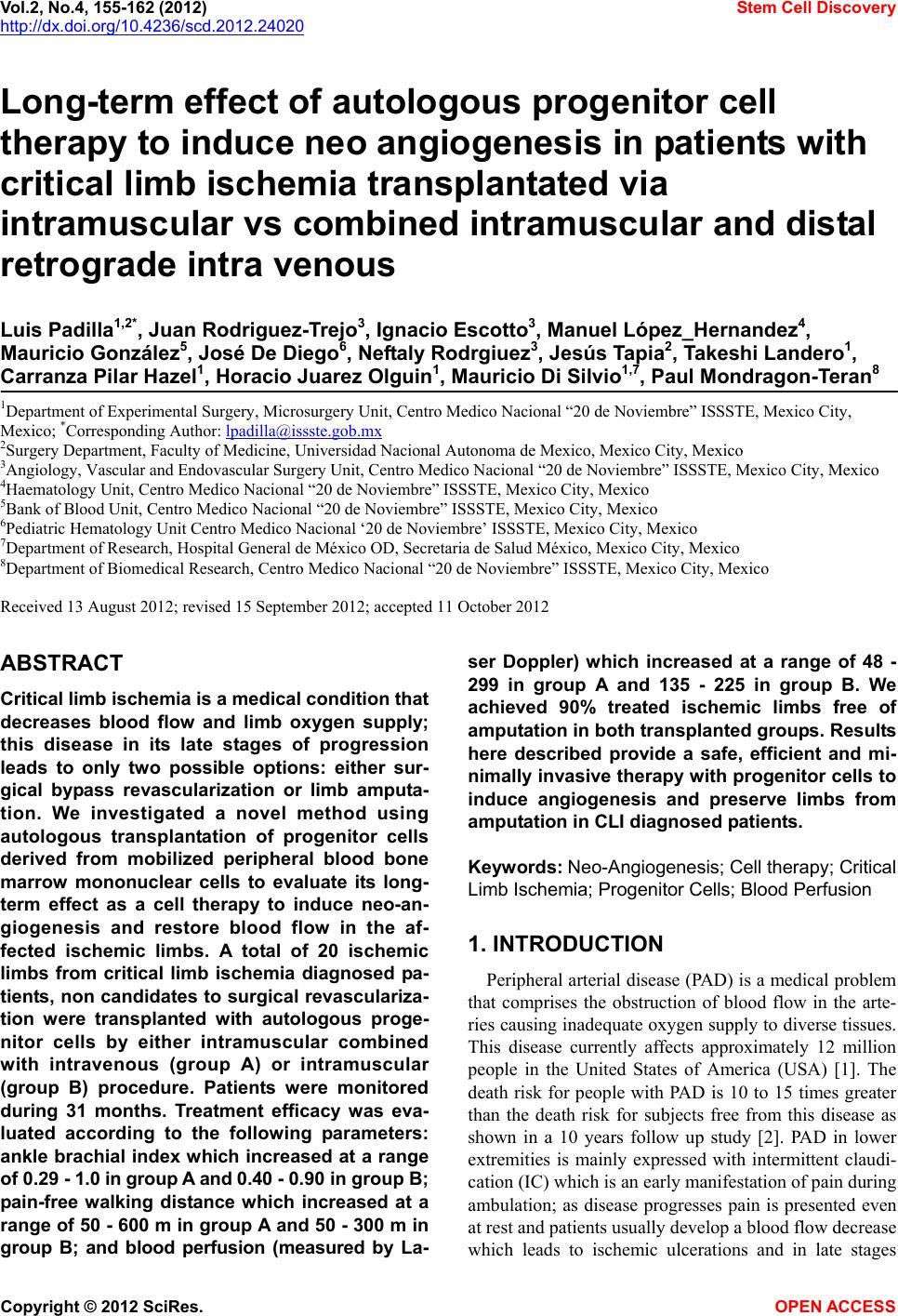
 Vol.2, No.4, 155-162 (2012) Stem Cell Discovery http://dx.doi.org/10.4236/scd.2012.24020 Long-term effect of autologous progenitor cell therapy to induce neo angiogenesis in patients with critical limb ischemia transplantated via intramuscular vs combined intramuscular and distal retrograde intra venous Luis Padilla1,2*, Juan Rodriguez-Trejo3, Ignacio Escotto3, Manuel López_Hernandez4, Mauricio González5, José De Diego6, Neftaly Rodrgiuez3, Jesús Tapia2, Takeshi Landero1, Carranza Pilar Hazel1, Horacio Juarez Olguin1, Mauricio Di Silvio1,7, Paul Mondragon-Teran8 1Department of Experimental Surgery, Microsurgery Unit, Centro Medico Nacional “20 de Noviembre” ISSSTE, Mexico City, Mexico; *Corresponding Author: lpadilla@issste.gob.mx 2Surgery Department, Faculty of Medicine, Universidad Nacional Autonoma de Mexico, Mexico City, Mexico 3Angiology, Vascular and Endovascular Surgery Unit, Centro Medico Nacional “20 de Noviembre” ISSSTE, Mexico City, Mexico 4Haematology Unit, Centro Medico Nacional “20 de Noviembre” ISSSTE, Mexico City, Mexico 5Bank of Blood Unit, Centro Medico Nacional “20 de Noviembre” ISSSTE, Mexico City, Mexico 6Pediatric Hematology Unit Centro Medico Nacional ‘20 de Noviembre’ ISSSTE, Mexico City, Mexico 7Department of Research, Hospital General de México OD, Secretaria de Salud México, Mexico City, Mexico 8Department of Biomedical Research, Centro Medico Nacional “20 de Noviembre” ISSSTE, Mexico City, Mexico Received 13 August 2012; revised 15 September 2012; accepted 11 October 2012 ABSTRACT Critical limb ischemia is a medical condition that decreases blood flow and limb oxygen supply; this disease in its late stages of progression leads to only two possible options: either sur- gical bypass revascularization or limb amputa- tion. We investigated a novel method using autologous transplantation of progenitor cells derived from mobilized peripheral blood bone marrow mononuclear cells to evaluate its long- term effect as a cell therapy to induce neo-an- giogenesis and restore blood flow in the af- fected ischemic limbs. A total of 20 ischemic limbs from critical limb ischemia diagnosed pa- tients, non candidates to surgical revasculariza- tion were transplanted with autologous proge- nitor cells by either intramuscular combined with intravenous (group A) or intramuscular (group B) procedure. Patients were monitored during 31 months. Treatment efficacy was eva- luated according to the following parameters: ankle brachial index which increased at a range of 0.29 - 1 . 0 i n g r ou p A a nd 0. 4 0 - 0.90 in group B; pain-free walking distance which increased at a range of 50 - 600 m in group A and 50 - 300 m in group B; and blood perfusion (measured by La- ser Doppler) which increased at a range of 48 - 299 in group A and 135 - 225 in group B. We achieved 90% treated ischemic limbs free of amputation in both transplanted groups. Results here described provide a safe, efficient and mi- nimally invasive therapy with progenitor cells to induce angiogenesis and preserve limbs from amput ation in CLI diagnosed patients. Keywords: Neo-Angiogenesis; Cell therapy; Critical Limb Ischemia; Progenitor Cells; Blood Perfusi on 1. INTRODUCTION Peripheral arterial disease (PAD) is a medical problem that comprises the obstruction of blood flow in the arte- ries causing inadequate oxygen supply to diverse tissues. This disease currently affects approximately 12 million people in the United States of America (USA) [1]. The death risk for people with PAD is 10 to 15 times greater than the death risk for subjects free from this disease as shown in a 10 years follow up study [2]. PAD in lower extremities is mainly expressed with intermittent claudi- cation (IC) which is an early manifestation of pain during ambulation; as disease progresses pain is presented even at rest and patients usually develop a blood flow decrease which leads to ischemic ulcerations and in late stages Copyright © 2012 SciRes. OPEN AC CESS  L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 156 gangrene requiring minor or major amputations, thus completing the natural story for critical limb ischemia (CLI) [3-5]. Diabetic patients are at a higher risk of de- veloping such CLI condition, since 30% of them will present it earlier in life, compared with non-diabetic population [6,7]; besides, PAD in diabetes mellitus dis- ease is more diffuse and severe, mainly affecting the lower extremities vasculature, while the non-diabetic patients with PAD have a higher aorto-iliac incidence [8,9]. CLI is a growing medical problem and has an es- timated incidence of 11% in the general population and 15% in adults over 55 years old; it also has been reported with an incidence of 500 to 1000 individuals per million each year [10]. The reported mortality rates due to CLI has been 20%, 35% 70% and up to 100% for 1, 2, 5 and 10 years respectively and at least 50% of patients will undergo major limb amputation within 6 to 12 months [11-13]. Current treatments for CLI patients include se- rum lipid levels reduction, antiplatelet (i.e. cilostasol) and antihypertensive drugs showing limited efficacy in CLI severe stages [14,15]. Although surgical revascu- larization remains as the most appropriate CLI therapy aimed to prevent limb loss, it is not suitable for at least 30% of patients because of extent of the disease and the lack of proper non damaged autologous vasculature; which leaves amputation as the only option if CLI has progressed beyond the point of salvage, vascular surgery is too risky or life expectancy is very low [16,17]. Inclu- iding only USA 100,000 major limb amputations are performed every year due to PAD with an annual cost of more than 13 billion US dollars [18]. Patients with CLI who are not eligible for revascularization have no effec- tive treatment option. Despite there is no Federal Drug Administration (FDA) approved therapy for these CLI patients, there has been several research groups reporting different strategies towards the establishment of pro- genitor cell therapies to allow the formation of new blood vessels (neo-angiogenesis) as a method to salvage ischemic limbs through autologous transplantation of progenitor cells derived from Bone Marrow-Mononu- clear Cells (BM-MNC) [18-23]. At least 40 research groups have obtained promising results with BM-MNC transplantation as progenitor cell therapy treatment for CLI patients, supporting evidence to establish this pro- cedure as an alternative to improve blood perfusion through neo-angiogenesis and to avoid amputation [3, 18]. To reach a maximum local cell concentration, Bartsch et al. [21], performed 13 intramuscular and intra-arterial transplantation of BM-MNC obtained from iliac crest bone marrow aspiration and purified through ficoll gra- dient technique, this procedure resulted in the improve- ment of the ankle brachial index (ABI); in contrast, the 12 patients in the control group with no cell transplant showed a statistically significant reduction of ABI and venous occlusion plethysmography at rest. Using a rat ischemic limb model we previously demonstrated the induction of effective neo-vascularization after bone marrow mononuclear cell (CD34+ and CD133+) trans- plantation into surgically induced fibrocollagenous tun- nels used as scaffolds to enhance cell survival and dif- ferentiation [24]. In a second experimental study, our group used dogs as an ischemic limb model confirming that transplantation of mobilized BM-MNC to peripheral blood through the use of Granulocyte Colony-Stimula- ting Factor (G-CSF) statistically significant increased angiogenesis as compared with cell transplant without G-CSF treatment [25]. Based on the evidence of neo-an- giogenesis as a result of mobilized BM-MNC autologous transplantation on these experimental models, we ob- tained the ethical and research institution committee ap- proval to start a clinical trial in humans for the progenitor cell therapy of CLI patients. In this report we analyze the efficacy of Mobilized BM-MNC transplantation com- paring combined procedure intramuscular and distal ret- rograde-intravenous (saphenous vein) transplantation (IM + IV) versus intramuscular (IM) standard transplan- tation procedure. 2. MATERIALS AND METHODS 2.1. Trial Profile The institutional board approved this prospective, con- trolled, randomized study; all enrolled patients provided written informed consent; all surgical procedures were conducted by senior expert surgeon (PL and RTJ). 2.2. Patient’s Inclusion Criteria Patients older than 18 years old presenting severe lower limb obstructive arterial disease classified as Fontaine stage IIb (Incapacitant intermittent claudica- tion), Fontaine stage III (pain at rest), Rutherford 4, with an ankle-brachial index (ABI) lower than 0.6, and who were non candidates to surgical bypass revascularization nor to endovascular procedure. 2.3. Patient’s Exclusion Criteria Patients with any neoplasia history in the last 10 years, patients with 1 year of life expectancy, patients with se- vere renal failure, severe malnutrition, or systemic chronic infectious disease at any clinical stage (Hepatitis B, C or HIV infection). The parameters to evaluate were procedure’s safety, treatment efficacy, ABI, blood perfusion and pain-free walking distance evolution. Patients of both experimental and control groups were monitored by measuring those Copyright © 2012 SciRes. OPEN AC CESS  L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 157 parameters at 0, 3, 12 months after progenitor cell trans- plant and at 18, 24 and 31 months after cell transplant therapy to evaluate the ratio of patients free of limb am- putation. 2.4. Study Design The study was designed as a randomized prospective controlled clinical trial and its main goal was to improve blood perfusion through neo-angiogenesis and to salvage patient’s ischemic limbs. 20 critical ischemic affected limbs (Fontaine II-b, Fontaine III and Rutherford 4) were evaluated in 14 patients non candidates to any form of revascularization who accepted to participate in the study and were included in the trial according to the estab- lished inclusion criteria. Limb ischemia diagnosis was based on Laser Doppler blood flow measurements (≤31 perfusion units), ankle brachial index (≤0.6) and pain- free walking distance (≤200 m). Patients were randomly divided into two main groups according to the following transplant strategy (as shown in Figure 1): Group A: 10 ischemic limbs from patients transplanted with progenitor cells using a combination of intramus- cular and distal retrograde-intravenous (saphenous vein) transplant. Group A is referred in this report as IM + IV (Intramuscular + Intravenous). Group B (control group): 10 ischemic limbs from pa- tients with progenitor cell intramuscular transplant. Group B is referred in this report as IM (intramuscular). 2.5. Cell Harvest Mobilization of Bone Marrow-Mononuclear Cells (B- M-MNC) to peripheral blood was achieved by a daily subcutaneous administration of 5 μg of Granulocyte Co- lony-Stimulating Factor (G-CSF) (Roche) during 5 con- secutive days at early morning. Mobilized Peripheral Blood Mononuclear Cells (M-PBMNC) were harvested on the 5th day of treatment by apheresis procedure using a continuous flow COBE-Spectra blood cell automated separator (COBE BCT, Lakewood CO., USA) and a dou- ble puncture technique using citrate plus glucose as an- ticoagulant and physiologic solution as primer. Aphe- resis was performed as a two-step procedure involving extraction and re-infusion using a Mahurkar or Niagara catheter and extraction/infusion flow speeds between 50 - 55 mL·min−1. For all patients a 60 mL standard amount of progenitor cell suspension per ischemic limb was ob- tained at the end of the apheresis procedure. 2.6. Cell Analysis CD34+ (hematopoietic) and CD133+ (angioblast) mouse anti human antibody (Beckton Dickinson, Ca, USA) cell marker were used to confirm progenitor cell phenotype- respectively by flowcytometry analysis using a FAC Figure 1. Study design. A total of 14 patients were re- cruited for this study achieving 10 ischemic limbs per group. Some of the patients presenting both ischemic limbs were included as one of the ischemic limbs in group A and the second ischemic limb in group B. SCalibur and Cell Quest Pro Software (Becton Dickinson, Ca, USA). Cell count was manually obtained using a haemocytometer. 2.7. Surgical Technique Ultrasound guided punctures on the affected limb muscle compartments were performed under epidural blockade and light sedation using a catheter needle BD Insyte (16GA-1.77IN) (17 × 45 mm). The 60 mL M-PBMN cell suspension per ischemic limb were dis- tributed as follows: 10 mL in the internal vastus com- partment, 10 mL in the external vastus compartment, 10 mL in the anterior tibial muscle compartment, 10 mL in the deep posterior muscle compartment and 10 mL in the superficial posterior muscle compartment. For group A, as part of the surgical procedure, the major saphenous vein was localized at the internal malleolus level, at this site a small incision was performed in order to dissect the vein and isolate it using chromic catgut 3 - 0 sutures. Af- terwards the vein was temporarily ocluded in a proximal direction and a catheter BD Insyte (16GA-1.77IN) (17 × 45 mm) was placed by direct puncture in order to per- form a regional distal retrograde heparinization (1 mL of heparine diluted 1:1000 + 9 mL saline solution). A ma- nual tourniquet was applied to avoid the venous flow to return for 10 minutes followed by the distal retrograde intravenous application of the last 10 mL cell suspension (Figure 2). For group B, 12 mL instead of 10 mL of M- PBMN cell suspension were distributed between the 5 muscles compartments as previously mentioned. Copyright © 2012 SciRes. OPEN AC CESS 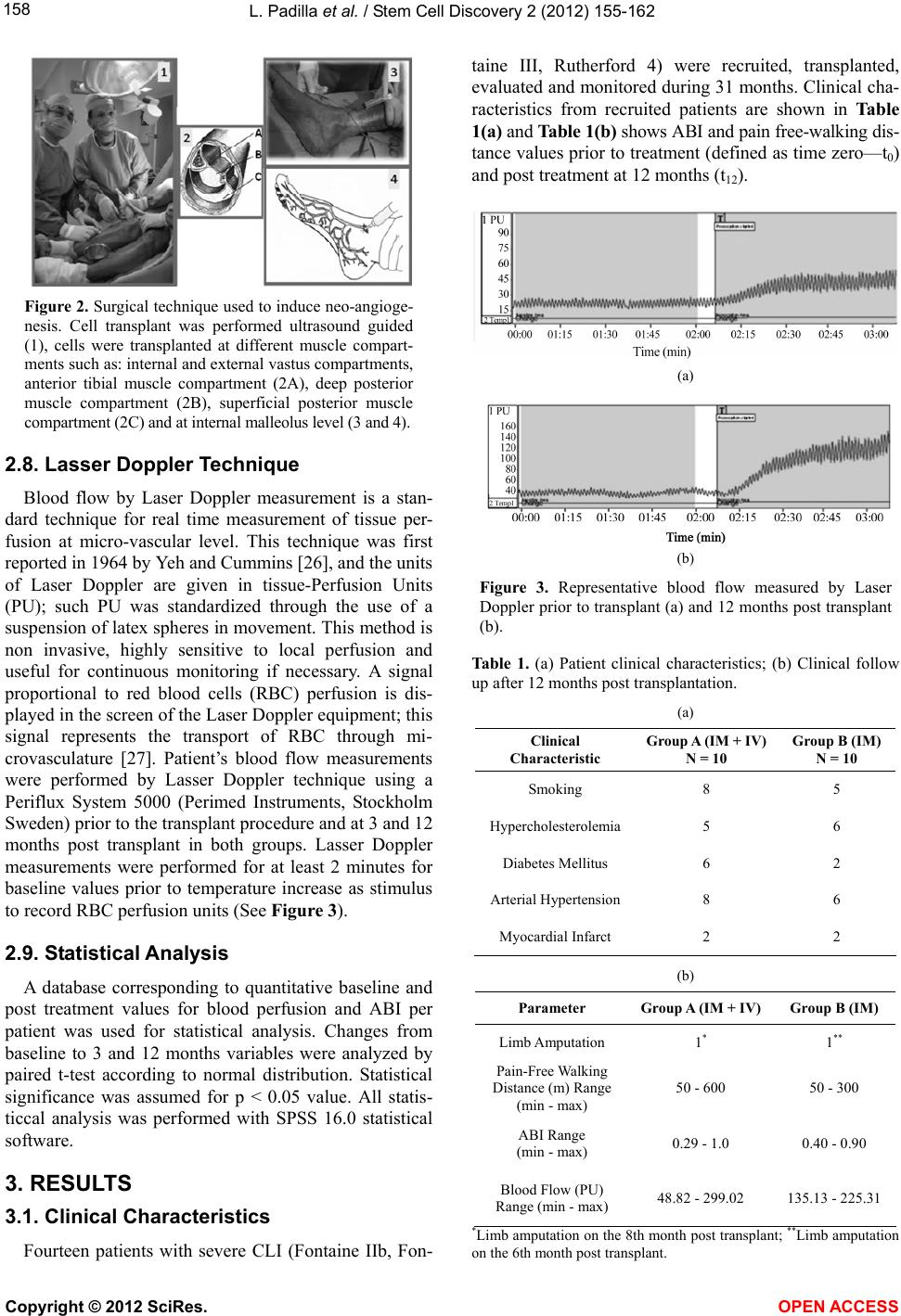 L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 158 Figure 2. Surgical technique used to induce neo-angioge- nesis. Cell transplant was performed ultrasound guided (1), cells were transplanted at different muscle compart- ments such as: internal and external vastus compartments, anterior tibial muscle compartment (2A), deep posterior muscle compartment (2B), superficial posterior muscle compartment (2C) and at internal malleolus level (3 and 4). 2.8. Lasser Doppler Technique Blood flow by Laser Doppler measurement is a stan- dard technique for real time measurement of tissue per- fusion at micro-vascular level. This technique was first reported in 1964 by Yeh and Cummins [26], and the units of Laser Doppler are given in tissue-Perfusion Units (PU); such PU was standardized through the use of a suspension of latex spheres in movement. This method is non invasive, highly sensitive to local perfusion and useful for continuous monitoring if necessary. A signal proportional to red blood cells (RBC) perfusion is dis- played in the screen of the Laser Doppler equipment; this signal represents the transport of RBC through mi- crovasculature [27]. Patient’s blood flow measurements were performed by Lasser Doppler technique using a Periflux System 5000 (Perimed Instruments, Stockholm Sweden) prior to the transplant procedure and at 3 and 12 months post transplant in both groups. Lasser Doppler measurements were performed for at least 2 minutes for baseline values prior to temperature increase as stimulus to record RBC perfusion units (See Figure 3). 2.9. Statistical Analysis A database corresponding to quantitative baseline and post treatment values for blood perfusion and ABI per patient was used for statistical analysis. Changes from baseline to 3 and 12 months variables were analyzed by paired t-test according to normal distribution. Statistical significance was assumed for p < 0.05 value. All statis- ticcal analysis was performed with SPSS 16.0 statistical software. 3. RESULTS 3.1. Clinical Characteristics Fourteen patients with severe CLI (Fontaine IIb, Fon- taine III, Rutherford 4) were recruited, transplanted, evaluated and monitored during 31 months. Clinical cha- racteristics from recruited patients are shown in Table 1(a) and Table 1(b) shows ABI and pain free-walking dis- tance values prior to treatment (defined as time zero—t0) and post treatment at 12 months (t12). (a) (b) Figure 3. Representative blood flow measured by Laser Doppler prior to transplant (a) and 12 months post transplant (b). Table 1. (a) Patient clinical characteristics; (b) Clinical follow up after 12 months post transplantation. (a) Clinical Characteristic Group A (IM + IV) N = 10 Group B (IM) N = 10 Smoking 8 5 Hypercholesterolemia5 6 Diabetes Mellitus 6 2 Arterial Hypertension 8 6 Myocardial Infarct 2 2 (b) Parameter Group A (IM + IV) Group B (IM) Limb Amputation 1* 1 ** Pain-Free Walking Distance (m) Range (min - max) 50 - 600 50 - 300 ABI Range (min - max) 0.29 - 1.0 0.40 - 0.90 Blood Flow (PU) Range (min - max) 48.82 - 299.02 135.13 - 225.31 *Limb amputation on the 8th month post transplant; **Limb amputation on the 6th month post transplant. Copyright © 2012 SciRes. OPEN AC CESS 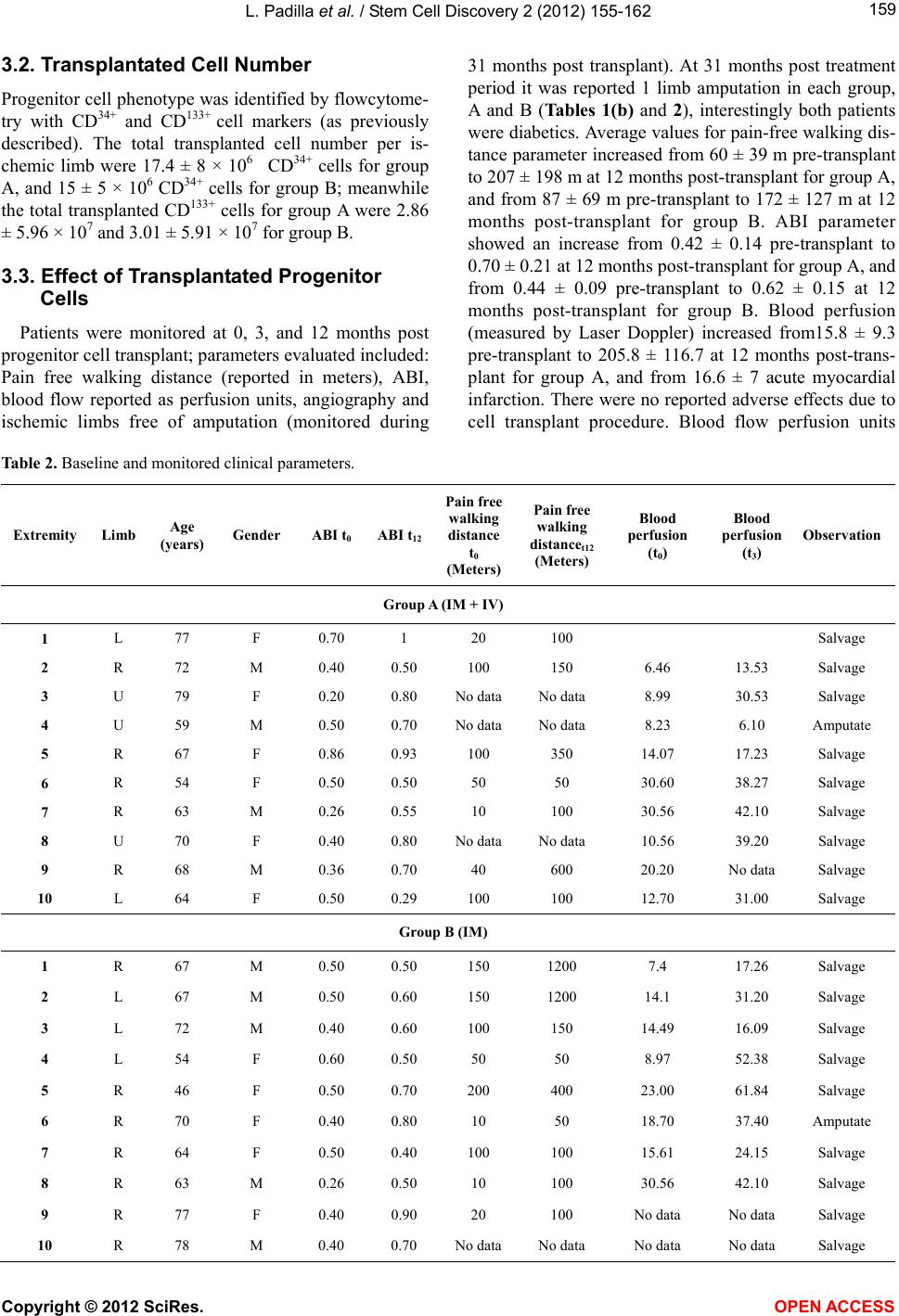 L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 Copyright © 2012 SciRes. OPEN AC CESS 159 3.2. Transplantated Cell Number Progenitor cell phenotype was identified by flowcytome- try with CD34+ and CD133+ cell markers (as previously described). The total transplanted cell number per is- chemic limb were 17.4 ± 8 × 106 CD34+ cells for group A, and 15 ± 5 × 106 CD34+ cells for group B; meanwhile the total transplanted CD133+ cells for group A were 2.86 ± 5.96 × 107 and 3.01 ± 5.91 × 107 for group B. 3.3. Effect of Transplantated Progenitor Cells Patients were monitored at 0, 3, and 12 months post progenitor cell transplant; parameters evaluated included: Pain free walking distance (reported in meters), ABI, blood flow reported as perfusion units, angiography and ischemic limbs free of amputation (monitored during 31 months post transplant). At 31 months post treatment period it was reported 1 limb amputation in each group, A and B (Tables 1(b) and 2), interestingly both patients were diabetics. Average values for pain-free walking dis- tance parameter increased from 60 ± 39 m pre-transplant to 207 ± 198 m at 12 months post-transplant for group A, and from 87 ± 69 m pre-transplant to 172 ± 127 m at 12 months post-transplant for group B. ABI parameter showed an increase from 0.42 ± 0.14 pre-transplant to 0.70 ± 0.21 at 12 months post-transplant for group A, and from 0.44 ± 0.09 pre-transplant to 0.62 ± 0.15 at 12 months post-transplant for group B. Blood perfusion (measured by Laser Doppler) increased from15.8 ± 9.3 pre-transplant to 205.8 ± 116.7 at 12 months post-trans- plant for group A, and from 16.6 ± 7 acute myocardial infarction. There were no reported adverse effects due to cell transplant procedure. Blood flow perfusion units Table 2. Baseline and monitored clinical parameters. Extremity Limb Age (years) Gender ABI t0 ABI t12 Pain free walking distance t0 (Meters) Pain free walking distancet12 (Meters) Blood perfusion (t0) Blood perfusion (t3) Observation Group A (IM + IV) 1 L 77 F 0.70 1 20 100 Salvage 2 R 72 M 0.40 0.50 100 150 6.46 13.53 Salvage 3 U 79 F 0.20 0.80 No dataNo data 8.99 30.53 Salvage 4 U 59 M 0.50 0.70 No dataNo data 8.23 6.10 Amputate 5 R 67 F 0.86 0.93 100 350 14.07 17.23 Salvage 6 R 54 F 0.50 0.50 50 50 30.60 38.27 Salvage 7 R 63 M 0.26 0.55 10 100 30.56 42.10 Salvage 8 U 70 F 0.40 0.80 No dataNo data 10.56 39.20 Salvage 9 R 68 M 0.36 0.70 40 600 20.20 No data Salvage 10 L 64 F 0.50 0.29 100 100 12.70 31.00 Salvage Group B (IM) 1 R 67 M 0.50 0.50 150 1200 7.4 17.26 Salvage 2 L 67 M 0.50 0.60 150 1200 14.1 31.20 Salvage 3 L 72 M 0.40 0.60 100 150 14.49 16.09 Salvage 4 L 54 F 0.60 0.50 50 50 8.97 52.38 Salvage 5 R 46 F 0.50 0.70 200 400 23.00 61.84 Salvage 6 R 70 F 0.40 0.80 10 50 18.70 37.40 Amputate 7 R 64 F 0.50 0.40 100 100 15.61 24.15 Salvage 8 R 63 M 0.26 0.50 10 100 30.56 42.10 Salvage 9 R 77 F 0.40 0.90 20 100 No data No data Salvage 10 R 78 M 0.40 0.70 No dataNo data No data No data Salvage 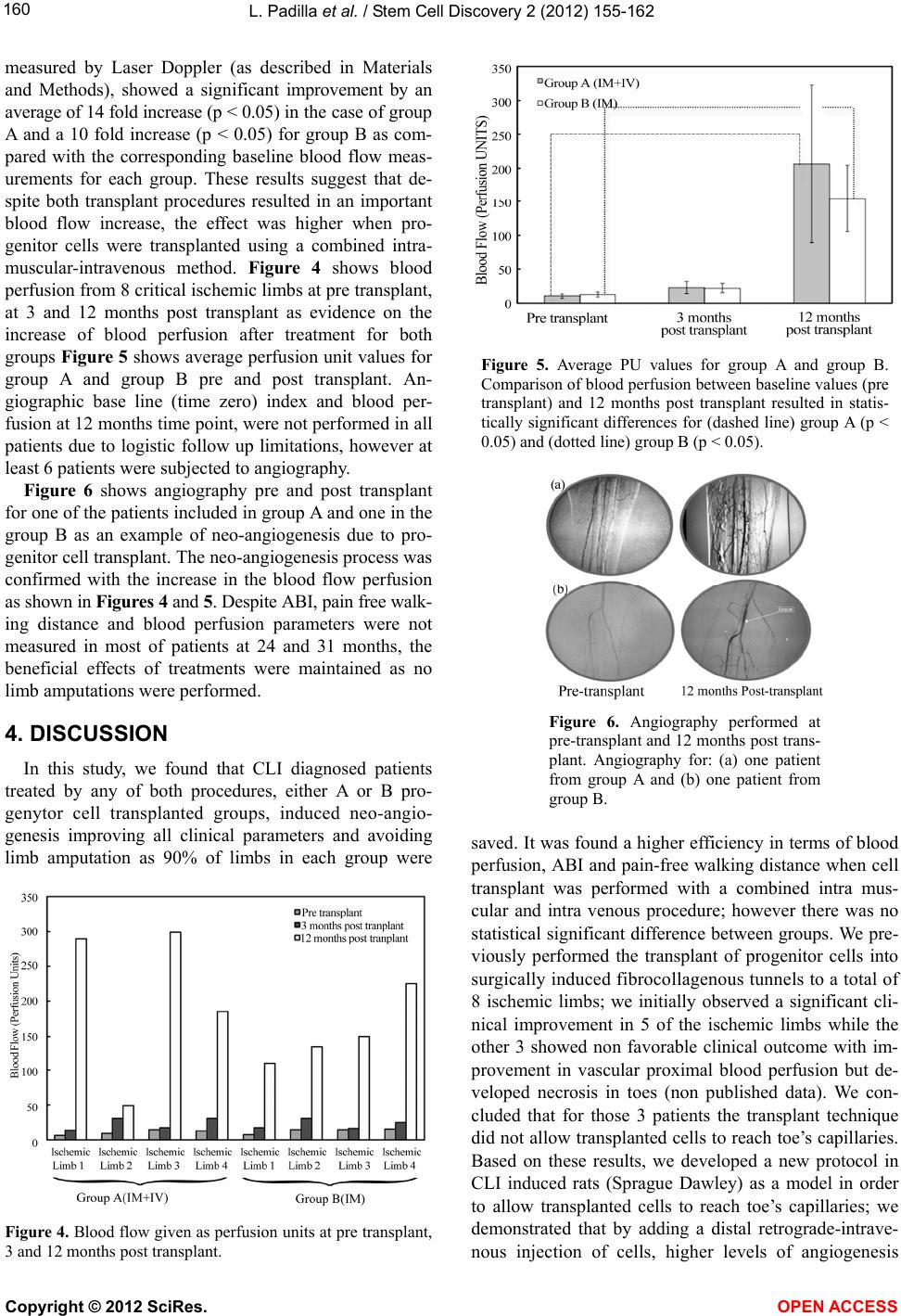 L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 160 measured by Laser Doppler (as described in Materials and Methods), showed a significant improvement by an average of 14 fold increase (p < 0.05) in the case of group A and a 10 fold increase (p < 0.05) for group B as com- pared with the corresponding baseline blood flow meas- urements for each group. These results suggest that de- spite both transplant procedures resulted in an important blood flow increase, the effect was higher when pro- genitor cells were transplanted using a combined intra- muscular-intravenous method. Figure 4 shows blood perfusion from 8 critical ischemic limbs at pre transplant, at 3 and 12 months post transplant as evidence on the increase of blood perfusion after treatment for both groups Figure 5 shows average perfusion unit values for group A and group B pre and post transplant. An- giographic base line (time zero) index and blood per- fusion at 12 months time point, were not performed in all patients due to logistic follow up limitations, however at least 6 patients were subjected to angiography. Figure 6 shows angiography pre and post transplant for one of the patients included in group A and one in the group B as an example of neo-angiogenesis due to pro- genitor cell transplant. The neo-angiogenesis process was confirmed with the increase in the blood flow perfusion as shown in Figures 4 and 5. Despite ABI, pain free walk- ing distance and blood perfusion parameters were not measured in most of patients at 24 and 31 months, the beneficial effects of treatments were maintained as no limb amputations were performed. 4. DISCUSSION In this study, we found that CLI diagnosed patients treated by any of both procedures, either A or B pro- genytor cell transplanted groups, induced neo-angio- genesis improving all clinical parameters and avoiding limb amputation as 90% of limbs in each group were Figure 4. Blood flow given as perfusion units at pre transplant, 3 and 12 months post transplant. Figure 5. Average PU values for group A and group B. Comparison of blood perfusion between baseline values (pre transplant) and 12 months post transplant resulted in statis- tically significant differences for (dashed line) group A (p < 0.05) and (dotted line) group B (p < 0.05). Figure 6. Angiography performed at pre-transplant and 12 months post trans- plant. Angiography for: (a) one patient from group A and (b) one patient from group B. saved. It was found a higher efficiency in terms of blood perfusion, ABI and pain-free walking distance when cell transplant was performed with a combined intra mus- cular and intra venous procedure; however there was no statistical significant difference between groups. We pre- viously performed the transplant of progenitor cells into surgically induced fibrocollagenous tunnels to a total of 8 ischemic limbs; we initially observed a significant cli- nical improvement in 5 of the ischemic limbs while the other 3 showed non favorable clinical outcome with im- provement in vascular proximal blood perfusion but de- veloped necrosis in toes (non published data). We con- cluded that for those 3 patients the transplant technique did not allow transplanted cells to reach toe’s capillaries. Based on these results, we developed a new protocol in CLI induced rats (Sprague Dawley) as a model in order to allow transplanted cells to reach toe’s capillaries; we demonstrated that by adding a distal retrograde-intrave- nous injection of cells, higher levels of angiogenesis Copyright © 2012 SciRes. OPEN AC CESS  L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 161 were produced as compared with intramuscular cell transplant. We also observed that mononuclear cells attach- ed to the smallest capillary segment used as a scaffold increased survival, proliferation, and cell differentiation towards endothelial cell type [28]. Tepper [29] and Law all [30] demonstrated that angiogenesis or vasculogene- sis induction requires the ischemic initial stimulus and the muscle inflammatory response that releases cytokines and growth factors (such as VEGF, bFGF, SDF-1, PDG- BB, IGF-1, TGF-B) that activate proliferation, migration and tubulization of endothelial cells. These findings support the use of muscle as the main target for progeny- tor cell transplant, however we showed that combined intramuscular and intravenous transplant technique pro- vides a higher transplant efficiency as demonstrated in our previous animal model report [26]. A similar 12 months follow up study reported by Van Tongeren et al. [31] demonstrated a higher efficiency when combined intramuscular and intra-arterial transplant strategy was used in comparison with only intramuscular transplant. The main results of our study is the evidence that both transplant procedures increases blood perfusion, ABI and pain free walking distance; showing slightly higher re- sults for combined intramuscular-intravenous group. More importantly, improvements achieved by progenitor cell transplant in this study, were maintained for up to 31 months achieving 90% of the limbs preserved from am- putationn. 5. CONCLUSION Progenitor cell therapy described in this study is a minimally invasive therapeutic option for patients with critical limb ischemia. Both, intramuscular and com- bined intramuscular-intravenous autologous progenitor cell transplant led to a substantial increase in blood flow perfusion, pain-free walking distance and ankle brachial index. Despite there was no statistical significance be- tween groups, a higher blood perfusion (measured by laser Doppler) was observed when progenitor cells were transplanted combining intramuscular-intravenous as com- pared with intra muscular procedure. Transplant of pro- genitor cells described here provides a safe and reliable cell therapy for CLI diagnosed patients who are not can- didates for invasive surgical revascularization. 6. ACKNOWLEDGEMENTS We would like to acknowledge to Reneé Acosta and Paola Lares Juárez for their help on the revision of the manuscript. REFERENCES [1] Ouriel, K. (2001) Detection of peripheral arterial disease in primary care. The Journal of the American Medical Association, 286, 1380-1381. doi:10.1001/jama.286.11.1380 [2] Criqui, M.H., Langer, R.D., Fronek, A., Feigelson, H.S., Klauber, M.R., McCann, T.J. and Bronwer, D. (1992) Mor- tality over a period of 10 years in patients with peripheral arterial disease. The New England Journal of Medicine, 326, 381-386. doi:10.1056/NEJM199202063260605 [3] Aranguren, X.L., Verfaillie, C.M. and Luttun, A. (2009) Emerging hurdles in stem cell therapy for peripheral vas- cular disease. Journal of Molecular Medicine, 87, 3-16. doi:10.1007/s00109-008-0394-3 [4] Ouriel, K. (2001) Peripheral arterial disease. The Lancet, 358, 1257-1264. doi:10.1016/S0140-6736(01)06351-6 [5] Luther, M., Lepantalo, M., Alback, A. and Mazke, S. (1996) Amputation rates as a measure of vascular surgical results. British Journal of Surgery, 83, 241-244. doi:10.1002/bjs.1800830229 [6] Coffman, J. D. (1991) Intermittent claudication—Be con- servative. The New England Journal of Medicine, 325, 577-578. [7] Beker, G.J., Furguson, J.G., Bakal, C.W., McKinnison, M.G.K., et al. (1993) Angioplasty, bypass surgery and am- putation for lower extremity peripheral arterial disase in Maryland: A closer look. Radiology, 186, 635-638. [8] Rutherford, R.B., Flanigon, D.P., Gupta, S.L., Johnsin, K., et al. (1986) Suggested standards for reports dealing with lower extremity ischemia. Journal of Vascular Surgery, 4, 80-94. [9] Deweese, J.A., Leather, R. and Porter, J. (1993) Practice guidelines: Lower extremity revascularization. Journal of Vascular Surgery, 18, 280-294. doi:10.1016/0741-5214(93)90609-P [10] Norgen, L., Hiatt, W.R., Dormandy, J.A., et al. (2007) TASC II working group inter society consensus for the management of pheripheral artherial disease (TASC II). European Journal of Vascular and Endovascular Surgery, 33, 1-75. [11] Di Stefano, R., Limbruno, U., Barone, D. and Balbarini, A. (2004) Therapeutic angiogenesis of critical lower limb ischemia review of the literature and prospects of re- search on stem cells. Italian Heart Journal, 5, 1-13. [12] Boccalon, H., Lehert, P. and Mosnier, M. (2000) Assess- ment of the prevalence of atherosclerotic lower limb arte- riopathy in France as a systolic index in a vascular risk population. Journal Des Maladies Vasculaires, 25, 38-46. [13] The European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products. (2002) Notes for guidance on clinical investigation of medicinal products for the treatment of peripheral arterial occlusive disease. CPMP/EWP714/98. [14] Marston, W.A., Davies, S.W., Armstrong, B., Farber, M.A., Mendes, R.C. and Fulton, J.J. (2006) Natural his- tory of limbs with arterial insufficiency and chronic ulce- ration treated without revascularization. Journal of Vas- cular Surgery, 44, 108-114. doi:10.1016/j.jvs.2006.03.026 [15] Hilleman, D.E. (1998) Management of peripheral arterial. Copyright © 2012 SciRes. OPEN AC CESS  L. Padilla et al. / Stem Cell Discovery 2 (2012) 155-162 Copyright © 2012 SciRes. OPEN AC CESS 162 Disease. American Journal of Health Promotion, 55, 521-527. [16] Dormady, J.A. and Rutherford, R.B. (2000) Management of peripheral arterial disease (PAD). TASC working group. Journal of Vascular Surgery, 31, S1-S278. [17] Aronow, W.S. (2005) Management of peripheral arterial disease. Cardiology in Review, 13, 61-68. doi:10.1097/01.crd.0000126082.86717.12 [18] Franz, R., Parks, A., Shah, K.J., Hankins, T., Hartman, J.F. and Wright, M.L. (2009) Use of autologus bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial dis- ease. Journal of Vascular Surgery, 50, 1378-1390. doi:10.1016/j.jvs.2009.07.113 [19] Huang, P.P., Yang, X.F., Li, S.Z., Wen, J.C., Zhang, Y. and Han, Z.C. (2007) Randomised comparison of G-CSF mo- bilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thrombosis and Haemostasis, 98, 1335-1342. [20] Amann, B., Luedeman, C., Ratei, R. and Schmidt-Lucke, A.J. (2009) Autologus bone marrow transplantation in- creases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. C ell Transplantation, 18, 371-380. doi:10.3727/096368909788534942 [21] Bartsch, T., Brehm, M., Zeus, T., Kogler, G., Wernet, P. and Strauer, B.E. (2007) Transplantation of autologus mononuclear bone marrow stem cells in patients with pe- riphera arterial disease (The TAM-PAD study). Clinical Research in Cardiology, 96, 891-899. doi:10.1007/s00392-007-0569-x [22] Powell, R.J., Comerota, A.J., Berceli, S.A., Guzman, R., Henry, D.T., Tzeng, E., Velazquez, O., Marston, W.A., Bartel, R.L., Longcore, A., Stern, T. and Watling, S. (2011) Interim results from the RESTORE-CLI, a randomized, double-blidn multicenter phase II trial comparing expanded autologus bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. Journal of Vascular Surgery, 54, 1032-1041. doi:10.1016/j.jvs.2011.04.006 [23] Cobellis, G., Silvestroni, A., Lillo, S., Sica, G., Botti, C., Maione, C., Schiavone, V., Rocco, S., Brando, G. and Sica, V. (2008) Long-term effects of repeated autologus transplantation of bone marrow cells in patients affected by peripheral arterial disease. Bone Marrow Transplanta- tion, 42, 667-672. doi:10.1038/bmt.2008.228 [24] Padilla, L., De la Garza, A.S., Villegas, F., Esperante, S., Rojas, E., Miranda, A., Figueroa, S., Schalch, P., Krotzsch, E. and Di Silvio, M. (2003) Administration of bone mar- row cells into surgically induced fibrocollagenous tunnels induces angiogenesis in ischemic rat hindlimb model. Microsurgery, 23, 568-574. doi:10.1002/micr.10208 [25] Padilla, L., Villegas, F., Glennie, G., Escotto, I., Schalch, P., Avila, G., Rodriguez-Trejo, J., Figueroa, S., De la Garza, A.S., Krotzsch, E. and Di Silvio, M. (2007) Bone marrow mononuclear cells stimulate angiogenesis when transplanted into surgically induced fibrocollagenous tun- nels: Results from a canine ischemic hinlimb model. Mi- crosurgery, 27, 91-97. doi:10.1002/micr.20289 [26] Yeh, Y. and Cummins, H. (1964) Localized fluid flows measurements with He-Ne laser spectrometer. Applied Physics Letters, 4, 176-178. doi:10.1063/1.1753925 [27] Kvernobo, K. and Slagsvold, C.E. (1998) Laser Doppler flowmetry in evaluation of lower limb resting skin circu- lation: A study in healthy controls and atherosclerotic pa- tients. Scandinavian Journal of Clinical & Laboratory Investigation, 48, 621-626. doi:10.3109/00365518809085781 [28] Padilla, S.L., Rodriguez, T.J., Escotto, S.L., De Diego, F.J., Rodriguez, R.N., Krötzsch, E., Villegas, A., Landero, T., Carranza, H., Goldberg, J. and Di Silvio, M. (2009) Progenitor mononuclear cell transplantation derived from the bone marrow through distal retrogressive endovenous route in order to induce angiogenesis. Cirujano General, 31, 213-218. [29] Tepper, O.M., Capla, J.M., Galiano, R.D. and Callaghan, M.J. (2009) Adult vasculogenesis occurs through in situ recruitment, proliferation and tubulization of circulating bone marrow derived cells. Blood, 105, 1068-1077. doi:10.1182/blood-2004-03-1051 [30] Lawall, H., Bramage, P. and Amann, B. (2010) Stem cell and progenitor cell therapy in peripheral arterial disease. Journal of Thrombosis and Haemostasis, 103, 696-709. [31] Van Tongeren, R. and Hamming, J. (2008) Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells; a clinical trial in patients with advanced limb ischemia. Journal of Cardiothoracic Surgery, 49, 51-58.
|