Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 758-762 JBiSE doi:10.4236/jbise.2010.38101 Published Online August 2010 (http://www.SciRP.org/journal/jbise/). Published Online August 2010 in SciRes. http:// www. scirp. org/journal/jbise Antimicrobial activity of the autochthonous compound Enoxil Lucian Lupaşcu1, Valeriu Rudic1,2, Viorica Cotos3, Tudor Lupaşcu4 1Institute of Microbiology and Biotechnology of the ASM, Chişinău, Moldavia; 2Academy of Sciences of Moldova (ASM), Chişinău, Moldavia; 3Hospital of Contagious Diseases “T. Ciorba”, Chişinău, Moldavia; 4Institute of Chemistry of the ASM, Chişinău, Moldavia. Email: lucian1978@mail.ru Received 24 February 2010; revised 18 March 2010; accepted 31 March 2010. ABSTRACT The paper presents data about the antimicrobial ac- tivity of the autochthonous compound of taninic source Enoxil. The minimal inhibitory and bacteri- cidal/fungicidal concentrations were established for some skin and wound infectious agents. It was estab- lished, based on Pseudomonas bacteria model, that the Enoxil compound suppress the activity of some important enzymes—a phenomenon that leads to the increase of bacterial sensibility to many tested anti- biotics. Keywords: Bacteria; Compound; Tannins; Wound; Infection 1. INTRODUCTION Skin and wound infections, although more characteristic for countries with low economic level and low hygiene standards [1], have lately become a serious concern for highly socio-economically developed countries, as well [2-5]. Of great concern are also the intrahospital plague infections, as they complicate the recovering process, produce anxiety, increase patient’s discomfort and may lead to death, being at the same time a serious threat to the medical worker [5,6]. The economical aspects of infections are also considerable [7]. The most frequent microorganisms in the derma or the purulent wound are the gram-positive bacteria Staphylococcus aureus and the gram-negative bacteria Escherichia coli, Pseudomo- nas aeruginosa [8]. Special attention is paid to pseudo- monadic infections as complications of combustions, traumas, tattoo procedures, cellulites, decubital chronic ulcers, foliculitis [9-11]. One of the traditional methods of fighting infections is the use of antibiotics. However, a concerning phenome- non is observed lately in the entire world, including the Republic of Moldova—resistance of microorganisms to these compounds [12,13]. A permanent and, often, abu- sive treatment with antibiotics had as a result the selec- tion of new virulences, aggressive and severe, with a high genetic resistance potential. This situation directs the specialists in the field towards the elaboration of antimicrobial compounds on the basis of novel action mechanisms [14]. Albeit the use of tannin extracts from plants for the treatment of various derma and plagues infections has been known for a long time, these don’t exhibit the expected effect for many microorganisms [15-17]. At the moment, the number of efficient medici- nal compounds, elaborated on the basis of tannin com- pounds is quite low, mostly due to the insolubility of many of them in aqueous or alcoholic solutions. The capacity of phenolic (tannin) extracts to disinfect and rapidly treat wounds is based on two fundamental phe- nomena—antimicrobial and antioxidant activities, de- termined by a series of mechanisms: capturing of iron ions from the substrate, thus depriving microorganisms of compounds necessary for their physiological activity; their binding to microbial proteins and formation of complexes; capturing of free radicals; absorption of oxygen radicals; inhibition of low density lipoproteins oxidation [18]. It is known that grape seeds are a rich source of so called enotannins–condensed tannins which represent a wide variety of natural substances with polyphenolic structure, remarkable due to the high con- tent of proanthocyanidins [19]. There are enotannins in industrial quantities in the Republic of Moldova. Taking into account that many tannins with antimicrobial prop- erties are insoluble in water, as well as the growing mi- crobial resistance to vegetal tannins, of great potential are researches regarding the possibility of structural modification of enotannins, increase of their oxidation number, in order to increase their efficiency and to use them for the treatment of skin and plague infections. The objective of present researches was to elucidate the antimicrobial activity of the autochthonous com-  L. Lupaşcu et al. / J. Biomedical Science and Engineering 3 (2010) 758-762 759 Copyright © 2010 SciRes. JBiSE pound Enoxil of tanninic origin. 2. MATERIALS AND METHODS Research was carried out at the Department of Microbi- ology, Immunology and Virusology of the State Univer- sity of Medicine and Pharmaceutics “N.Testemiteanu”. The autochthonous compound Enoxil served as the antimicrobial remedy, obtained by hydro-solubilization of enotannins using chemical and physico-chemical procedures [20]. As microbial cultures, served several pathogens with severe implications in many contagious diseases—bacteria Staphylococcus aureus, Escherichia coli, Salmonella abony, P.aeruginosa, Proteus vulgaris and the fungus Candida albi cans. In order to determine the level of activity of the tested compound, the values of minimal inhibiting concentra- tion (MIC) and the minimal bactericidal/fungicidal con- centration (MBC/MFC) were used [21]. As test indices of the action of Enoxil on the P.aeruginosa bacteria, served a range of important biochemical parameters – indices of bacterial viability and pathogenicity: synthesis of cytochrome oxidase, citrate reductase, haemolysins, pyocyanin, odour presence, and as the index of sensibil- ity towards antibiotics—zone of inhibition (mm) of the culture, specific to each antibiotic. The sensibility of the P.aeruginosa bacteria was determined using the me- thod of antibiotics diffusion in gelose from roundels [22] that contained the following antibiotics homolo- gated in the Republic of Moldova: cloramfenicol (30 mkg), pefloxaciline (5 mkg), erythromycin (15 mkg), cefu- roxim (30 mkg), cefoxitin (30 mkg), cefalotine (30 mkg), piperacillin (30 mkg), imipenem (10 mkg), ciprofloxacin (5 mkg), tobramycin (10 mkg), gentamicin (10 mkg), tetracycline (30 mkg). The standardized nutritive me- dium Miuller-Hinton was used for testing. The boxes with bacteria and antibiotics were maintained at 37°C, for 24 hours, with a subsequent determination of the result of antibioticogram. The acute toxicity research during the enteral admini- stration of the Enoxil compound was made on mouses and white rats. It was established that the 500, 1000 and 2000 mg/kg body weight doses do not provoke modifi- cations in the animals behaviour and neither their death. The cronical toxicity was established by the administra- tion of the Enoxil in 100 and 300 mg/kg body weight doses at the male rats during 30 days. Data were statistically analysed using the soft package STATISTICA 7. 3. RESULTS AND DISCUSSIONS During the research was established that the animals that took the 300 mg/kg dose were more adinamic and were dead in 5-21 days time, but those administrated the 100 mg/kg dose survived. At macroscopical examination of the internal organs were not attested any pathological modifications. After undertaken the cronical toxicity study was established that the Enoxil compound in the 100 mg/kg dose do not modify esentially the level of creatinin and cholesterol. Investigations on the action of Enoxil compound on bacteria E.coli, S.abony, S.aureus, P.vulgaris, P.aerugi- nosa and the fungus C.albicans resulted in the determi- nation of the MIC (Figure 1(a)) and the minimal bacte- ricidal/fungicidal concentration (MBC/MFC) (Figure 1(b)). Thus, Enoxil presents antibacterial, as well as antifungal properties at relatively low concentrations. The minimal (a) (b) Figure 1. (a) Minimal inhibiting concentration and (b) Bactericidal/fungicidal of Enoxil for several mi- croorganisms. On the hexagonal perimeter: 1–E.coli, 2–S.abony, 3–S.aureus, 4–P.vulgaris, 5–P. aeruginosa, 6–C.albicans. On the vertical–concentrations, %.  760 L. Lupaşcu et al. / J. Biomedical Science and Engineering 3 (2010) 758-762 Copyright © 2010 SciRes. JBiSE inhibiting concentration of Enoxil for the tested bacteria was shown to be in the range 0.15-0.3%, while the bac- tericidal one—in the range 0.3-0.6%. For the fungus C.albicans the minimal inhibiting concentration equals to 0.3%, while the fungicidal one—to 0.6%. The values MIC and MBC/MFC for the microorgan- isms taken into the study presented differences of an order of magnitude, except for the E.coli bacteria, when these indexes coincided. As a result of the correlation analysis, it was determined that the degree of depend- ence (r) between MIC and MBC/MFC is significant and positive, equal to 0.66. Regression analysis, which has a predictive value and represents the mathematical relation of the dependence, demonstrated that its equation is: y = 0.0923 + 0.3385 x (p ≤ 0.05). Data presented in Table 1 shows that under the action of Enoxil at the determined static concentration (0.15%) took place the suppression of activities of oxidases, cit- rate reductases and haemolysin. At the same time, the bacterial culture didn’t produce any pyocyanin and in- dole, and it didn’t develop any specific odours. In these conditions, the maintenance of the mobility is a proof of bacteria viability, which reveals the correctitude of the test. Thus, the static concentration of Enoxil produces significant perturbations at the biochemical level inside the bacterial cell, which probably significantly dimin- ishes the capacity of substrates decomposition, as well as its pathogenic potential. Treatment of the bacterial culture with Enoxil in- creased the sensitivity (S) for the majority of investi- gated antibiotics, with manifested resistance (R) only for erythromycin and cefalotine (Table 2). In the case of tetracycline, a reaction of medium sen- sitivity was observed (RS). In the case of blank, the cul- ture manifested sensitivity only for pefloxaciline and imipenem, while in the case of using Enoxil, the sensi- bility was exhibited towards the majority of antibiotics, except for erythromycin and cefalotine. It should me mentioned that Enoxil introduced sensibility into the bacteria towards several antibiotics considered inactive for P.aeruginosa, for example cefoxitin. The analysis of the similitude degree of the compounds using the clus- terian method of dendrograms construction, demon- strated that the tested antibiotics were distributed in big clusters (Figure 2(a)), quite different, which reveals that Table 1. Influence of the static concentration of Enoxil on several biochemical and functional indices of the P.aeruginosa bacterium. Indicator Blank (+/–) Enoxil (+/–) Oxidase + – Citrate reductase + – Haemolysin + – Indole – – Pyocyanin + – Odour + – Mobility + + Table 2. Influence of the static concentration of Enoxil on the sensibility of the culture P. aerugino sa towards several antibiotics. Untreated culture (blank) Treated culture Nr. Antibiotic Inhibition zon e, mm Reaction Inhibition zone, mm Reaction 1 Cloramfenicol 0.0 R 26.0 ± 0.6* S 2 Pefloxaciline 14.0 ± 0.6 R 26.3 ± 0.3* S 3 Erythromycin 0.0 R 13.0 ± 0.6* R 4 Cefuroxim 0.0 R 20.0 ± 1.2* S 5 Cefoxitin 0.0 R 22.0 ± 0.6* S 6 Cefalotine 0.0 R 0.0 R 7 Piperacillin 0.0 R 20.0 ± 1.2* S 8 Imipenem 30.0 ± 1.2 S 29.3 ± 1.5* S 9 Ciprofloxacin 0.0 R 24.0 ± 0.6* S 10 Tobramycin 0.0 R 17.0 ± 1.2* S 11 Gentamicin 0.0 R 16.0 ± 0.6* S 12 Tetracycline 0.0 R 19.0 ± 0.7* R-S * - difference from the blank with statistic support at p < 0.05. 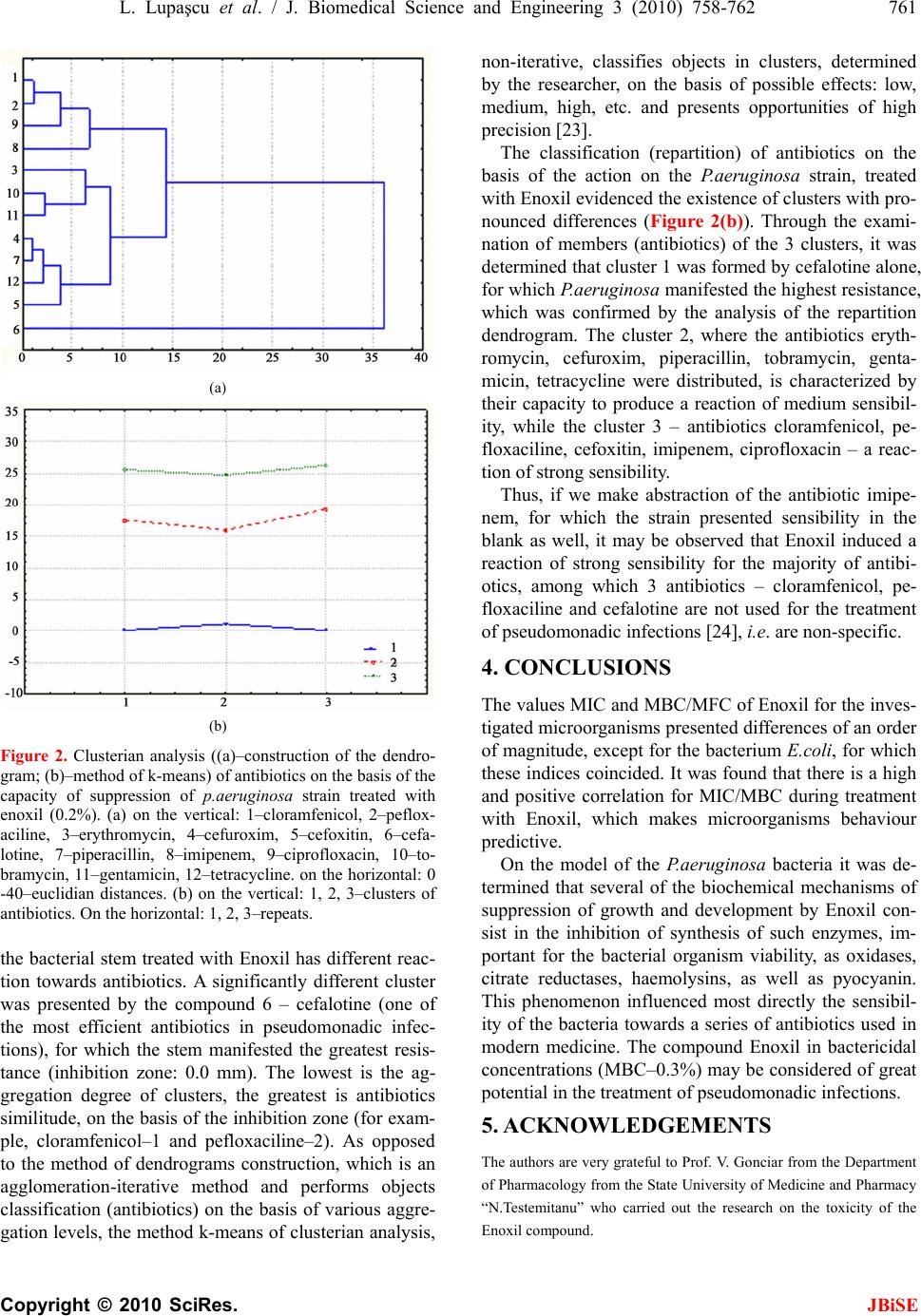 L. Lupaşcu et al. / J. Biomedical Science and Engineering 3 (2010) 758-762 761 Copyright © 2010 SciRes. JBiSE (a) (b) Figure 2. Clusterian analysis ((a)–construction of the dendro- gram; (b)–method of k-means) of antibiotics on the basis of the capacity of suppression of p.aeruginosa strain treated with enoxil (0.2%). (a) on the vertical: 1–cloramfenicol, 2–peflox- aciline, 3–erythromycin, 4–cefuroxim, 5–cefoxitin, 6–cefa- lotine, 7–piperacillin, 8–imipenem, 9–ciprofloxacin, 10–to- bramycin, 11–gentamicin, 12–tetracycline. on the horizontal: 0 -40–euclidian distances. (b) on the vertical: 1, 2, 3–clusters of antibiotics. On the horizontal: 1, 2, 3–repeats. the bacterial stem treated with Enoxil has different reac- tion towards antibiotics. A significantly different cluster was presented by the compound 6 – cefalotine (one of the most efficient antibiotics in pseudomonadic infec- tions), for which the stem manifested the greatest resis- tance (inhibition zone: 0.0 mm). The lowest is the ag- gregation degree of clusters, the greatest is antibiotics similitude, on the basis of the inhibition zone (for exam- ple, cloramfenicol–1 and pefloxaciline–2). As opposed to the method of dendrograms construction, which is an agglomeration-iterative method and performs objects classification (antibiotics) on the basis of various aggre- gation levels, the method k-means of clusterian analysis, non-iterative, classifies objects in clusters, determined by the researcher, on the basis of possible effects: low, medium, high, etc. and presents opportunities of high precision [23]. The classification (repartition) of antibiotics on the basis of the action on the P.aeruginosa strain, treated with Enoxil evidenced the existence of clusters with pro- nounced differences (Figure 2(b)). Through the exami- nation of members (antibiotics) of the 3 clusters, it was determined that cluster 1 was formed by cefalotine alone, for which P.aeruginosa manifested the highest resistance, which was confirmed by the analysis of the repartition dendrogram. The cluster 2, where the antibiotics eryth- romycin, cefuroxim, piperacillin, tobramycin, genta- micin, tetracycline were distributed, is characterized by their capacity to produce a reaction of medium sensibil- ity, while the cluster 3 – antibiotics cloramfenicol, pe- floxaciline, cefoxitin, imipenem, ciprofloxacin – a reac- tion of strong sensibility. Thus, if we make abstraction of the antibiotic imipe- nem, for which the strain presented sensibility in the blank as well, it may be observed that Enoxil induced a reaction of strong sensibility for the majority of antibi- otics, among which 3 antibiotics – cloramfenicol, pe- floxaciline and cefalotine are not used for the treatment of pseudomonadic infections [24], i.e. are non-specific. 4. CONCLUSIONS The values MIC and MBC/MFC of Enoxil for the inves- tigated microorganisms presented differences of an order of magnitude, except for the bacterium E.coli, for which these indices coincided. It was found that there is a high and positive correlation for MIC/MBC during treatment with Enoxil, which makes microorganisms behaviour predictive. On the model of the P.aeruginosa bacteria it was de- termined that several of the biochemical mechanisms of suppression of growth and development by Enoxil con- sist in the inhibition of synthesis of such enzymes, im- portant for the bacterial organism viability, as oxidases, citrate reductases, haemolysins, as well as pyocyanin. This phenomenon influenced most directly the sensibil- ity of the bacteria towards a series of antibiotics used in modern medicine. The compound Enoxil in bactericidal concentrations (MBC–0.3%) may be considered of great potential in the treatment of pseudomonadic infections. 5. ACKNOWLEDGEMENTS The authors are very grateful to Prof. V. Gonciar from the Department of Pharmacology from the State University of Medicine and Pharmacy “N.Testemitanu” who carried out the research on the toxicity of the Enoxil compound.  762 L. Lupaşcu et al. / J. Biomedical Science and Engineering 3 (2010) 758-762 Copyright © 2010 SciRes. JBiSE REFERENCES [1] Dogra, S. and Kumar, B. (2003) Epidemiology of skin diseases in school children: A study from Northern India. Pediatric Dermatology, 20(6), 470-473. [2] Collier, M. (2002) Wound-bed management: Key princi- ples for practice. Professional Nurse, 18(4), 221-225. [3] Cooper, R. (2002) Wound microbiology: Past, present, and future. British Journal of Nursing, 11(22), 4-6. [4] Miller, M. (2001) Wound infection unravelled. Journal of Community Nursing, 15(3), 31-33. [5] Nosocomial Infection National Surveillance Service (2002) Surveillance of surgical site infection in English hospitals: A national surveillance and quality improvement pro- gramme. Health Protection Agency, London. [6] Singh, N.P., Goyal, R., Manchanda, V., et al. (2003) Changing trends in bacteriology of burns in the burns unit. Burns, 29(2), 129-132. [7] Prisacari, V. and Paraschiv, A. (2008) Contrubutions in the epidemiological surveillance systems optimization within nosocomial septico-purulent infections of surgi- cal profile. Epidemiology and Microbiology. Mat. Con- gress VI of Hygienists, Epidemiologists and Microbi- ologists from the Republic of Moldova, Chişinău, 2, 22-25. [8] Rodgers, G., Mortensen, J., Fisher, M., et al. (2000) Pre- dictors of infectious complications after burn injuries in children. Pediatric Infectious Disease Journal, 19(10), 990-995. [9] Gang, R.K., Bang, R.L., Sanyal, S.C., et al. (1999) Pseu- domonas aeruginosa septicaemia in burns. Burns, 25(7), 611-616. [10] Kehheth, T. (2008) Pseudomonas aeruginosa. http://www. textbookofbacteriology.net/pseudomonas.html [11] Rozhavin, M.A. (1983) Some biological properties of the P.aeruginosa melanin. Journal of Microbiology, in Rus- sian, 1, 45-47. [12] Valencia, I., Kirsner, R. and Kerdel, F. (2004) Micro- biologic evaluation of skin wounds: Alarming trend to- ward antibiotic resistance in an inpatient dermatology service during a 10-year period. Journal of the Ameri- can Academy of Dermatology, 50(6), 845-849. [13] Visnevchi, L. and Bradu, V. (2008) Antibiotical microor- ganisms resistance modification, tested in the microbi- ological laboratory of PRMC Ungheni. Epidemiology and Microbiology. Mat. Congress VI of Hygienists, Epi- demiologists and Microbiologists from the Republic of Moldova, Chişinău, 2, 264-265. [14] Schwaber, M.J., Cosgrove, S.E., Gold, H.S., et al. (2004) Fluoroquinolones protective against cephalosporin resis- tance in gram-negative nosocomial pathogens. http://www. medscape.com/viewarticle/466484 [15] Asres, K., Mazumder, A. and Bucar, F. (2006) Antibacte- rial and antifungal activities of extracts of combretum molle. Ethiopian Medical Journal, 44(3), 269-277. [16] Santos, S.C. and Mello, J.C.P. (2004) Taninos. Farma- cognosia da Planta ao Medicamento, in Portuguese, 5th Edition, Porto Alegre, Florianópolis, 615-656. [17] Timbola, A.K., Szpoganicz, B., Branco, A., et al. (2002) A new flavonol from leaves of Eugenia jambolana. Fi- toterapia, 73(2), 174-176. [18] Chung, K., Lu, Z. and Chou, M. (1998) Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food and Chemical Toxi- cology, 36(12), 1053-1060. [19] Kulciţki, V., Vlad, P., Duca, G., et al. (2007) Investigation of grape seed proantocyanidins. Chemistry Journal of Moldova, 2(1), 36-50. [20] Lupascu, T. and Lupascu, L. (2006) The obtaining pro- cedure of the watersoluble enotannins. Patent Nr. 3125. MD. BOPI: 8. [21] Andrews, J.M. (2001) Determination of minimum in- hibitory concentrations. Journal of Antimicrobial Che- motherapy, 48(1), 5-16. [22] National Committee for Clinical Laboratory Standards (NCCLS) (2002) Performance standards for antimicro- bial susceptibility testing. 12th Informational Supplement M100–S12 NCCLS, Wayne. [23] Halafian, A.A. (2008) Modern statistical methods of medical research. In Russian, LKI Publishers, Moscow. [24] Matcovschi, C., Procopisin, V. and Parii, B. (2006) Phar- macotherapeutical guide. Central Printing, Chişinău. |

