P. KANCHANA, C. SEKAR 987
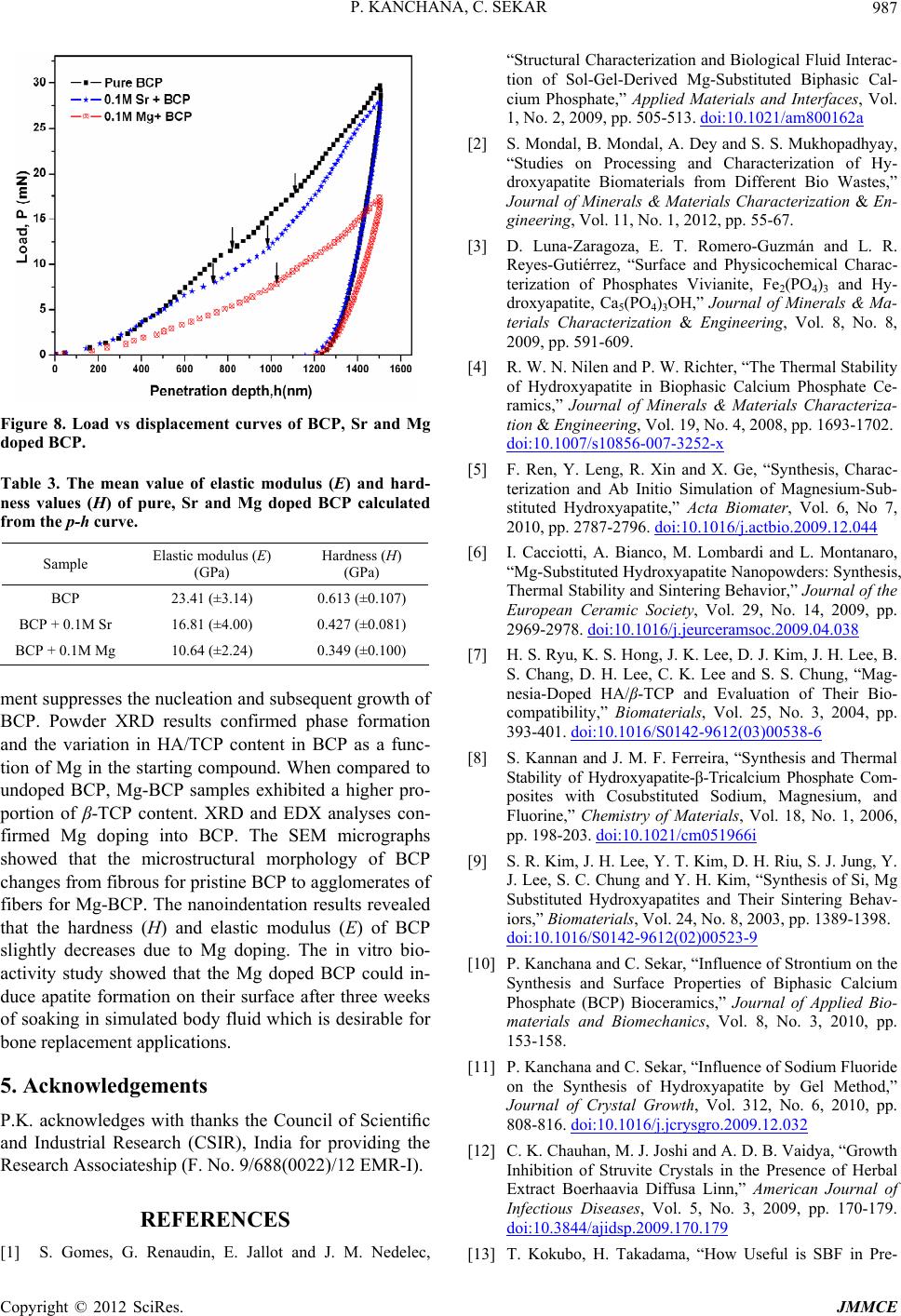
Figure 8. Load vs displacement curves of BCP, Sr and Mg
doped BCP.
Table 3. The mean value of elastic modulus (E) and hard-
ness values (H) of pure, Sr and Mg doped BCP calculated
from the p-h curve.
Sample Elastic modulus (E)
(GPa)
Hardness (H)
(GPa)
BCP 23.41 (±3.14) 0.613 (±0.107)
BCP + 0.1M Sr 16.81 (±4.00) 0.427 (±0.081)
BCP + 0.1M Mg 10.64 (±2.24) 0.349 (±0.100)
ment suppresses the nucleation and subsequent growth of
BCP. Powder XRD results confirmed phase formation
and the variation in HA/TCP content in BCP as a func-
tion of Mg in the starting compound. When compared to
undoped BCP, Mg-BCP samples exhibited a higher pro-
portion of β-TCP content. XRD and EDX analyses con-
firmed Mg doping into BCP. The SEM micrographs
showed that the microstructural morphology of BCP
changes from fibrous for pristine BCP to agglomerates of
fibers for Mg-BCP. The nanoindentation results revealed
that the hardness (H) and elastic modulus (E) of BCP
slightly decreases due to Mg doping. The in vitro bio-
activity study showed that the Mg doped BCP could in-
duce apatite formation on their surface after three weeks
of soaking in simulated body fluid which is desirable for
bone replacement applications.
5. Acknowledgements
P.K. acknowledges with thanks the Council of Scientific
and Industrial Research (CSIR), India for providing the
Research Associateship (F. No. 9/688(0022)/12 EMR-I).
REFERENCES
[1] S. Gomes, G. Renaudin, E. Jallot and J. M. Nedelec,
“Structural Characterization and Biological Fluid Interac-
tion of Sol-Gel-Derived Mg-Substituted Biphasic Cal-
cium Phosphate,” Applied Materials and Interfaces, Vol.
1, No. 2, 2009, pp. 505-513. doi:10.1021/am800162a
[2] S. Mondal, B. Mondal, A. Dey and S. S. Mukhopadhyay,
“Studies on Processing and Characterization of Hy-
droxyapatite Biomaterials from Different Bio Wastes,”
Journal of Minerals & Materials Characterization & En-
gineering, Vol. 11, No. 1, 2012, pp. 55-67.
[3] D. Luna-Zaragoza, E. T. Romero-Guzmán and L. R.
Reyes-Gutiérrez, “Surface and Physicochemical Charac-
terization of Phosphates Vivianite, Fe2(PO4)3 and Hy-
droxyapatite, Ca5(PO4)3OH,” Journal of Minerals & Ma-
terials Characterization & Engineering, Vol. 8, No. 8,
2009, pp. 591-609.
[4] R. W. N. Nilen and P. W. Richter, “The Thermal Stability
of Hydroxyapatite in Biophasic Calcium Phosphate Ce-
ramics,” Journal of Minerals & Materials Characteriza-
tion & Engineering, Vol. 19, No. 4, 2008, pp. 1693-1702.
doi:10.1007/s10856-007-3252-x
[5] F. Ren, Y. Leng, R. Xin and X. Ge, “Synthesis, Charac-
terization and Ab Initio Simulation of Magnesium-Sub-
stituted Hydroxyapatite,” Acta Biomater, Vol. 6, No 7,
2010, pp. 2787-2796. doi:10.1016/j.actbio.2009.12.044
[6] I. Cacciotti, A. Bianco, M. Lombardi and L. Montanaro,
“Mg-Substituted Hydroxyapatite Nanopowders: Synthesis,
Thermal Stability and Sintering Behavior,” Journal of the
European Ceramic Society, Vol. 29, No. 14, 2009, pp.
2969-2978. doi:10.1016/j.jeurceramsoc.2009.04.038
[7] H. S. Ryu, K. S. Hong, J. K. Lee, D. J. Kim, J. H. Lee, B.
S. Chang, D. H. Lee, C. K. Lee and S. S. Chung, “Mag-
nesia-Doped HA/β-TCP and Evaluation of Their Bio-
compatibility,” Biomaterials, Vol. 25, No. 3, 2004, pp.
393-401. doi:10.1016/S0142-9612(03)00538-6
[8] S. Kannan and J. M. F. Ferreira, “Synthesis and Thermal
Stability of Hydroxyapatite-β-Tricalcium Phosphate Com-
posites with Cosubstituted Sodium, Magnesium, and
Fluorine,” Chemistry of Materials, Vol. 18, No. 1, 2006,
pp. 198-203. doi:10.1021/cm051966i
[9] S. R. Kim, J. H. Lee, Y. T. Kim, D. H. Riu, S. J. Jung, Y.
J. Lee, S. C. Chung and Y. H. Kim, “Synthesis of Si, Mg
Substituted Hydroxyapatites and Their Sintering Behav-
iors,” Biomaterials, Vol. 24, No. 8, 2003, pp. 1389-1398.
doi:10.1016/S0142-9612(02)00523-9
[10] P. Kanchana and C. Sekar, “Influence of Strontium on the
Synthesis and Surface Properties of Biphasic Calcium
Phosphate (BCP) Bioceramics,” Journal of Applied Bio-
materials and Biomechanics, Vol. 8, No. 3, 2010, pp.
153-158.
[11] P. Kanchana and C. Sekar, “Influence of Sodium Fluoride
on the Synthesis of Hydroxyapatite by Gel Method,”
Journal of Crystal Growth, Vol. 312, No. 6, 2010, pp.
808-816. doi:10.1016/j.jcrysgro.2009.12.032
[12] C. K. Chauhan, M. J. Joshi and A. D. B. Vaidya, “Growth
Inhibition of Struvite Crystals in the Presence of Herbal
Extract Boerhaavia Diffusa Linn,” American Journal of
Infectious Diseases, Vol. 5, No. 3, 2009, pp. 170-179.
doi:10.3844/ajidsp.2009.170.179
[13] T. Kokubo, H. Takadama, “How Useful is SBF in Pre-
Copyright © 2012 SciRes. JMMCE