers of diesel engines will not accept blends with viscosities that fall below the range limit of viscosity for diesel fuel. The viscosities of each binary blend of soy-biodiesel and petroleum diesel of different percent mixture com- position decreased non-linearly with temperature as shown in Figure 2. The viscosity for each of the different blend was very high at low temperature and decreased as the temperature increased. Also at a fixed temperature, there was a decrease in viscosities of soy-biodiesel and petroleum diesel blend as percent volume of petroleum diesel in the mixture increased. The viscosity for this blend falls within the limit of tested viscosity of blends for diesel engines used across the globe (varying from 5.226 at 20˚C to 1.845 at 90˚C). Figure 3 shows the variation in viscosity of ternary blends of soybean oil, soy-biodiesel and petroleum diesel, of different percent volume mixture composition with temperature. From the figure, it could be seen that viscos- ity was high at low temperature, however, decreased as the temperature increased. That is, viscosity decreases non- linearly with temperature. Also at a fixed temperature, there was a decrease in viscosities of the ternary blend as the percent volume of petroleum diesel in the mixture in- creases. The viscosity for this ternary blend falls within reasonable limit of tested viscosity of blends that can be used for diesel engines used across the globe (varying from 5.508 at 20˚C to 2.264 at 90˚C). This was not far from the viscosity variation for the binary blend of bio- diesel and petroleum diesel. Further investigation by prac- tical testing of this blend on diesel engines shall be carried out in our next research work to ascertain if it will possible to use it on diesel engines wit hout much m odifi cations. Figures 4 and 5 show the variations in densities of bi- nary blends of soybean oil and soy-biodiesel; soybean oil and petroleum diesel fuel; and soy-biodiesel and petro- leum diesel fuel, respectively, (all of different percent volume mixture composition) with temperature. Density or specific gravity has been described as one of the most basic or important parameters of fuel as certain perform- ance indicators such as heating value and cetane number are correlated with it [5,30,32]. Compression ignition engines are designed to inject fuel into the combustion chamber by volume rather than mass and it is desirable to maintain diesel density with in a tigh t tolerance to ach iev e optimal air to fuel ratios. The results from each of Fig- ures 4 and 5 revealed that for each of the binary blends, densities were high at low temperature and decreases as the temperature increased. That is, densities decrease non-linearly with temperature. Also at a fixed tempera- ture, there was decrease in densities of soybean oil and soy-biodiesel blends as the percent volume of soy-bio- diesel in the mixtures increased. This decrease in densi- ties was also observed for the soybean oil and petroleu m diesel blend, and soy-biodiesel and petroleum diesel blend as the volume fraction of petroleum diesel increased in the mixtures. Figure 6 also shows the temperature and composition dependent behavior of the densities of ternary blends of soy-biodiesel, soybean oil and petroleum diesel fuel. The results as revealed in the figure were similar to those obtained for the binary blends. However, the densities of binary blend of biodiesel and petro diesel falls within range limits for international standard of biodiesel and the ternary blends show density closely related to that of biodiesel. Further investigation will ascertain its suitab il- ity for use. 0 1 2 3 4 5 6 7 8 290 300 310320 330 340 350 360 370 380 390 400 Viscosity(mpa‐s) Tempe rature(K) B100 B5B10 B20 B30 Thirdorder polynomial Figure 2. Effect of temperature on viscosities of binary blends of soy biodiesel with petroleum diesel oil at different volume fractions. Copyright © 2012 SciRes. ACES 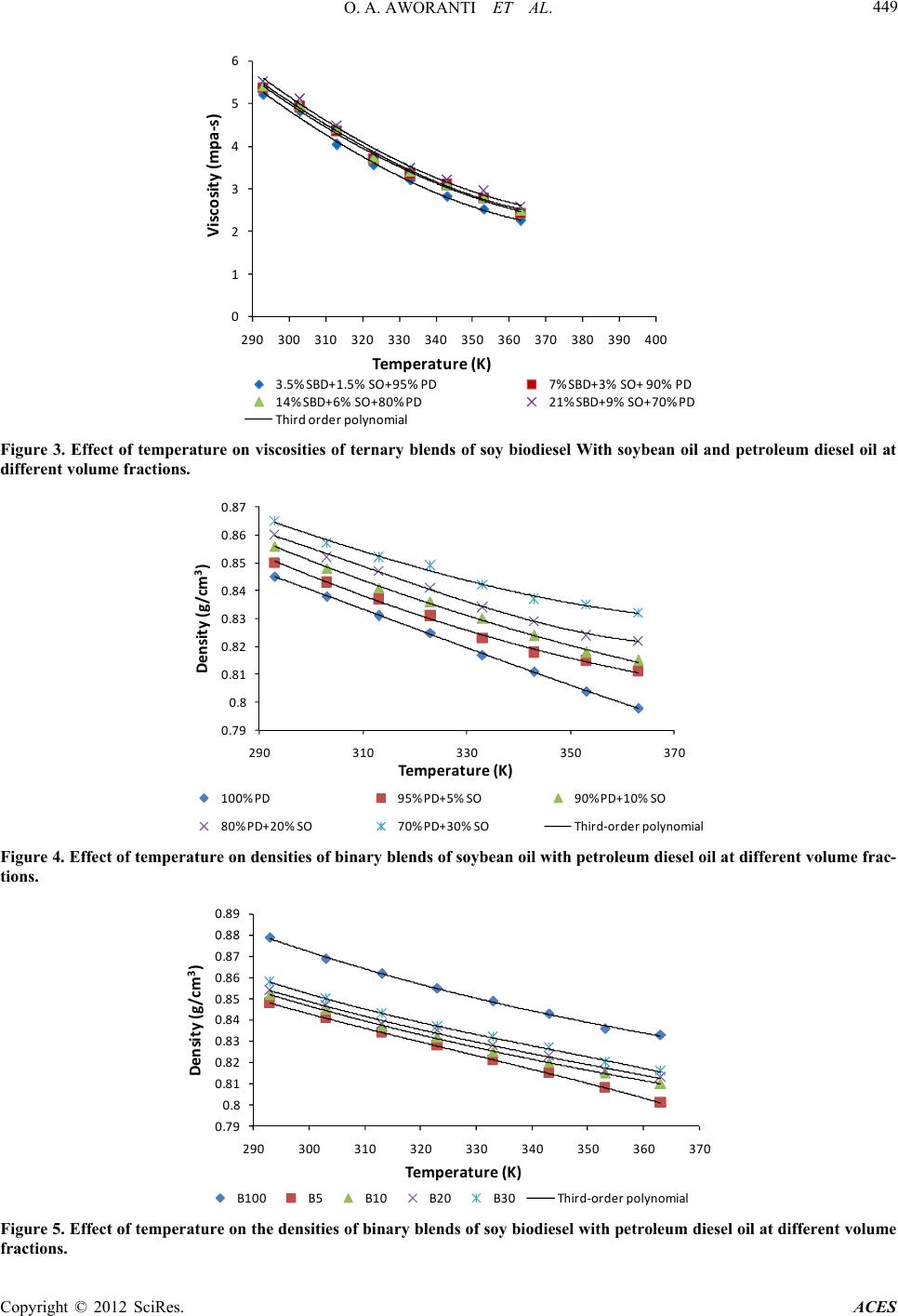 O. A. AWORANTI ET AL. 449 0 1 2 3 4 5 6 290 300 310 320 330 340 350 360 370 380 Viscosity(mpa‐s) Tem perature(K) 390 400 3.5%SBD+1.5%SO+95%PD 7%SBD+3%SO+90%PD 14%SBD+6%SO+80%PD21%SBD+9%SO+7 0%PD Thi rdorderpolynomial Figure 3. Effect of temperature on viscosities of ternary blends of soy biodiesel With soybean oil and petroleum diesel oil at different volume fractions. 0.79 0.8 0.81 0.82 0.83 0.84 0.85 0.86 0.87 290 310 330 350 Den sit y(g/cm 3 ) Tem perature(K) 370 100%PD95%PD+5%SO 90%PD+10%SO 80%PD+20%SO 70%PD+30%SO Third‐order polynomial Figure 4. Effect of temperature on densities of binary blends of soybean oil with petroleum diesel oil at different volume frac- tions. 0.79 0.8 0.81 0.82 0.83 0.84 0.85 0.86 0.87 0.88 0.89 290 300 310 320 330 340 350 Density(g/cm 3 ) Tem perature(K) 360 370 B100 B5 B10 B20 B30 Third ‐orderpolynomial Figure 5. Effect of temperature on the densities of binary blends of soy biodiesel with petroleum diesel oil at different volume fractions. Copyright © 2012 SciRes. ACES 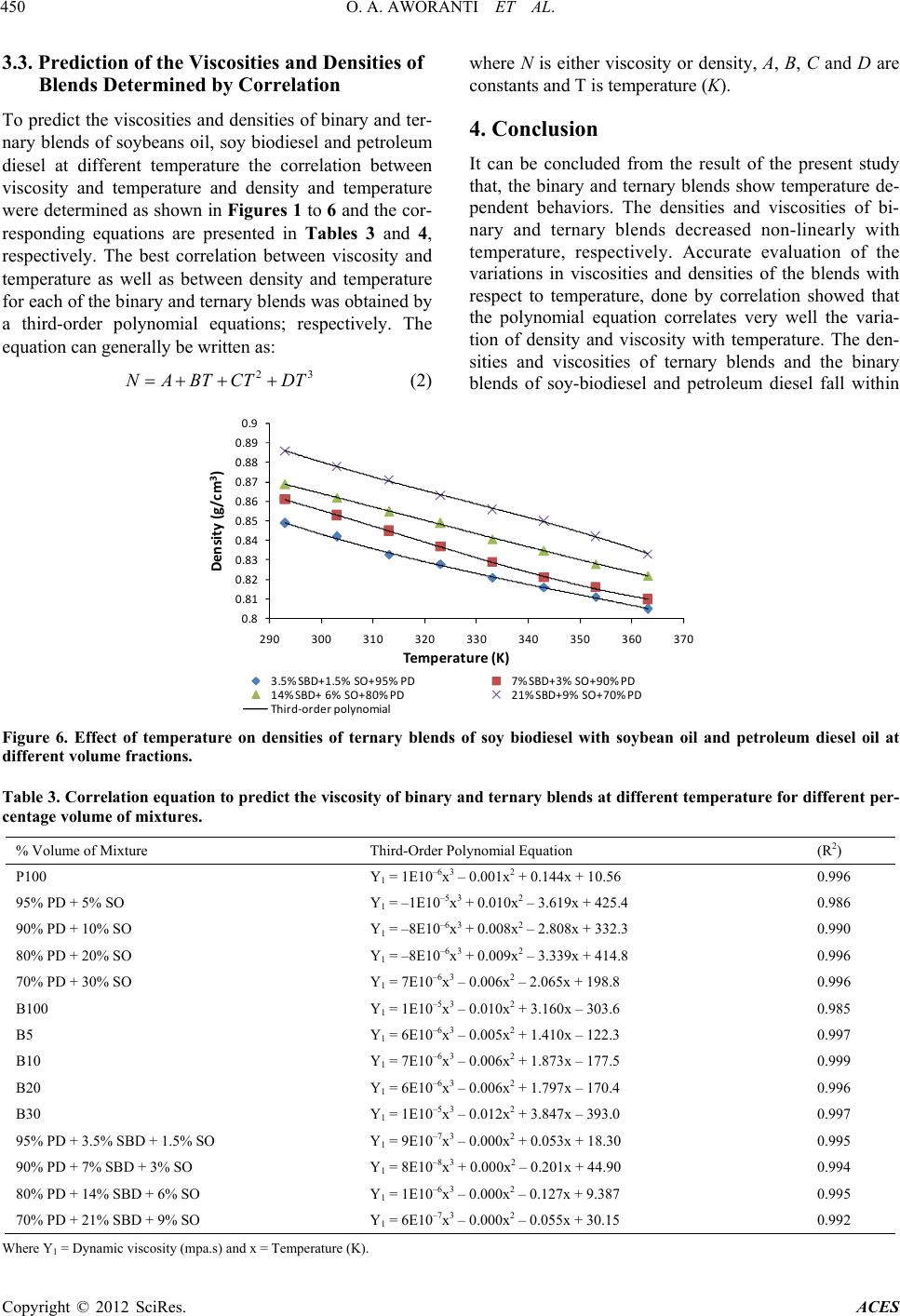 O. A. AWORANTI ET AL. Copyright © 2012 SciRes. ACES 450 23 CT DT 3.3. Prediction of the Viscosities and Densities of Blends Determined by Correlation where N is either viscosity or density, A, B, C and D are constants and T is temperature (K). To predict the viscosities and densities of binary and ter- nary blends of soybeans oil, soy biodiesel and petroleum diesel at different temperature the correlation between viscosity and temperature and density and temperature were determined as shown in Figures 1 to 6 and the cor- responding equations are presented in Tables 3 and 4, respectively. The best correlation between viscosity and temperature as well as between density and temperature for each of the binary an d ternary b lends wa s obtained by a third-order polynomial equations; respectively. The equation can generally be written as: 4. Conclusion It can be concluded from the result of the present study that, the binary and ternary blends show temperature de- pendent behaviors. The densities and viscosities of bi- nary and ternary blends decreased non-linearly with temperature, respectively. Accurate evaluation of the variations in viscosities and densities of the blends with respect to temperature, done by correlation showed that the polynomial equation correlates very well the varia- tion of density and viscosity with temperature. The den- sities and viscosities of ternary blends and the binary lends of soy-biodiesel and petroleum diesel fall within NABT (2) b 0.8 0.81 0.82 0.83 0.84 0.85 0.86 0.87 0.88 0.89 0.9 290 300 310 320 330 340 350 360 370 Density(g/cm 3 ) Temperature(K) 3.5%SBD+1.5%SO+95%PD 7%SBD+3%SO+90%PD 14%SBD+6%SO+ 80%PD21%SBD+9%SO+70%PD Third‐ord erpolynomial Figure 6. Effect of temperature on densities of ternary blends of soy biodiesel with soybean oil and petroleum diesel oil at different volume fractions. Table 3. Correlation equation to predict the viscosity of binary and ternary blends at different temperature for different per- centage volume of mixtures. % Volume of Mixture Third-Order Polynomial Equation (R2) P100 Y1 = 1E10–6x3 – 0.001x2 + 0.144x + 10.56 0.996 95% PD + 5% SO Y1 = –1E10–5x3 + 0.0 1 0 x2 – 3.619x + 425.4 0.986 90% PD + 10% SO Y1 = –8E10–6x3 + 0.0 0 8 x2 – 2.808x + 332.3 0.990 80% PD + 20% SO Y1 = –8E10–6x3 + 0.0 0 9 x2 – 3.339x + 414.8 0.996 70% PD + 30% SO Y1 = 7E10–6x3 – 0.006x2 – 2.065x + 198. 8 0.996 B100 Y1 = 1E10–5x3 – 0.010x2 + 3.160x – 3 03.6 0.985 B5 Y1 = 6E10–6x3 – 0.005x2 + 1.410x – 1 22.3 0.997 B10 Y1 = 7E10–6x3 – 0.006x2 + 1.873x – 17 7.5 0.999 B20 Y1 = 6E10–6x3 – 0.006x2 + 1.797x – 17 0.4 0.996 B30 Y1 = 1E10–5x3 – 0.012x2 + 3.847x – 39 3.0 0.997 95% PD + 3.5% SBD + 1.5% SO Y1 = 9E10–7x3 – 0.000x2 + 0.053x + 18.30 0.9 95 90% PD + 7% SBD + 3% SO Y1 = 8E10–8x3 + 0.000x2 – 0. 201x + 44.90 0.994 80% PD + 14% SBD + 6% SO Y1 = 1E10–6x3 – 0.000x2 – 0.127x + 9.387 0.995 70% PD + 21% SBD + 9% SO Y1 = 6E10–7x3 – 0.000x2 – 0.055x + 30.15 0.992 Where Y1 = Dynamic viscosity (mpa .s) and x = Tem perature (K). 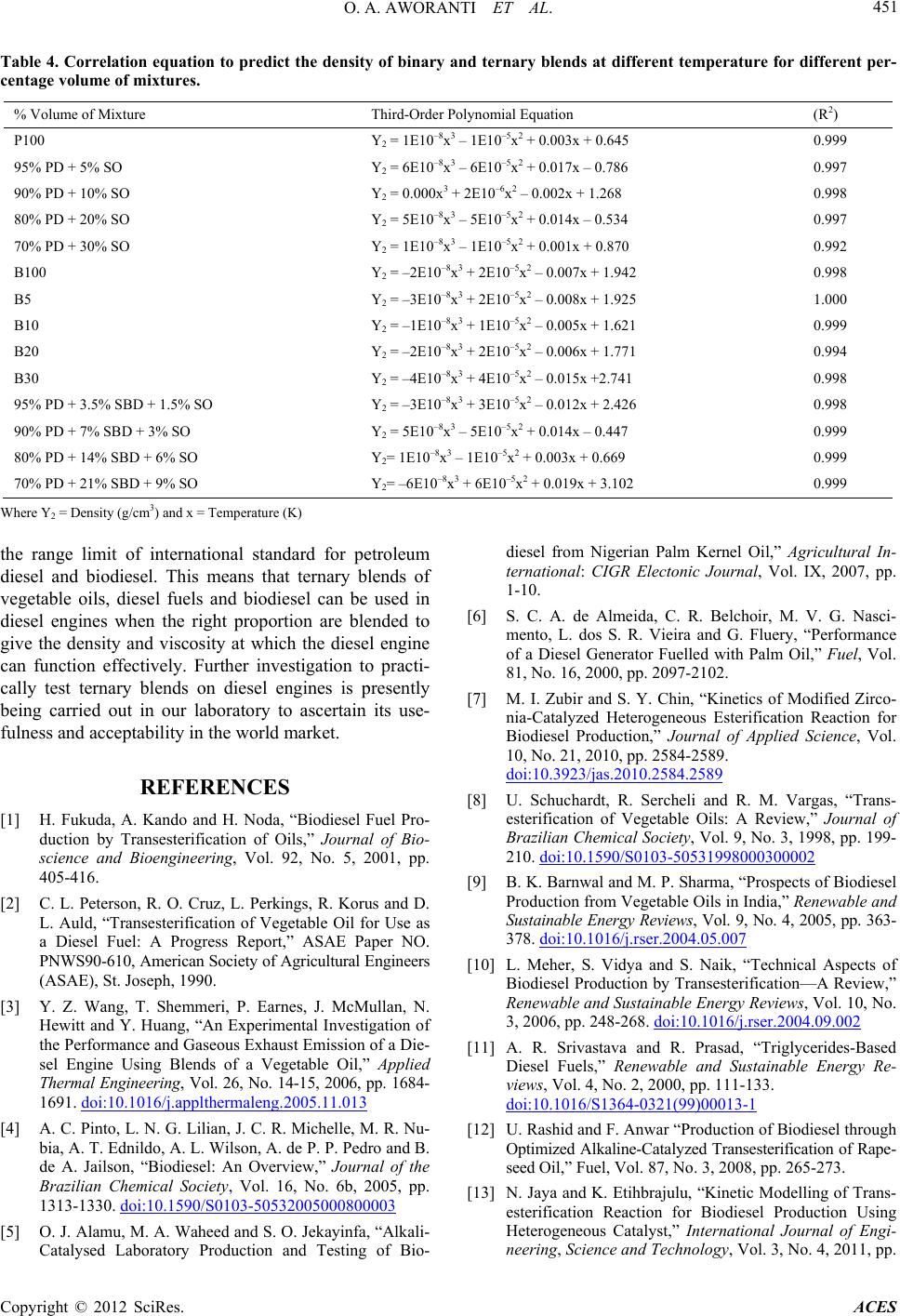 O. A. AWORANTI ET AL. 451 Table 4. Correlation equation to predict the density of binary and ternary blends at different temperature for different per- centage volume of mixtures. % Volume of Mixture Third-Order Polynomial Equation (R2) P100 Y2 = 1E10–8x3 – 1E10–5x2 + 0.003x + 0.645 0.999 95% PD + 5% SO Y2 = 6E10–8x3 – 6E10–5x2 + 0.017x – 0.786 0.997 90% PD + 10% SO Y2 = 0.000x3 + 2E10–6x2 – 0.002x + 1.268 0.998 80% PD + 20% SO Y2 = 5E10–8x3 – 5E10–5x2 + 0.014x – 0.534 0.997 70% PD + 30% SO Y2 = 1E10–8x3 – 1E10–5x2 + 0.001x + 0.870 0.992 B100 Y2 = –2E10–8x3 + 2E10–5x2 – 0.007x + 1.942 0.998 B5 Y2 = –3E10–8x3 + 2E10–5x2 – 0.008x + 1 .925 1.000 B10 Y2 = –1E10–8x3 + 1E10–5x2 – 0.005x + 1.621 0.999 B20 Y2 = –2E10–8x3 + 2E10–5x2 – 0.006x + 1.771 0.994 B30 Y2 = –4E10–8x3 + 4E10–5x2 – 0.015x +2.741 0.998 95% PD + 3.5% SBD + 1.5% SO Y2 = –3E10–8x3 + 3E10–5x2 – 0.012x + 2.426 0 .998 90% PD + 7% SBD + 3% SO Y2 = 5E10–8x3 – 5E10–5x2 + 0.014x – 0. 4 47 0.999 80% PD + 14% SBD + 6% SO Y2= 1E10–8x3 – 1E10–5x2 + 0.003x + 0.669 0.999 70% PD + 21% SBD + 9% SO Y2= –6E10–8x3 + 6E10–5x2 + 0. 019x + 3.102 0.999 Where Y2 = Density (g/cm3) and x = T emperature (K) the range limit of international standard for petroleum diesel and biodiesel. This means that ternary blends of vegetable oils, diesel fuels and biodiesel can be used in diesel engines when the right proportion are blended to give the density and viscosity at which the diesel engine can function effectively. Further investigation to practi- cally test ternary blends on diesel engines is presently being carried out in our laboratory to ascertain its use- fulness and acceptability in the world market. REFERENCES [1] H. Fukuda, A. Kando and H. Noda, “Biodiesel Fuel Pro- duction by Transesterification of Oils,” Journal of Bio- science and Bioengineering, Vol. 92, No. 5, 2001, pp. 405-416. [2] C. L. Peterson, R. O. Cruz, L. Perkings, R. Korus and D. L. Auld, “Transesterification of Vegetable Oil for Use as a Diesel Fuel: A Progress Report,” ASAE Paper NO. PNWS90-610, American Society of Agricultural Engineers (ASAE), St. Joseph, 1990. [3] Y. Z. Wang, T. Shemmeri, P. Earnes, J. McMullan, N. Hewitt and Y. Huang, “An Experimental Investigation of the Performance and Gaseous Exhaust Emission of a Die- sel Engine Using Blends of a Vegetable Oil,” Applied Thermal Engineering, Vol. 26, No. 14-15, 200 6, pp. 1684- 1691. doi:10.1016/j.applthermaleng.2005.11.013 [4] A. C. Pinto, L. N. G. Lilian, J. C. R. Michelle, M. R. Nu- bia, A. T. Ednildo, A. L. Wilson, A. de P. P. Pedro and B. de A. Jailson, “Biodiesel: An Overview,” Journal of the Brazilian Chemical Society, Vol. 16, No. 6b, 2005, pp. 1313-1330. doi:10.1590/S0103-50532005000800003 [5] O. J. Alamu, M. A. Waheed and S. O. Jekayinfa, “Alkali- Catalysed Laboratory Production and Testing of Bio- diesel from Nigerian Palm Kernel Oil,” Agricultural In- ternational: CIGR Electonic Journal, Vol. IX, 2007, pp. 1-10. [6] S. C. A. de Almeida, C. R. Belchoir, M. V. G. Nasci- mento, L. dos S. R. Vieira and G. Fluery, “Performance of a Diesel Generator Fuelled with Palm Oil,” Fuel, Vol. 81, No. 16, 2000, pp. 2097-2102. [7] M. I. Zubir and S. Y. Chin, “Kinetics of Modified Zirco- nia-Catalyzed Heterogeneous Esterification Reaction for Biodiesel Production,” Journal of Applied Science, Vol. 10, No. 21, 2010, pp. 2584-2589. doi:10.3923/jas.2010.2584.2589 [8] U. Schuchardt, R. Sercheli and R. M. Vargas, “Trans- esterification of Vegetable Oils: A Review,” Journal of Brazilian Chemical Society, Vol. 9, No. 3, 1998, pp. 199- 210. doi:10.1590/S0103-50531998000300002 [9] B. K. Barnwal and M. P. Sharma, “Prospects of Biodiesel Production from Vegetable Oils in India,” Renewable and Sustainable Energy Reviews, Vol. 9, No. 4, 2005, pp. 363- 378. doi:10.1016/j.rser.2004.05.007 [10] L. Meher, S. Vidya and S. Naik, “Technical Aspects of Biodiesel Production by Transesterification—A Review,” Renewable and Sustainable Energy Reviews, Vol. 10, No. 3, 2006, pp. 248-268. doi:10.1016/j.rser.2004.09.002 [11] A. R. Srivastava and R. Prasad, “Triglycerides-Based Diesel Fuels,” Renewable and Sustainable Energy Re- views, Vol. 4, No. 2, 2000, pp. 111-133. doi:10.1016/S1364-0321(99)00013-1 [12] U. Rashid and F. Anwar “Production of Biodiesel through Optimized Alkaline-Catalyzed Transesterification of Rape- seed Oil,” Fuel, Vol. 87, No. 3, 2008, pp. 265-273. [13] N. Jaya and K. Etihbrajulu, “Kinetic Modelling of Trans- esterification Reaction for Biodiesel Production Using Heterogeneous Catalyst,” International Journal of Engi- neering, Science and Technology, Vol. 3, No. 4, 2011, pp. Copyright © 2012 SciRes. ACES  O. A. AWORANTI ET AL. 452 3463-3466. [14] Y. Shimada, H. Watanabe, A. Sugihara and Y. Tominaga, “Enzymatic Alcoholysis for Biodiesel Fuel Production and Application of the Reaction to Oil Processing,” Jour- nal of Molecular Catalysis B: Enzyme, Vol. 17, No. 3-5, 2002, pp. 133-142. doi:10.1016/S1381-1177(02)00020-6 [15] Y. Zhang, M. A. Dube, D. D. Mclean and M. Kates, “Biodiesel Production from Waste Cooking Oil: 1. Proc- ess Design and Technological Assessment,” Bioresource Technology, Vol. 89, No. 1, 2003, pp. 1-16. doi:10.1016/S0960-8524(03)00040-3 [16] D. Royon, M. Daz, G. Ellenrieder and S. Locatelli, “En- zymatic Production of Biodiesel from Cotton Seed Oil Using t-Butanol as a Solvent,” Bioresource Technology, Vol. 98, No. 3, 2007, pp. 648-653. doi:10.1016/j.biortech.2006.02.021 [17] A. Demirbas, “Biodiesel Fuels from Vegetable Oils via Catalytic and Noncatalytic Supercritical Alcohol Trans- esterification and Other Methods: A Survey,” Energy Conversion and Management, Vol. 44, No. 13, 2003, pp. 2093-2109. doi:10.1016/S0196-8904(02)00234-0 [18] R. D. Abigor, P. O. Uadia,T. A. Foglia, M. J. Hasa, K. C. Jones, E. Okpefa, J. U. Obibuzor and M. E. Bafor, “Li- pase Catalysed Production of Biodiesel Fuel from Some Nigerian Lauric Oils,” Biochemical Society Transactions, Vol. 28, No. 6, 2000, pp. 979-981. doi:10.1042/BST0280979 [19] Y. Watanabe, Y. Shimada, A. Sugihara and Y. Tominaga, “Conversion of Degummed Soybean Oil to Biodiesel Fuel with Immobilized Candida antarctica Lipase,” Jour- nal of Molecular Catalysis B: Enzyme, Vol. 17, No. 3-5, 2002, pp. 151-155. doi:10.1016/S1381-1177(02)00022-X [20] O. Kose, M. Tuter and H. A. Akosoy, “Immobilized Can- dida antarctica Lipase-Catalysed Alcoholysis of Cotton Seed Oil in a Solvent-Free Medium,” Bioresource Tech- nology, Vol. 83, No. 2, 2002, pp. 125-129. doi:10.1016/S0960-8524(01)00203-6 [21] S. Dmytoyshyn, A. Dalai, S. Chandhari, H. Mishra and M. Reaney, “Synthesis and Characterization of Vegetable Oil Derived Esters: Evaluation for Their Diesel Additional Properties,” Bioresou rce Technology, Vol. 92, No. 1, 2004, pp. 55-64. doi:10.1016/j.biortech.2003.07.009, [22] S. E. Agarry, A. O. Ajani, O. A. Aworanti and B. O. Solomon, “Alkali Catalyzed Production of Biodiesel Fuel from Nigerian Citrus Seeds Oil,” Proceedings of the 40th Annual Conference of Nigerian Society of Chemical En- gineers, Harnessing and Optimizing Nigeria’s Energy Resources in the New Decade, Port Harcourt, 16-18 No- vember 2010, pp. 145-155. [23] O. O. Fasina and Z. Colley, “Viscosity and Specific Heat of Vegetable Oils as a Function of Temperature: 35˚C to 180˚C,” International Journal of Food Properties, Vol. 11, No. 4, 2008, pp. 738-746. doi:10.1080/10942910701586273 [24] M. Dak, R. C. Verma and M. K. Jain, “Mathematical Models for Prediction of Heological Parameters of Pine- apple Juice,” International Journal Food Engineering, Vol. 4, No. 3, 2008, pp. 1-17. doi:10.2202/1556-3758.1285 [25] I. Stanciu, “A New Viscosity-Temperature Relationship for Vegetable Oil,” Journal of Petroleum Technology and Alternative Fuels, Vol. 3, No. 2, 2012, pp. 19-23. [26] J. Toth, Z. Simon, P. Medveczky, L. Gombos, B. Jelinek, L. Szilagyi, L. Graf and A. Malnasi-Csizmadia, “Site Di- rected Mutagenesis at Position 193 of Human Trypsin 4 Alters the Rate of Conformational Change during Activa- tion: Role of Local Internal Viscosity in Protein Dynam- ics,” Structural Functional Genetics, Vol. 67, No. 4, 2004, pp. 1119-1127. doi:10.1002/prot.21398 [27] C. Kapseu, G. J. Kayem, D. Balesdent and L. Schuf- fenecker, “Estimation of Dynamic Viscosities of Vegeta- ble Oils,” Journal of American Oil Chemical Society, Vol. 68, No. 2, 1991, pp. 128-133. doi:10.1007/BF02662333 [28] W. Lang, S. Sokhansanj and F. W. Sosulski, “Modelling the Temperature Dependence of Kinematic Viscosity for Refined Canola Oil,” Journal of American Oil Chemical Society, Vol. 69, No. 10, 1992, pp. 1054-1062. doi:10.1007/BF02541080 [29] J. F. Toro-Vazquez and R. Infante-Guerrero, “Regres- sional Models That Describe oil Absolute Viscosity,” Journal of American Oil Chemical Society, Vol. 70, No. 11, 1993, pp. 1115-1122. doi:10.1007/BF02632152 [30] M. Ahmad, S. Rashid, M. A. Khan, M. Zafar, S. Sultana and S. Gulzar, “Optimization of Base Catalysed Trans- esterification of Peanut Oil Biodiesel,” African Journal of Biotechnology, Vol. 8, No. 3, 2009, pp. 441-446. [31] Z. J. Predojevic and B. D. Skrbic, “Alkali Catalysed Pro- duction of Biodiesel from Waste Frying Oil,” Journal of Serbian Chemical Society, Vol. 74, No. 8-9, 2009, pp. 993-1007. doi:10.2298/JSC0909993P [32] E. A. Ajav and O. A. Akingbehin, “A Study of Some Fuel Properties of Local Ethanol Blended with Diesel Fuel,” Agricultural Engineering International: The CIGR Jour- nal of Scientific Research and Development, Vol. IV, 2002, pp. 1-9. Copyright © 2012 SciRes. ACES
|