R. N. PATIL ET AL. 1017
0
50000
100000
150000
200000
250000
0 2040608
Impedance @ (0.1 Hz)
Coating Thickness (µm)
0
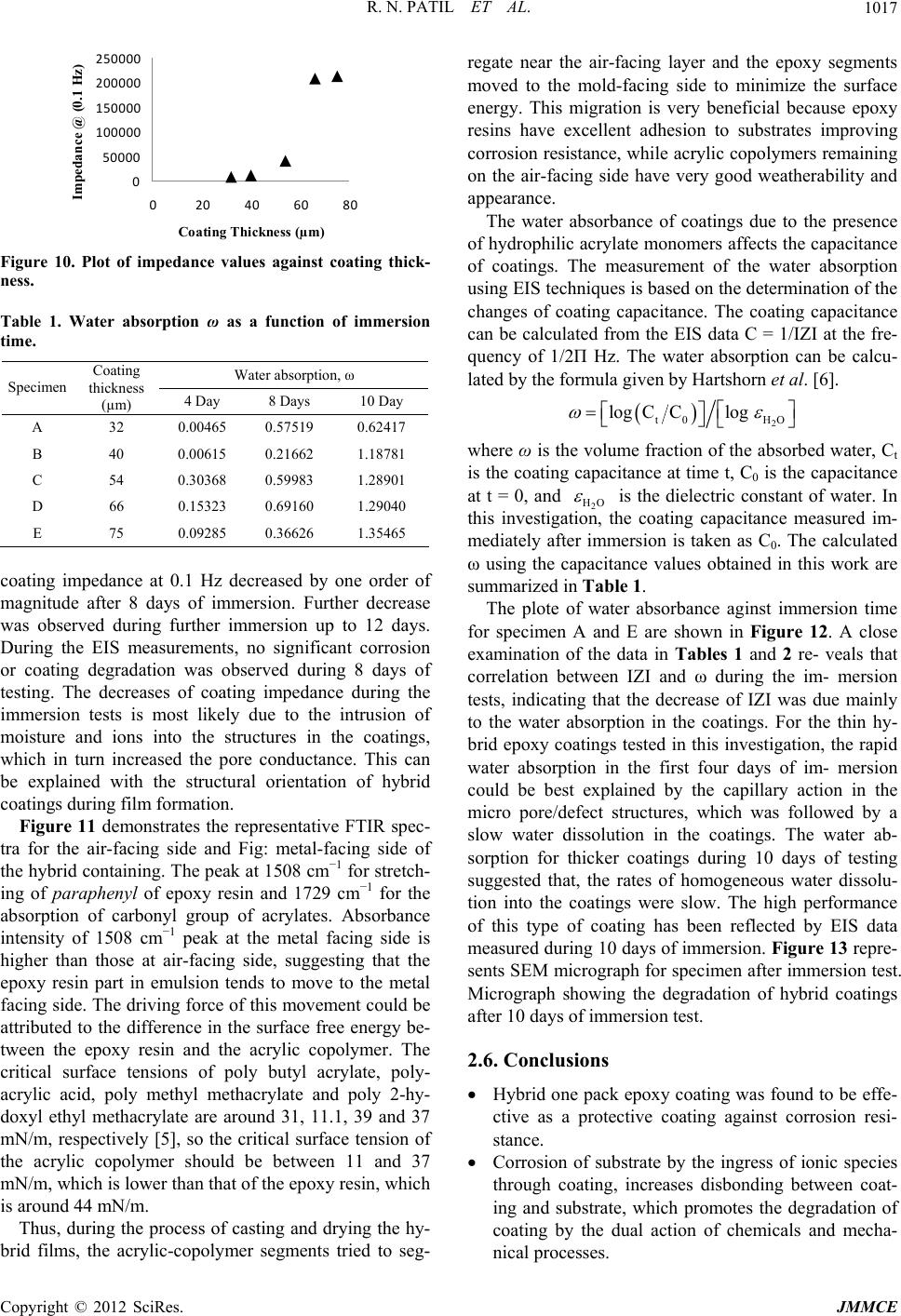
Figure 10. Plot of impedance values against coating thick-
ness.
Table 1. Water absorption ω as a function of immersion
time.
Water absorption, ω
Specimen
Coating
thickness
(µm) 4 Day 8 Days 10 Day
A 32 0.00465 0.57519 0.62417
B 40 0.00615 0.21662 1.18781
C 54 0.30368 0.59983 1.28901
D 66 0.15323 0.69160 1.29040
E 75 0.09285 0.36626 1.35465
coating impedance at 0.1 Hz decreased by one order of
magnitude after 8 days of immersion. Further decrease
was observed during further immersion up to 12 days.
During the EIS measurements, no significant corrosion
or coating degradation was observed during 8 days of
testing. The decreases of coating impedance during the
immersion tests is most likely due to the intrusion of
moisture and ions into the structures in the coatings,
which in turn increased the pore conductance. This can
be explained with the structural orientation of hybrid
coatings during film formation.
Figure 11 demonstrates the representative FTIR spec-
tra for the air-facing side and Fig: metal-facing side of
the hybrid containing. The peak at 1508 cm−1 for stretch-
ing of paraphenyl of epoxy resin and 1729 cm−1 for the
absorption of carbonyl group of acrylates. Absorbance
intensity of 1508 cm−1 peak at the metal facing side is
higher than those at air-facing side, suggesting that the
epoxy resin part in emulsion tends to move to the metal
facing side. The driving force of this movement could be
attributed to the difference in the surface free energy be-
tween the epoxy resin and the acrylic copolymer. The
critical surface tensions of poly butyl acrylate, poly-
acrylic acid, poly methyl methacrylate and poly 2-hy-
doxyl ethyl methacrylate are around 31, 11.1, 39 and 37
mN/m, respectively [5], so the critical surface tension of
the acrylic copolymer should be between 11 and 37
mN/m, which is lower than that of the epoxy resin, which
is around 44 mN/m.
Thus, during the process of casting and drying the hy-
brid films, the acrylic-copolymer segments tried to seg-
regate near the air-facing layer and the epoxy segments
moved to the mold-facing side to minimize the surface
energy. This migration is very beneficial because epoxy
resins have excellent adhesion to substrates improving
corrosion resistance, while acrylic copolymers remaining
on the air-facing side have very good weatherability and
appearance.
The water absorbance of coatings due to the presence
of hydrophilic acrylate monomers affects the capacitance
of coatings. The measurement of the water absorption
using EIS techniques is based on the determination of the
changes of coating capacitance. The coating capacitance
can be calculated from the EIS data C = 1/IZI at the fre-
quency of 1/2Π Hz. The water absorption can be calcu-
lated by the formula given by Hartshorn et al. [6].
2
t0 HO
logCClog
where ω is the volume fraction of the absorbed water, Ct
is the coating capacitance at time t, C0 is the capacitance
at t = 0, and 2
HO
is the dielectric constant of water. In
this investigation, the coating capacitance measured im-
mediately after immersion is taken as C0. The calculated
ω using the capacitance values obtained in this work are
summarized in Table 1.
The plote of water absorbance aginst immersion time
for specimen A and E are shown in Figure 12. A close
examination of the data in Tables 1 and 2 re- veals that
correlation between IZI and ω during the im- mersion
tests, indicating that the decrease of IZI was due mainly
to the water absorption in the coatings. For the thin hy-
brid epoxy coatings tested in this investigation, the rapid
water absorption in the first four days of im- mersion
could be best explained by the capillary action in the
micro pore/defect structures, which was followed by a
slow water dissolution in the coatings. The water ab-
sorption for thicker coatings during 10 days of testing
suggested that, the rates of homogeneous water dissolu-
tion into the coatings were slow. The high performance
of this type of coating has been reflected by EIS data
measured during 10 days of immersion. Figure 13 repre-
sents SEM micrograph for specimen after immersion test.
Micrograph showing the degradation of hybrid coatings
after 10 days of immersion test.
2.6. Conclusions
Hybrid one pack epoxy coating was found to be effe-
ctive as a protective coating against corrosion resi-
stance.
Corrosion of substrate by the ingress of ionic species
through coating, increases disbonding between coat-
ing and substrate, which promotes the degradation of
coating by the dual action of chemicals and mecha-
nical processes.
Copyright © 2012 SciRes. JMMCE