 Open Journal of Pathology, 2012, 2, 133-139 Published Online October 2012 (http://www.SciRP.org/journal/ojpathology) http://dx.doi.org/10.4236/ojpathology.2012.24024 Copyright © 2012 SciRes. OJPathology 1 Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis Mónica Belinda Romero-Guadarrama1*, María Esther Gutiérrez Díaz-Ceballos1, Fiacro Jiménez-Ponce2, Samantha Thingen-Velarde3 1Unit Pathology Hospital General de México, Medicine School, Autonomous University of Mexico, Mexico City , Mexico; 2Neurosur- gery Unit Hospital General de México, Mexico City, Mexico; 3Hemathology Service, Hospital General de México, Mexico City, Mexico. Email: *monicaromero@att.net.mx Received June 14th, 2012; revised July 12th, 2012; accepted July 23rd, 2012 ABSTRACT Background: Diffuse large B-cell lymphomas of the central nervous system (DLBCL CNS) represent less than 1% of all lymphomas and between 2% and 3% of all cerebral tumors. They occur in adults of 60 years of age or more. The objective of this work is to describe the clinical-pathological characteristics, the immunophenotype and the differential diagnosis. Clinical Case: From the files of the surgical pathology unit we found four cases of primary diffuse large B cell lymphoma of the central nervous system in a 6-year period. Three corresponded to women over 47 years of age and the other to a 42-year-old man. The time of evolution was between 2 and 4 months. The symptoms were headache, blurred vision, hemiparesis, and seizures. Localization was in the pineal region, the frontal, parietal regions, and the right thalamus. Morphologically, large lymphoid cells with a diffuse growth pattern and necrosis were observed. Im- munohistochemical markers, such as CD 20 and bcl2 were positive, one was positive to CD3. Expression of bcl6 and CD 10 was positive in one case, and MUM-1 was positive in three cases. All the cases were negative for Epstein-Barr virus. Conclusions: The diffuse large-B cell lymphoma of the central nervous system is rare. Its average age of presen- tation is at 60 years or older. The localization is in the pineal, frontal, parietal and thalamic regions. Three cases were originated by activated B lymphocyte (MUM-1 expression) and other from the Germinal Center (GC) (CD 10 expres- sion). The clinical course was bad. The four patients died shortly after the diagnosis. Keywords: Primary Lymphoma; Central Nervous System 1. Introduction Primary lymphomas of the central nervous system are immunophenotype B lymphomas with an aggressive clinical course and, in general, correspond to diffuse large B cell lymphomas and Burkitt’s lymphoma. Rare cases of small lymphocyte lymphomas have been re- ported [1]. The average age of presentation in non-immunocom- promised patients is around 60 years, when occurring in patients with HIV syndrome the age of presentation is 5 to 10 years earlier. Symptoms depend on the anatomical location, although they are frequently located in the cerebral hemispheres, around 60% are found in the su- pratentorial area. [2] Symptoms are focal and/or are caused by the increase in intracranial pressure that can be rapidly progressing. Radiological studies such as MRI (Magnetic Reso- nance Image) or CAT (Computed Axial Tomography) scan of the brain reveal heterogeneous lesions with signs of central necrosis; in 20% to 40% of cases, multiple le- sions can be observed. Diagnosis is histopathological and tissue can be obtained by either stereotactic biopsy or craniotomy. Most of these lymphomas present cells that resemble blasts, with a diffuse infiltration pattern and frequently, cells are distributed around the blood vessels, with a concentric pattern accompanied by an increase in re- ticulin fibers. Most of these tumors can be classified as centroblastic. They express markers for B cells, such as CD 20, CD 22, or CD 79ª, and an important percentage (89%) ex- press MUM-1. Prognosis is poor and differential diagnosis must be made from other tumors of the central nervous system. The objective of this study is to present the clinicopa- thological, immunophenotype characteristics and the dif- *Corresponding a uthor. 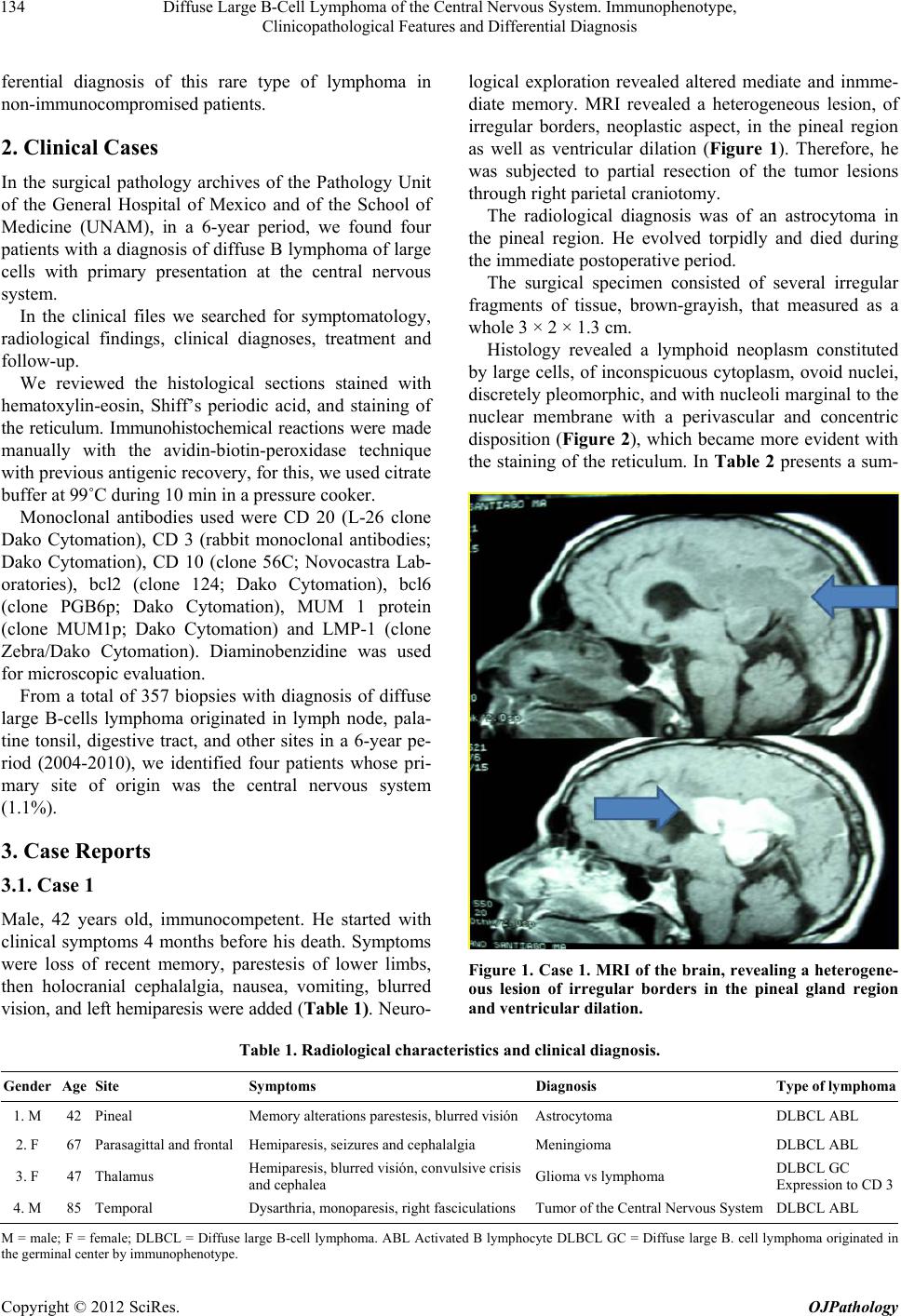 Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis 134 ferential diagnosis of this rare type of lymphoma in non-immunocompromised patients. 2. Clinical Cases In the surgical pathology archives of the Pathology Unit of the General Hospital of Mexico and of the School of Medicine (UNAM), in a 6-year period, we found four patients with a diagnosis of diffuse B lymphoma of large cells with primary presentation at the central nervous system. In the clinical files we searched for symptomatology, radiological findings, clinical diagnoses, treatment and follow-up. We reviewed the histological sections stained with hematoxylin-eosin, Shiff’s periodic acid, and staining of the reticulum. Immunohistochemical reactions were made manually with the avidin-biotin-peroxidase technique with previous antig enic recov ery, fo r this, we us ed citrate buffer at 99˚C during 10 min in a pressure cooker. Monoclonal antibodies used were CD 20 (L-26 clone Dako Cytomation), CD 3 (rabbit monoclonal antibodies; Dako Cytomation), CD 10 (clone 56C; Novocastra Lab- oratories), bcl2 (clone 124; Dako Cytomation), bcl6 (clone PGB6p; Dako Cytomation), MUM 1 protein (clone MUM1p; Dako Cytomation) and LMP-1 (clone Zebra/Dako Cytomation). Diaminobenzidine was used for microscopic evaluation. From a total of 357 biopsies with diagnosis of diffuse large B-cells lymphoma originated in lymph node, pala- tine tonsil, digestive tract, and other sites in a 6-year pe- riod (2004-2010), we identified four patients whose pri- mary site of origin was the central nervous system (1.1%). 3. Case Reports 3.1. Case 1 Male, 42 years old, immunocompetent. He started with clinical symptoms 4 months before his death. Symptoms were loss of recent memory, parestesis of lower limbs, then holocranial cephalalgia, nausea, vomiting, blurred vision, and left hemiparesis were added (Table 1). Ne uro- logical exploration revealed altered mediate and inmme- diate memory. MRI revealed a heterogeneous lesion, of irregular borders, neoplastic aspect, in the pineal region as well as ventricular dilation (Figure 1). Therefore, he was subjected to partial resection of the tumor lesions through right parietal craniotomy. The radiological diagnosis was of an astrocytoma in the pineal region. He evolved torpidly and died during the immediate postoperative period. The surgical specimen consisted of several irregular fragments of tissue, brown-grayish, that measured as a whole 3 × 2 × 1.3 cm. Histology revealed a lymphoid neoplasm constituted by large cells, of inconspicuous cytoplasm, ovoid nuclei, discretely pleomorphic, and with nucleoli margin al to the nuclear membrane with a perivascular and concentric disposition (Figure 2), which became more evident with the staining of the reticulum. In Table 2 presents a sum- Figure 1. Case 1. MRI of the brain, revealing a heter ogene- ous lesion of irregular borders in the pineal gland region and ventricular dilation. Table 1. Radiological characteristics and clinical diagnosis. Gender Age Site Symptoms Diagnosis Type of lymphoma 1. M 42 Pineal Memory alterations parestesis, blurre d visión Astrocytoma DLBCL ABL 2. F 67 Parasagittal and frontal Hemiparesis, seizures and cephalalgia Meningioma DLBCL ABL 3. F 47 Thalamus Hemiparesis, blurred visión, convulsive crisis and cephalea Glioma vs lym ph oma DLBCL GC Expression to CD 3 4. M 85 Temporal Dysarthria, monoparesis, right fasciculations Tumor of the Central Nervous System DLBCL ABL M = male; F = female; DLBCL = Diffuse large B-cell lymphoma. ABL Activated B lymphocyte DLBCL GC = Diffuse large B. cell lymphoma originated in the germin al center by immunophenotype. Copyright © 2012 SciRes. OJPathology 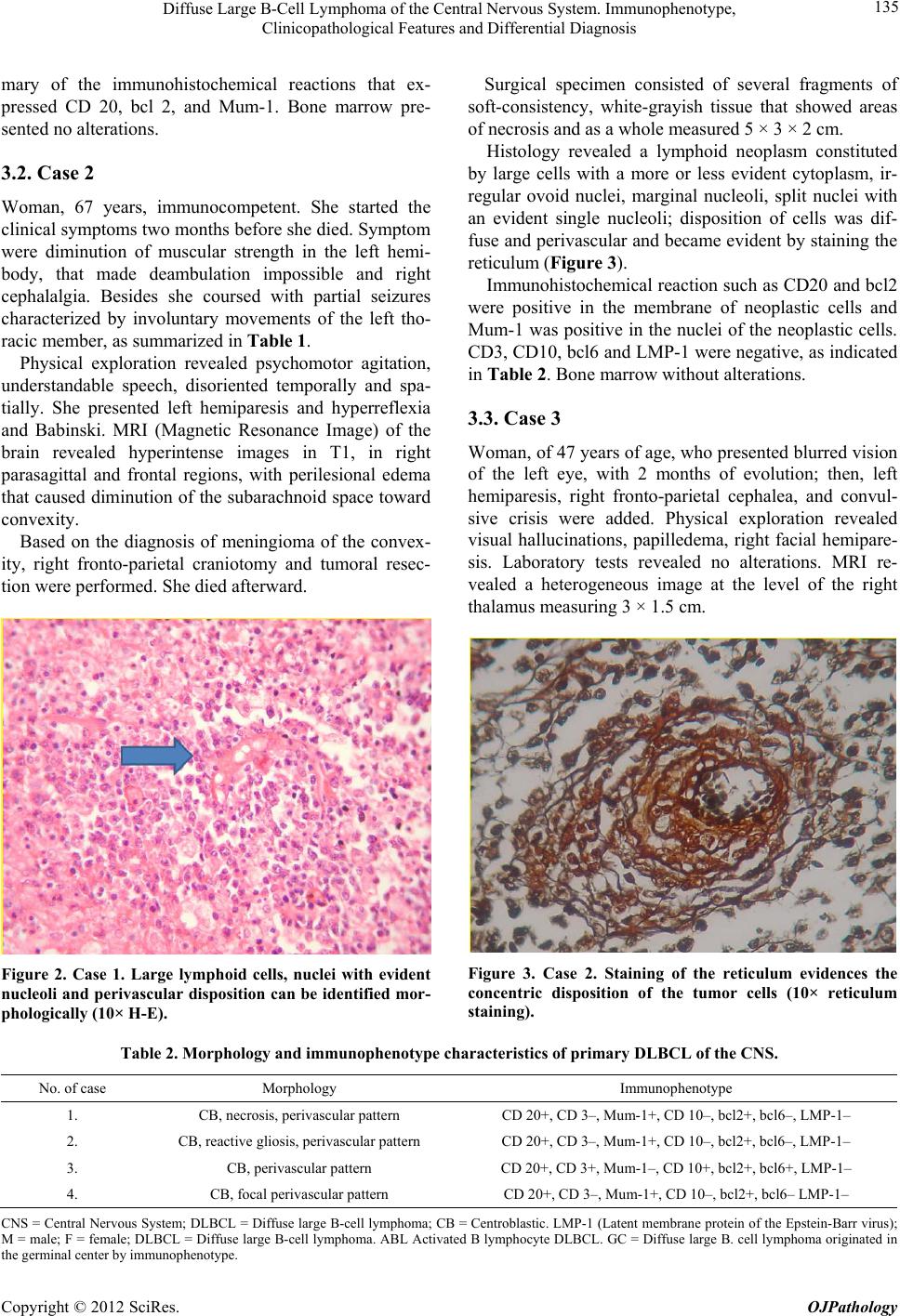 Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis 135 mary of the immunohistochemical reactions that ex- pressed CD 20, bcl 2, and Mum-1. Bone marrow pre- sented no alterations. 3.2. Case 2 Woman, 67 years, immunocompetent. She started the clinical symptoms two months before she died. Symptom were diminution of muscular strength in the left hemi- body, that made deambulation impossible and right cephalalgia. Besides she coursed with partial seizures characterized by involuntary movements of the left tho- racic member, as summarized in Table 1. Physical exploration revealed psychomotor agitation, understandable speech, disoriented temporally and spa- tially. She presented left hemiparesis and hyperreflexia and Babinski. MRI (Magnetic Resonance Image) of the brain revealed hyperintense images in T1, in right parasagittal and frontal regions, with perilesional edema that caused diminution of the subarachnoid space toward convexity. Based on the diagnosis of meningioma of the convex- ity, right fronto-parietal craniotomy and tumoral resec- tion were performed. She died afterward. Figure 2. Case 1. Large lymphoid cells, nuclei with evident nucleoli and perivascular disposition can be identified mor- phologically (10× H-E). Surgical specimen consisted of several fragments of soft-consistency, white-grayish tissue that showed areas of necrosis and as a whole measured 5 × 3 × 2 cm. Histology revealed a lymphoid neoplasm constituted by large cells with a more or less evident cytoplasm, ir- regular ovoid nuclei, marginal nucleoli, split nuclei with an evident single nucleoli; disposition of cells was dif- fuse and perivascular and became evid ent by staining the reticulum (Figure 3). Immunohistochemical reaction such as CD20 and bcl2 were positive in the membrane of neoplastic cells and Mum-1 was positive in the nuclei of the neoplastic cells. CD3, CD10, bcl6 and LMP-1 were negative, as indicated in Table 2. Bone marrow without alterations. 3.3. Case 3 Woman, of 47 years of age, who presented blurred vision of the left eye, with 2 months of evolution; then, left hemiparesis, right fronto-parietal cephalea, and convul- sive crisis were added. Physical exploration revealed visual hallucinations, papilledema, right facial hemipare- sis. Laboratory tests revealed no alterations. MRI re- vealed a heterogeneous image at the level of the right thalamus measuring 3 × 1.5 cm. Figure 3. Case 2. Staining of the reticulum evidences the concentric disposition of the tumor cells (10× reticulum staining). Table 2. Morphology and immunophenotype characteristics of primary DLBCL of the CNS. No. of case Morphology Immunophen otype 1. CB, necrosis, perivascular pattern CD 20+, CD 3–, Mum-1+, CD 10–, bcl2+, bcl6–, LMP-1– 2. CB, reactive gliosis, perivascular pattern CD 20+, CD 3–, Mum-1+, CD 10–, bcl2+, bcl6–, LMP-1– 3. CB, perivascular pattern CD 20+, CD 3+, Mum-1–, CD 10+, bcl2+, bcl6+, LMP-1– 4. CB, focal perivascular pattern CD 20+, CD 3–, Mum-1+, CD 10–, bcl2+, bcl6 – LMP-1– CNS = Central Nervous System; DLBCL = Diffuse large B-cell lymphoma; CB = Centroblastic. LMP-1 (Latent membrane protein of the Epstein-Barr virus); M = male; F = female; DLBCL = Diffuse large B-cell lymphoma. ABL Activated B lymphocyte DLBCL. GC = Diffuse large B. cell lymphoma originated in the germin al center by immunophenotype. Copyright © 2012 SciRes. OJPathology  Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis 136 Clinical diagnosis was of glioma vs basal nuclei lym- phoma, as indicated in Table 1. Cerebral biopsy was performed through stereotaxy. Morphologically it was similar to the two previous cases, however many of the neoplastic cells expressed CD 20 and CD 3 (Figures 4 and 5), as well as germinal center markers (CD 10 and bcl6) as shown in Table 2. Biopsy or the bone marrow revealed myelofibrosis grade I, without infiltration. The patient evolved well after high doses of intrave- nous methotrexate (10 doses of 25 mg) and rescue with folinic acid 24 h afterward. Clinical follow up was of 4 months, with persistence of cephalalgia and eventual death. Figure 4. Case 3. Anti-CD 30 immunohistochemical reac- tion that is positive in the cytoplasmic membrane of the neoplastic cells (40× Inmunoperoxidase). Figure 5. Case 3. Aberrant expression of CD 3 in neoplastic cells that are distributed around the blood vessels (40× In- munoperoxidase). 3.4. Case 4 Woman of 85 years, with antecedents of systemic arterial hypertension and chronic obstructive pulmonary disease. She started with clinical symptoms one month before diagnosis. Symptoms were dysarthria, right monoparesis and facial fasciculations of the same side. Simple CAT scan of the brain and MRI revealed left parietal tumor (Figure 6), craniotomy was performed based on a clini- cal-radiological diagnosis of tumor of the central nervous system, as indicated in Table 1. Partial resection of the tumor was performed through craniotomy. Several irregular fragments of tissue measuring 4 × 3 × 2 cm were received. Histology revealed a lymphoid tu- mor constituted by large cells, scarce cytoplasm, and irregular nuclei perivascularly distributed. Immnohisto- chemical markers were positive for CD 20, bcl2, and MUM-1 (Table 2). Post-operative period with clinical evolution of three months and eventual death. 4. Discussion Primary lymphomas of the central nervous system (PLCNS) are non-Hodgkin extranodal lymphomas that occur in the craniospinal axis and most are highly ma- lignant type B neoplasms [1,2]. They present at this site probably due to the tropism of lymphoid cells [3]. The biological mechanism of selective tropism is still un- known, and the genesis of this lymphoma in the central Figure 6. Case 4. MRI revealing a hyperintense homogene- ous image, at the fronto-parietal level. Copyright © 2012 SciRes. OJPathology  Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis 137 nervous system is not completely understood; a probable hypothesis is that the tumor cells or iginate in extracranial sites [4]. The immunophenotype and the presence of rearranged, somatically mutated immunoglobulin (IG) genes with evidence for ongoing mutation suggest PCNSL tumor cells are derived from a germinal centre (GC) exit B cell [5]. Tumor manifestation is often diffuse, multifocal and frequently affects the supratentorial cerebral parenchyma. They occur in all age groups, with an incidence peak in the fifth or sixth decade of life in patients without AIDS [6]. In the present study, in a period of 6 years, four pa- tients presented as primary site of the diffuse large B-cells lymphoma (DLBCL) the central nervous system, representing 1.1% of all the diffuse large cell lymphomas at diverse sites, including the lymph node, in our institu- tion. Average age was of 60 years, being more frequent in women, which agrees with the correspondin g literature of the American continent; other authors have found men to be more affected [7,8]. Clinical sympthomatology curred 2 to 4 months before the diagnosis, hence the start is fast with just a few months before diagnosis. In the series by Bataille et al., the most common symptoms were neurological focality (70%) and neuropsychiatric alterations (43%); [9] in the present study, these were cephalea, hemiparesis, visual alterations and convulsive crises. The site most affected is the frontal lobe; in this series location was variable presenting in the pineal region, frontal lobe, parasagittal region, temporal lobe, and in the thalamic region, as summarized in Table 1. In more than half the cases, le- sions are solitary (65%), although multifocal lesions can be observed (35%) [10,11]. The CAT scan reveals iso- dense and hyperintense lesions, and with MRI they ap- pear isointense and hypointense and can present per- ilesional edema and mass effect. In our patients, these changes in MRI were heterogeneous, without a pre- dominating pattern, lesions were localized in three, and one showed multifocal lesions. DLBCLs presenting in the CNS are generally intraparenchymatous and with periventricular affection, the latter is a very important piece of information for the differential diagnosis be- tween primary and secondary lymphomas, since the latter affect mainly the leptomeninges [12]. Morphologically, neoplastic cells are large, barely evident cytoplasm and nuclei that resemble centroblasts, as can be seen in Fig- ure 2, although they can exhibit an immunoblast mor- phology [1] with a diffuse growth pattern and perivascu lar disposition and concentric accentuation (Figure 3), tumor cells alternate with small reactive lymphocytes, macrophages, activated microglial cells, and reactive astrocytes [13]. They express pan-B markers such as CD 20 and CD 79ª (Figure 4), they can express bcl2 and CD 10, not related to t (14; 18) (q32; q21). Expression of bcl-6 has been associated with a favorable prognosis. However, strong expression of RF4/MUM-1 has been observed in approximately 90% of cases [14,15]. Our results agree with previously reported data in other series. DLBCLs in the SNC frequently contain complex cytogenetic anomalies, diagnosis is based on the combination of histology, immunophenotype, and mo- lecular clonality [16]. The aberrant expression of B-cell-associated antigens has been identified in benign proliferations of T cells and non-Hodgkin lymphomas with the immunophenotype T [17,18]. It is not rare that low-grade non-Hodgkin lym- phomas co-express T-cell-associated antigens, particu- larly CD 5 and CD 43 [19,20]. There are T markers, such as CD 5, that are expressed in DL BCL without any other specification in lymph node and in other variants like large cell intravascular lymphomas [21,22] Cases of B lymphomas with aberrant co-expression to T cell-asso- ciated antigens, such as CD2, CD 4, CD 7, CD 8, and CD 45 RO have been reported [23-25]. In a recent study, Wang et al. [26] found four cases of DLBCL with aber- rant expression to CD 3, in one of the cases, the lym- phoma occurred in the CNS in a 52-year-old woman; in the present study, expression of CD 3 was observed in a 47-year-old woman (Case 3) (Figure 5); the biological significance and the mechanism for the CD 3 expression in DLBCL is not known; hence, we believe that other pan B markers such as CD 79ª, CD 138, PAX 5, and Mum-1 must be determined or molecular biology tech- niques must be implemented for the correct determina- tion of the cell lineage of these unusual lymphomas. In the present series, three DLBCL in the SNC expressed Mum-1 and all expressed bcl-2 markers of bad prognosis; in a recent publication of 17 cases, expression of bcl2 and STAT-3 (Signal transducer and activator of trans- duction) was studied, emphasizing the low expression of this marker as a subtype of diffuse large B cell lym- phoma originated from activated lymphocytes [27]. Among the most significantly up-regulated genes in PCNSL were SPPI, TF, DDR1, TUBB2B, SERPINA3, S100B, and CA2. More than 460 expressed sequence tags were differentially expressed between PCNSL [27]. Regarding prognosis, several variables have been identi- fied that affect survival negatively such as: age over 60 years, high serum levels of lactic dehydrogenase, hyper- proteinorachia, and affectation of deep cerebral structures like periventricular regions, basal ganglia, brain stem, and cerebellum [28]. Differential diagnosis must be made with multiform glioblastoma, primary neuroectodermic tumor, metasta- ses of anaplastic carcinoma and melanoma. Immunohis- Copyright © 2012 SciRes. OJPathology  Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis 138 tochemistry with the follow monoclonal antibodies: cy- tokeratin, HMB 45, acid glial glycoprotein, epithelial membrane antigen and CD 99 are highly valuables for the correct classification of this type of neoplasia [1]. Regarding treatment, a better survival has been shown with the combination of two treatments (methotrexate and radiotherapy) at not too high doses to avoid or re- duce the neurological sequelae of these treatments [29]. In conclusion, we present the clinicopathological characteristics of four cases of DBCL in the CNS, three of them classified as originating from activated lympho- cyte B, and the other from the germinal center (GC) with aberrant expression of CD3 (pan-T marker). REFERENCES [1] A. C. Feller and J. Diebold, “Histopathology of Nodal and Extranodal Non-Hodgkin’s Lymphomas. Based on the WHO Classification,” 3rd Completely Revised and Updated Edition, Springer-Verlag, Berlin, Heidelberg, New York, 2004, pp. 330-334. [2] S. Swerdlow, E. Campo, L. N. Harris, S. E. Jaffe, A. S. Pileri, H. Stein, et al., “WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues,” 4th Edition, International Agency for Research of Cancer, Lyon, 2008, pp. 240-241. [3] T. Batchelor and J. S. Loeffler, “Primary CNS Lym- phoma,” Journal of Clinical Oncology, Vol. 24, No. 8, 2006, pp. 1281-1288. doi:10.1200/JCO.2005.04.8819 [4] L. Jiang, A. L. Marlow, J. S. Cooper, V. Ch. Roemeling, M. D. Menke, A. J. Copland, et al., “Selective Central Nervous System Tropism of Primary Central Nervous System Lymphoma,” International Journal of Clinical and Experimental Pathology, Vol. 3, No. 8, 2010, pp. 763-767. [5] M. Montesinos-Rongen, S. Reiner and D. Marina, “Pri- mary Lumphoma of the Central Nervous System: Just DLBCL or Not?” Blood, Vol. 113, No. 1, 2009, pp. 7-10. doi:10.1182/blood-2008-04-149005 [6] F. H. Hochberg and D. C. Mill er, “Primary Central Ne rv- ous System Lymphoma,” Journal of Neurosurgery, Vol. 68, 1998, pp. 835-853. doi:10.3171/jns.1988.68.6.0835 [7] T. M. Alécio, J. M. Alécio, P. H. Aguilar and R. Ramina, “Primary Central Nervous System Lymphomas in Im- munocompetents Patients,” Neurocirugia, Vol. 17, 2006, pp. 46-53. [8] M. Schabet, “Epidemiology of Primary CNS Lym- phoma,” Journal of Neuro-Oncology, Vol. 43, No. 3, 1999, pp. 199-201. doi:10.1023/A:1006290032052 [9] B. Bataille, V. Delwail, E. Menet, P. V. Marcq, P. In- grand, M. Wager, et al., “Primary Intracerebral Malignant Lymphoma. Report of 248 Cases,” Journal of Neuro- Oncology, Vol. 92, No. 2, 2000, pp. 261-266. [10] W. Kuker, T. Nagele, A. Korfel, S. Hecky, E. Thiel, M. Bamberg, et al., “Primary Central Nervous System Lym- phomas (PCNSL) MRI Features at Presentation in 100 Patients,” Journal of Neuro-Oncology, Vol. 72, No. 2, 2005, pp. 169-177. doi:10.1007/s11060-004-3390-7 [11] A. Coulon, F. Lafitte, K. Hoang-Xuan, N. Martin-Du- verneuil, K. Mokhtari, J. Blustajn, et al., “Radio-Graphic Findings in 37 Patients of Primary CNS Lymphoma in Immunocompetent Patients,” European Radiology, Vol. 12, 2002, pp. 329-340. doi:10.1007/s003300101037 [12] N. Erdag, R. M. Bhorade, R. A. Alberico, N. Yousuf and M. R. Patel, “Primary Lymphoma of the Central Nervous System: Typical and Atypical CT and MR Imaging Ap- pearances,” American Journal of Roentgenology, Vol. 176, No. 5, 2001, pp. 1319-1326. [13] G. Guinto, I. Felix, N. Arechiga, V. Arteaga and K. Kovacs, “PCNSL in Immunocompetent Patients,” His- tology and Histopathology, Vol. 19, 2004, pp. 963-972. [14] K. M. Braaten, R. A. Betensky, L. de Leval, Y. Okada, F. H. Hochberg, D. N. Lous, et al., “BCL-6 Expression Pre- dicts Improved Survival in Patients with Primary Central Nervous System Lymphoma,” Clinical Cancer Research, Vol. 9, No. 3, 2003, pp. 1063-1069. [15] S. Camilleri-Broet, E. Criniere, P. Broet, V. Delwail, K. Mokhtari, A. Moreau, et al., “A Uniform Activated B-Cell-Like Immunophenotype Might Explain the Poor Prognosis of Primary Central Nervous System Lympho- mas Analysis of 83 Cases,” Blood, Vol. 107, No. 1, 2006, pp. 190-196. doi:10.1182/blood-2005-03-1024 [16] C. H. Lin, K. T. Kuo, S. S. Chuang, S. H. Kuo, J. H. Chang, K. C. Chang, et al., “Comparison of the Expres- sion and Prognostic Significance of Differential Markers between Diffuse Large B-Cell Lymphoma of Central Nervous System Origin and Peripheral Nodal Origin,” Clinical Cancer Research, Vol. 12, 2006, pp. 1152-1156. doi:10.1158/1078-0432.CCR-05-1699 [17] K. M. Algino, R. W. Thomason, D. E. King, M. M. Mon- tiel and F. E. Craig, “CD 20 (Pan-B Cell Antigen) Ex- pression on Bone Marrow Derived T Cells,” American Journal of Clinical Pathology, Vol. 106, No. 1, 1996, pp. 76-81. [18] S. J. Hamilton-Dutoit, G. Pallesen, “B Cell Associated Monoclonal Antibody L 26 May Occasionally Label T Cell Lymphomas,” APMIS, Vol. 97, No. 11, 1989, pp. 1033-1036. doi:10.1111/j.1699-0463.1989.tb00514.x [19] J. Linder, L. Y. Ye, D. S. Harrington, J. O. Armitage, D. D. Weissenburger, “Monoclonal Antibodies Marking B- Cell Non Hodgkin’s Lymphoma in Paraffin Embedded Tissues,” Mo dern Pat holog y, Vol. 1, No. 1, 1988, pp. 29- 34. [20] D. A. Arber and L. M. Weiss, “CD 43: A Review,” Ap- plied Immunohistochemistry, Vol. 1, 1993, pp. 88-96. [21] D. A. Arber and L. M. Weiss, “CD 5: A Review,” Ap- plied Immunohistochemistry, Vol. 3, 1995, pp. 1-22. [22] H. S. Khalidi, R. K. Brynes, P. Borwne, C. H. Koo, H. Battifora and L. J. Medeiros, “Intravascular Large B-Cell Lymphoma: The CD 5 Antigen Is Expressed by a Subset of Cases,” Modern Pathology, Vol. 11, No. 10, 1998, pp. 983-988. [23] P. J. Kurtin, K. S. Hobday, S. Ziesmer and B. L. Caron, Copyright © 2012 SciRes. OJPathology  Diffuse Large B-Cell Lymphoma of the Central Nervous System. Immunophenotype, Clinicopathological Features and Differential Diagnosis Copyright © 2012 SciRes. OJPathology 139 “Demonstration of Distinct Antigen Profiles of Small B-Cell Lymphomas by Paraffin Section Immunohisto- chemistry,” American Journal of Clinical Pathology, Vol. 112, No. 3, 1999, pp. 319-329. [24] T. Inaba, C. Shimazaki, T. Sumikuma, A. Okano, M. Hatsuse and A. Okamoto, “T-Cell Associated Antigen- Positive B-Cell Lymphoma,” Leukemia & Lymphoma, Vol. 42, No. 6, 2001, pp. 1161-1171. doi:10.3109/10428190109097741 [25] A. Matolcsy, A. Chadburn and D. M. Knowles, “De Novo CD 5 Positive and Richter’s Syndrome-Associated Dif- fuse Large B Cell Lymphomas Are Genotypically Dis- tinct,” American Journal of Pathology, Vol. 147, No. 1, 1995, pp. 207-216. [26] J. Wang, Ch. Chen, S. Lau, R. I. Raghavan, E. H. Rowsell, J. Said, et al., “CD-3 Positive Large B-Cell Lymphoma,” American Journal of Surgical Pathology, Vol. 33, No. 4, 2009, pp. 505-512. doi:10.1097/PAS.0b013e318185d231 [27] N. Vajpayee, J. Hussain, I. Tolocica, R. E. Hutchison and A. Gajra, “Expression of Signal Transducter and Activa- tor of Transcription 3 (STAT 3) in Primary Central Nervous System Diffuse Large B-Cell Lymphoma: A Retrospective Analysis of 17 Cases,” Journal of Neuro- Oncology, Vol. 100, No. 2, 2010, pp. 294-253. doi:10.1007/s11060-010-0188-7 [28] A. J. Ferreri, J. Y. Blay , M. Reni, F. Pasini, M. Spina, A. Ambrosetti, et al., “Pronostic Scoring System for Primary CNS Lymphomas: The International Extra-Nodal Study Group Experience,” Journal of Clinical Oncology, Vol. 21, No. 2, 2003, pp. 266-272. doi:10.1200/JCO.2003.09.139 [29] R. Yamanaka, K. Morii, Y. Shinbo, J. Homma, M. Sano, N. Tsuchiya, et al., “Results of Treatment of 112 Cases of Primary CNS Lymphoma,” Japanese Journal of Clinical Oncology, Vol. 38, No. 5, 2008, pp. 373-380. doi:10.1093/jjco/hyn027 Abbreviations DLBCLCNS = Diffuse Large B Cell Lymphoma of the Central Nervous System. HIV = Human Immunodeficiency Virus CNS = Central Nervous System DLBCL = Diffuse Large B-Cell Lymphoma CB = Centroblastic LMP-1 = Latent Membrane Protein of the Epstein-Barr Virus MRI = Magnetic Resonance Imagen CAT = Computarizade Axial Tomography M = Male F = Female DLBCL = Diffuse Large B-Cell Lymphoma. ABL = Activated B Lymphocyte DLBCL GC = Diffuse Large B. Cell Lymphoma Originated in the Germinal Center by Immunophenotype
|